Abstract
Background: Accurate brain tissue segmentation from magnetic resonance (MR) images is an important step in analysis of cerebral images. There are software packages which are used for brain segmentation. These packages usually contain a set of skull stripping, intensity non-uniformity (bias) correction and segmentation routines. Thus, assessment of the quality of the segmented gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) is needed for the neuroimaging applications.
Methods: In this paper, performance evaluation of three widely used brain segmentation software packages SPM8, FSL and Brainsuite is presented. Segmentation with SPM8 has been performed in three frameworks: i) default segmentation, ii) SPM8 New-segmentation and iii) modified version using hidden Markov random field as implemented in SPM8-VBM toolbox.
Results: The accuracy of the segmented GM, WM and CSF and the robustness of the tools against changes of image quality has been assessed using Brainweb simulated MR images and IBSR real MR images. The calculated similarity between the segmented tissues using different tools and corresponding ground truth shows variations in segmentation results.
Conclusion: A few studies has investigated GM, WM and CSF segmentation. In these studies, the skull stripping and bias correction are performed separately and they just evaluated the segmentation. Thus, in this study, assessment of complete segmentation framework consisting of pre-processing and segmentation of these packages is performed. The obtained results can assist the users in choosing an appropriate segmentation software package for the neuroimaging application of interest.
Keywords: MRI, Brain, Segmentation, SPM, FSL, Brainsuite
Introduction
With progress in magnetic resonance (MR) imaging techniques, structural and functional brain imaging are playing an important role in neuroscience and experimental medicine. Increasing anatomical scans of the human brain have emerged automated cerebral MR image analysis tools. These tools are applied to characterizing differences in the shape and neuroanatomical configuration of different brains. In particular, qualitative or quantitative information extraction from cerebral MR images relies on accurate and reliable brain tissue segmentation. The goal of medical image segmentation is to separate an image into a number of different and disjoint sets of voxels where each set corresponds to the real anatomy of the patient.
Segmentation of cerebrospinal fluid (CSF), gray matter (GM) and white matter (WM) from MR images is a challenging task. The main difficulties are tissue intensities non-uniformity (bias), noise artifacts and partial volume effect (PVE). A number of techniques have been proposed for automatic segmentation of GM, WM and CSF from cerebral MR images: statistical-based segmentation [1-4]geometrical-based segmentation [5-7], atlas-based segmentation [8-12] and learning-based segmentation methods [13]. Based on these methods, several tools have been developed by researchers to automate brain tissue segmentation. However, assessment of the quality of the segmented GM, WM and CSF is needed to compare segmentation methods. The challenge in tissue segmentation now lies in having a robust classification approach based on image intensity values representing GM, WM and CSF.
Actually, there are software packages which are most widely used in neuroimaging analysis. These packages usually contain a set of skull stripping, intensity non-uniformity (bias) correction and automated segmentation routines. There are three software packages wildly used in the neuroimaging community for structural and functional brain imaging study. These packages are: SPM [8], written by the Wellcome Department of Imaging Neuroscience at University College London, UK, FMRIB Software Library (FSL) [14], written by the Analysis Group, FMRIB, Oxford, UK, and BrainSuite [15] written by the Laboratory of Neuro Imaging at the University of California Los Angeles and the Biomedical Imaging Research Group at the University of Southern California.
There are different studies to assess the accuracy of these software packages in skull stripping. However, there is a few studies investigate GM, WM and CSF segmentation using complete package. Tsang et al. [16], compared the segmentation algorithms that are deployed in the two widely used software packages SPM5 and FSL. Klauschen et al. [17] conducted an experiment to evaluate tissue segmentation using SPM, FSL and Freesurfer. They assumed that skull stripping and bias is corrected separately using FSL. Thus, they evaluated the segmentation algorithm of the packages.
In these studies, the assessment of the segmentation packages was performed based on the same skull-striping and intensity non-uniformity correction methods. Therefore, they only compared the effects of the tissue segmentation method. The neuroscientists usually select and apply a package for skull striping, intensity non-uniformity correction and segmentation for brain volumetry or functional analysis. However, the previous comparison studies just evaluated part of these packages such as segmentation [17] or registration [18]. On the other hand, the latest versions of these packages perform the segmentation in different methods which are not studied in former studies. Therefore, comparison of complete segmentation framework consisting of pre-processing and segmentation of these packages is necessary.
In this study, we investigated the accuracy of the latest version of software packages SPM, FSL and Brainsuite for brain tissue segmentation which are the most widely used brain tissue segmentation software packages. Furthermore, different brain tissue segmentation methods developed in these packages, i.e., New-segmentation in SPM8, are studies and compared. The comparison is performed using a variety of independent datasets, and the most widely used metrics in the literature. According to the authors knowledge such complete comprehensive analysis has not been previously carried out and can be used by users of these software packages.
A description of the datasets used, brief review on the segmentation routines applied in these three packages and details of the quantitative measures used in validation are described in the next section. The results of experiments are described in the Results section. Finally, the discussion is given in the Discussion section.
Materials and Methods
MRI Brain Data
In order to assess the performance of the methods, validation was performed based on simulated images using Brainweb simulator and real MR images.
BrainWeb-Simulated Brain Data
The simulated 3D MR images (181´217´181 voxels of 1mm 3 isotropic resolution) which are used as test data, are provided by the BrainWeb simulated brain database from the McGill University. This database provides realistic simulations of MRI acquisition with different levels of intensity non-uniformity and noise. Simulated MR images are generated based on an anatomical model of normal brain. Eighteen datasets with different noise level (n) range between 0%, 1%, 3%, 5%, 7% and 9% and intensity non-uniformity (rf) with 0%, 20% and 40% are used.
IBSR-Real Data
In order to evaluate the performance of the methods on real MR data, the cerebral MR datasets from 20 normal subjects provided by Center for Morphometric Analysis at Massachusetts General Hospital (IBSR) are used. The real MR brain data and their hand-guided expert segmentation results are available at these datasets. IBSR provides performance results from five other automatic segmentation methods making it convenient to compare the results with those reported by others. These 20 datasets involve different levels of difficulty such as low contrast scans, relatively smaller brain volumes and considerable intensity non-uniformity.
Segmentation Methods
There are number of tools available for brain tissue segmentation with different usages. In this study, the accuracy of three fully automated brain tissue segmentation packages SPM, FSL and Brainsuite were compared. These packages incorporate tools for intensity non-uniformity correction and skull-stripping/masking procedures. FSL and BrainSuite perform skull-stripping and intensity non-uniformity correction as pre-processing for the segmentation procedure, while SPM does these steps concurrently during segmentation.
SPM
The SPM (Statistical Parametric Mapping) software SPM8 is a MATLAB software package implementing statistical methods for analysis of functional and structural neuroimages. Brain tissue segmentation with SPM8 can be performed in three frameworks: i) default segmentation, ii) SPM8 New-segmentation and iii) modified version using hidden Markov random field as an additional spatial constraint as implemented in SPM-VBM toolbox.
SPM8 default Segmentation
The default segmentation routine implemented in SPM [8] is based on a unified segmentation model that performs tissue segmentation, registration and intensity non-uniformity (bias) correction all in the same model. The principal idea of this method is to model image intensities as a mixture of k Gaussians, where each Gaussian cluster is modelled by its mean ( µk), variance ( σk) and a mixing proportion. In the unified model, the tissue probability maps (TPMs) are used as a priori information of the tissue classes. The Bayes rule is employed to produce the posterior probability of each tissue class. In SPM, the segmentation routine automatically segments the input MR image into GM, WM, and CSF. However, the classification is probabilistic in the sense that a probability value of belonging to each of the classes is assigned to each voxel in the output images. When generating binary images, voxels corresponding to grater tissue probability in the maps are counted as members of that particular class.
The recent version of SPM software SPM8 also takes advantage of different improved models and a more robust initial affine transformation for registration, an extended set of tissue probability maps, a different treatment of the mixing proportions and the capability of working with multispectral data. In this study, all of the segmentations by SPM8 are performed using the default atlas (a modified version of the ICBM Tissue Probabilistic Atlas, available at http://www.loni.ucla.edu/ICBM/ICBM_Probabilistic.html) and parameters for this version.
SPM8 New-segmentation
In SPM8 a New-segmentation routine is implemented which is an extension of the default segmentation routine. The algorithm is essentially the same as default unified segmentation with the following modifications:
A different treatment of the mixing proportions.
The use of an improved registration model.
The ability to use multispectral data.
An extended set of tissue probability maps, which allows a different treatment of non-brain voxels.
A more robust initial affine registration.
The New-segmentation routine can segment the brain into six tissue classes: GM, WM, CSF, bone, soft tissue, and air/background.
SPM8-VBM
VBM is a collection of extensions to the segmentation algorithm of SPM which utilizes hidden Makrov random field as post processing [19]. This modification to the algorithm helps in determining the probability a given voxel belong to a tissue class which is achieved by calculating the MRF energy for a given voxel, based on its proximity to the surrounding voxels.
FSL
FMRIB’s Software Library (FSL) is a software package developed by members of the Oxford Centre for Functional MRI of the Brain (Oxford University) which is composed of image analysis and statistical tools for neuroimage data study [14]. Although FSL has many different modules for functional and structural MRI data analysis, in this section we are only going to focus in FMRIB Automated Segmentation Tool (FAST) which developed for segmentation of brain tissues. The FSL-FAST segmentation routine is based on a Hidden Markov Random Field (HMRF) model that is optimized using the Expectation-Maximization algorithm [20].
In this study, FSL version 4.1 is employed for the whole process of registration, skull stripping and brain tissue segmentation. Since the FSL-FAST brain tissue segmentation requires skull stripped version of input MRI data, as the first step of tissue segmentation, skull stripping is performed using the FSL’s own brain extraction tool. Then, in the second step, FAST tool using probability maps as its default settings is used to segment the brain into three tissue classes of GM, WM and CSF and performing bias correction.
Skull stripping using BET
Intracranial segmentation commonly referred to as “skull-stripping” removes extra-cerebral tissues such as skull, eyeballs, and skin. Skull-stripping facilitates image processing such as surface rendering, cortical flattening, image registration, and tissue segmentation. Thus, as the first step of the FSL segmentation, the “Brain Extraction Tool version 2.1 (BET)” integrated in FSL software is used to perform skull stripping and remove non-brain parts of the image.
Brain tissue segmentation using FAST
MR imaging suffer from non-homogeneity in radio-frequency field which results in non-biological intensity non-uniformities across the imaged brain. Thus, in the second step FAST toolbox version 4.1 (FMRIB’s Automated Segmentation Tool) integrated in FSL software is used to segment GM, WM and CSF, whilst also correcting for intensity non-uniformity. Accurate intensity non-uniformity correction requires segmentation knowledge while perfect segmentation requires intensity non-uniformity to be corrected. The segmentation routine implemented in FAST toolbox is based on HMRF model and associated expectation-maximization algorithm. In this method, the histogram of input image is modeled as a mixture of Gaussians with mean and variance for each class. The segmentation allows a reconstruction of the image; subtracting this from the real image gives an estimate of the non-uniformity. This whole process is then iterated between segmentation and intensity non-uniformity correction until convergence [14, 20]. The resulting outputs are intensity non-uniformity corrected version and segmented GM, WM and CSF from input data.
BrainSuite
BrainSuite is a suite of image analysis tools designed to process MRI data of the human head. Brain tissue segmentation routine of BrainSuite starts by skull-striping using brain surface extractor (BSE) tool followed by bias field corrector (BFC) tool for intensity non-uniformities correction and partial volume classifier (PVC) tool for tissue segmentation.
Skull stripping using BSE
The brain tissue segmentation in Brainsuite is started by applying BSE tool to remove non-brain tissues from input cerebral MRI data. It operates by using an edge detector to find a boundary between the brain and the skull by using mathematical morphological operators to enhance the result. The edge detection result is improved by the application of an isotropic diffusion filter before the edge detection is performed. The parameters that have been chosen are presented in table 1 for applied data sets.
Table 1.
The parameters that have been chosen for each simulated and real dataset.
| Part | Item | BrainWeb | IBSR |
|---|---|---|---|
| BSE | Diffusion Iterations | 8 | 3 |
| Diffusion Constant | 55 | 25 | |
| Edge Constant | 0.65 | 0.64 | |
| Erosion Size | 4 | 1 | |
| BFC | Histogram Radius | 12 | 12 |
| Sample Spacing | 16 | 16 | |
| Control Point Spacing | 64 | 64 | |
| Spline Stiffness | 0.0001 | 0.0001 | |
| ROI Shape | Cubiod | Cubiod | |
| Lower Limit | 0.9 | 0.9 | |
| Upper Limit | 1.1 | 1.1 | |
| PVC | Spatial Prior | 0.1 | 0.1 |
Intensity non-uniformities correction using BFC
After skull stripping, BFC tool is used to correct the image intensity non-uniformities that are due to magnetic field variations. It estimates a correction field for the brain region based on a series of local estimates of the tissue gain variation.
Brain tissue segmentation using PVC
As the last step of the brain tissue segmentation, the PVC tool is used for tissue classification. It is performed by partial volume classifier. It assigns an integer tissue label to each voxel in the image. These labels correspond to the type of tissue that is estimated to be in that voxel. It can generate labeling with three tissue classes output that are GM, WM and CSF and also with six tissue classes that are composed of combination of these voxels.
Evaluation method
Similarity metrics
Two similarity metrics are used for quantitative evaluation of the proposed method: Dice coefficient [21] or similarity index and Jaccard coefficient [22]. These metrics represent spatial overlap between two binary images and their values range between 0 (no overlap) and 1 (perfect agreement) as they are expressed as percentage in the following:
Success and error rate
The sensitivity (true positive fraction, TPF) refers to the ability to correctly identify appropriate tissue in the segmented mask. The higher sensitivity shows the lower missed true tissue voxels. It is defined as follows.
The specificity (true negative fraction, TNF) refers to the ability of the proposed segmentation method to correctly remove non-desired voxels. The higher Specificity shows the lower missed true non-desired voxels.
Sensitivity and specificity are the measures that are computed based on true positive (TP), false negative (FN) and false positive (FP), true negative (TN).
Results
In order to assess the relative accuracy of three brain tissue segmentation tools: SPM8, FSL and Brainsuite, they are applied to segment GM, WM and CSF from simulated and IBSR real MR images.
Comparison based on simulated data
The resulting GM,WM and CSF tissues from segmentation of simulated MR image with intensity non-uniformity rf=0% and noise level n=0% using brain tissue segmentation tools: SPM8 (including SPM8-Seg, SPM8-VBM, SPM8-NewSeg), FSL and Brainsuite are shown in figure 1. Figure 2 illustrates the quantitative assessment of the accuracy of the segmented GM,WM and CSF from simulated MR images with intensity non-uniformity levels 0%, 20% and 40% in different noise levels 0%, 1%, 3%, 5%, 7% and 9% based on Dice similarity metric.
Figure 1.
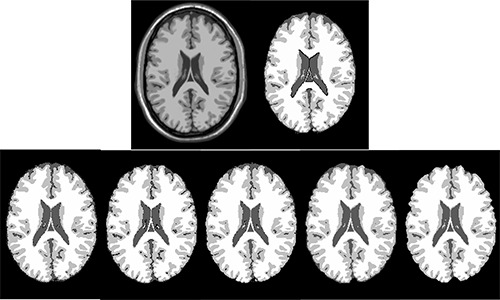
The segmented GM, WM and CSF from simulated MR image with n=0% and rf=0%. The first row shows the input MR image and the ground truth for GM, WM and CSF. The second row from left to right shows the segmentation results using SPM8-Seg, SPM8-VBM, SPM8-NewSeg, FSL and Brainsuite, respectively.
Figure 2.
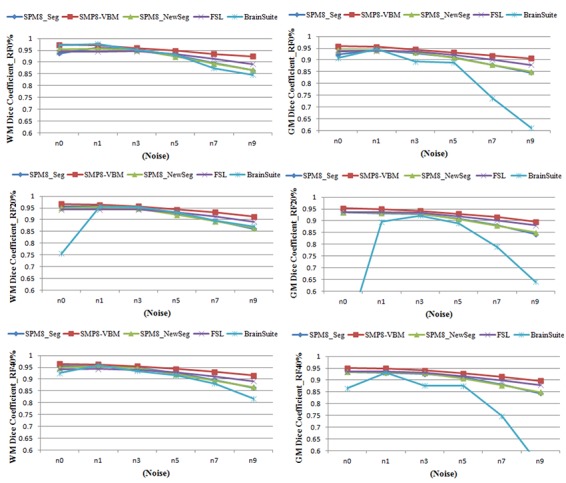
Quantitative evaluation of the segmented GM and WM from simulated MR images based on Dice similarity metric using three packages: SPM, FSL and Brainsuite.
Table 2 shows the mean similarity based on Dice and Jaccard metrics for segmented GM, WM and CSF from 18 simulated MR images. Furthermore, the mean sensitivity and specificity are shown. Here, SPM8 and FSL results are relatively close when compared with Brainsuite. However, SPM8-VBM performs better than two other packages. This can be seen from figure 2 where SPM8-VBM segmented accurately the WM and GM even in high level of noise and intensity non-uniformity.
Table 2.
Comparison of segmentation accuracy of SPM8, FSL and Brainsuite using Brainweb simulated MR images.
| Tissue | Software Packages | Dice | Jaccarde | Sensitivity | Specificity |
|---|---|---|---|---|---|
| WM | SPM8-Seg | 0.92±0.04 | 0.86±0.06 | 0.92±0.02 | 0.99±0.01 |
| SPM8-VBM | 0.95±0.02 | 0.90±0.03 | 0.96±0.01 | 0.99±0.01 | |
| SPM8-NewSeg | 0.92±0.03 | 0.86±0.06 | 0.94±0.04 | 0.99±0.01 | |
| FSL | 0.93±0.02 | 0.86±0.03 | 0.89±0.02 | 0.99±0.01 | |
| Brainsuite | 0.91±0.06 | 0.84±0.09 | 0.90±0.10 | 0.99±0.01 | |
| GM | SPM8-Seg | 0.90±0.03 | 0.83±0.06 | 0.90±0.04 | 0.99±0.01 |
| SPM8-VBM | 0.93±0.02 | 0.87±0.04 | 0.92±0.03 | 0.99±0.01 | |
| SPM8-NewSeg | 0.91±0.03 | 0.83±0.06 | 0.91±0.03 | 0.99±0.01 | |
| FSL | 0.92±0.02 | 0.85±0.04 | 0.95±0.02 | 0.98±0.01 | |
| Brainsuite | 0.80±0.16 | 0.68±0.20 | 0.74±0.21 | 0.99±0.01 | |
| CSF | SPM8-Seg | 0.62±0.05 | 0.46±0.05 | 0.96±0.04 | 0.94±0.01 |
| SPM8-VBM | 0.76±0.02 | 0.61±0.02 | 0.66±0.02 | 0.99±0.01 | |
| SPM8-NewSeg | 0.74±0.07 | 0.59±0.09 | 0.79±0.04 | 0.98±0.01 | |
| FSL | 0.74±0.06 | 0.6±0.08 | 0.66±0.09 | 0.99±0.01 | |
| Brainsuite | 0.46±0.16 | 0.31±0.13 | 0.37±0.15 | 0.99±0.01 |
In order to investigate the significant differences between segmentation methods of SPM8, t-test was used to find significant difference between SPM8-Seg and SPM8-NewSeg. However, we could not find a significance difference between them.
Comparison based on Real IBSR data
The extracted GM, WM and CSF tissues from a selected real MR image using the applied brain tissue segmentation tools are shown in figure 3. Figure 4 shows the accuracy of segmented GM and WM from IBSR real MR images using Dice similarity metric. Furthermore, since the segmentation accuracy provided by the IBSR is measured by the Jaccard similarity metric, the mean Dice and Jaccarde similarity metrics are shown in table 3.
Figure 3.
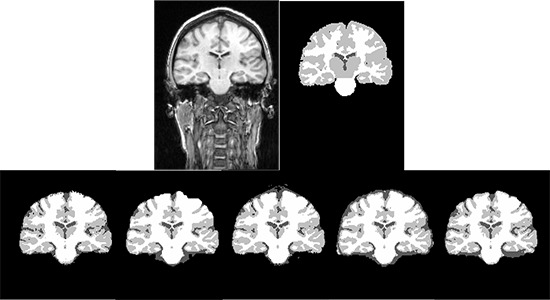
The segmented GM, WM and CSF from a selected subject from IBSR real MR images. The first row shows the input MR image and the ground truth for GM, WM and CSF. The second row from left to right shows the segmentation results using SPM8-Seg, SPM8-VBM, SPM8-NewSeg, FSL and Brainsuite, respectively.
Figure 4.
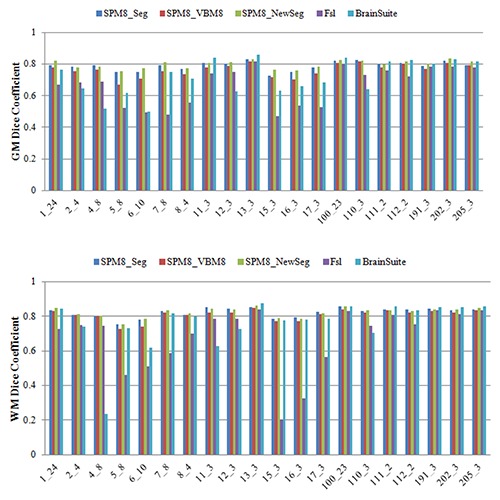
Dice similarity metric for segmented WM and GM from IBSR real MR images
Table 3.
Comparison of segmentation accuracy of SPM8, FSL and Brainsuite using IBSR real MR images
| Tissue | Software Packages | Dice | Jaccarde | Sensitivity | Specificity |
|---|---|---|---|---|---|
| WM | SPM8-Seg | 0.82±0.03 | 0.70±0.04 | 0.81±0.02 | 0.99±0.01 |
| SPM8-VBM | 0.81±0.03 | 0.68±0.04 | 0.87±0.05 | 0.99±0.01 | |
| SPM8-NewSeg | 0.82±0.03 | 0.70±0.04 | 0.85±0.02 | 0.99±0.01 | |
| FSL | 0.68±0.18 | 0.54±0.18 | 0.86±0.19 | 0.97±0.01 | |
| Brainsuite | 0.76±0.14 | 0.63±0.15 | 0.79±0.18 | 0.99±0.01 | |
| SPM8-Seg | 0.79±0.03 | 0.65±0.04 | 0.73±0.05 | 0.99±0.01 | |
| GM | SPM8-VBM | 0.76±0.04 | 0.62±0.05 | 0.67±0.05 | 0.99±0.01 |
| SPM8-NewSeg | 0.80±0.02 | 0.66±0.03 | 0.73±0.03 | 0.99±0.01 | |
| FSL | 0.66±0.12 | 0.51±0.13 | 0.67±0.06 | 0.98±0.02 | |
| Brainsuite | 0.72±0.11 | 0.57±0.13 | 0.64±0.15 | 0.99±0.01 |
Similar to segmented WM and GM from simulated images, here, SPM8 performed better than FSL and Brainsuite for real MR images. On the other words, comparing the results of IBSR dataset against the results of the Brainweb dataset showed that the performance of the SPM8 algorithm was consistent for both datasets.
However, comparison of the results obtained using SPM8 toolboxes showed that the SPM8-NewSeg segmentation algorithm provided higher similarity values when overlaid against the ground truth of the expert segmented tissue images. This result for SPM8 was confirmed by t-test with p<0.05.
Brain tissue segmentation
Brain (consisting of GM and WM) extraction is one of the most time-consuming steps in neuroimage analysis. While numerous brain extraction methods have been developed to perform this step automatically, their output varies and may affect the results of subsequent image analysis. Thus, the extracted brain using three popular neuroimage analysis tools SPM8, FSL and BrainSuite were compared. The brain mask was created as the binarized sum of the GM and WM after segmentation.
Figure 5 shows the accuracy of segmented brain in term of Dice metric for simulated MR image in different noise and intensity non-uniformity levels. Table 4 shows quantitative results in term of mean similarity for segmented brain using different tools. It can be seen that SPM8 performs better than other tools in term of accuracy. Comparison between segmentation toolboxes of SPM8 shows that the SPM8-VBM which utilizes MRF has better performance. On the other hand, the t-test showed no scientific difference between SPM8-Seg and SPM8-NewSeg tools integrated in SPM8.
Figure 5.
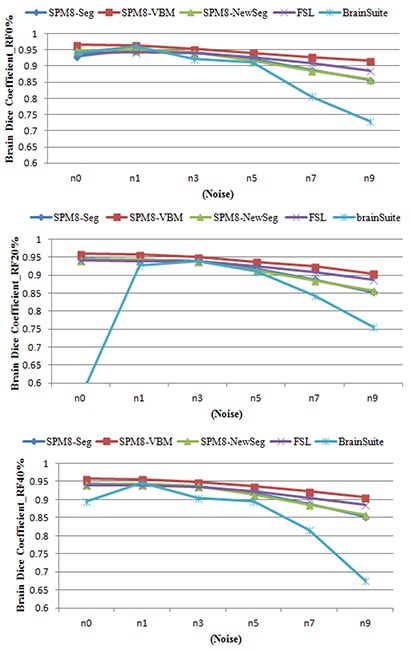
Dice similarity metric for segmented Brain from Brainwebsimulated MR images.
Table 4.
Comparison of brain segmentation accuracy using SPM8, FSL and Brainsuite based on BrainwebSimulated dataset and IBSR real dataset
| Tissue | Software Packages | Dice | Jaccarde | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Brainweb | SPM8-Seg | 0.91±.03 | 0.86±.06 | 0.91±0.02 | 0.99±0.01 |
| SPM8-VBM | 0.94±.02 | 0.89±.03 | 0.94±0.02 | 0.99±0.01 | |
| SPM8-NewSeg | 0.91±.03 | 0.84±.06 | 0.92±0.03 | 0.99±0.01 | |
| FSL | 0.92±.02 | 0.86±.04 | 0.92±0.02 | 0.99±0.01 | |
| Brainsuite | 0.85±.11 | 0.76±.14 | 0.82±0.16 | 0.99±0.01 | |
| IBSR | SPM8-Seg | 0.80±.03 | 0.88±.03 | 0.77±0.02 | 0.99±0.01 |
| SPM8-VBM | 0.79±.04 | 0.88±.03 | 077±0.05 | 0.99±0.01 | |
| SPM8-NewSeg | 0.81±.02 | 0.89±.04 | 0.72±0.02 | 0.99±0.01 | |
| FSL | 0.67±.15 | 0.89±.03 | 0.76±0.08 | 0.97±0.02 | |
| Brainsuite | 0.74±.12 | 0.89±.13 | 0.72±0.14 | 0.99±0.01 |
The same comparison was made to assess the accuracy of segmented brain using real IBSR MR images. The resulting Dice coefficients of segmented brain (consisting of GM and WM) were shown in figure 6. Table 4 shows the mean similarity between the segmented brain and corresponding ground truth based on Dice and Jaccard similarity metrics. Similar to the results obtained using simulated images, the SPM8 toolbox provide better results in compared to the two other packages.
Figure 6.
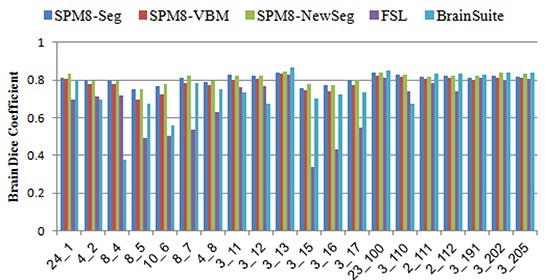
Dice similarity metric for segmented Brain from IBSR real MR images.
Discussion and Conclusion
Segmentation of brain tissues GM, WM and CSF from MR images is challenging task because of some difficulties like tissue intensities non-uniformity, noise artifacts and partial volume effect. Beside of different techniques that have been proposed for automatic segmentation of brain tissues from cerebral MR images, there are software packages which are most widely used in neuroimaging analysis. Among them, SPM8, FSL and BrainSuite software packages are usually applied by scientists for structural and functional analysis of brain.
In this paper, we quantitatively evaluated the segmentation performance of these software packages through a series of experiments. Unlike the existing evaluations that perform preprocessing such as skull striping and intensity non-uniformity by the same tool and just the segmentation routine is evaluated, in this study, whole segmentation consisting of pre-processing and tissue segmentation were performed by each tool. Standard procedures with parameters that give best results were used.
Our analysis was performed based on Brainweb simulated MR images and IBSR real MR images. The results show variation in segmentation performance of the tools for brain tissue segmentation in term of accuracy. Difference in accuracy is the fact that these tools are designed for slightly different purpose, even though all can be used for m brain tissue segmentation.
While SPM is designed to perform segmentation of brain tissues consisting of GM, WM and CSF, the FSL and Brainsuite can segment sub-cortical structures also. On the other words, FSL and Brainsuite are general propose tools in comparison with SPM.
Comparison of the tools based on simulated and real MR images show that performance of the Brainsuite was influenced by noise and intensity non-uniformity. The FSL performance was influenced by the noise of the image and little by the image intensity non-homogeneity. The accuracy of segmented GM and WM were reduced about 5% by increasing the noise from 0% to 9% in the simulated MR images. Finally, the obtained results show that similar to two other tools, the segmentation was influenced by noise but little by image intensity non-homogeneity. However, applying MRF as post-processing in VBM toolbox of SPM reduce the influence of noise and improves the segmentation accuracy of GM and WM. The little influence of intensity non-homogeneity on the segmentation results is results of intensity non-homogeneity estimation during segmentation procedure in SPM8 and FSL.
On the other hand, the accuracy of the tools in brain segmentation was evaluated. The qualitative evaluation using Dice and Jaccard metrics shows SPM8 performs better than others. Beside, based on t-test, no significant difference between SPM8-Seg and SPM8-NewSeg was found.
Conflict of Interest: None
References
- 1.Bezdek JC, Hall LO, Clarke LP. Review of MRI Segmentation Techniques using Pattern Recognition. Med Phys. 1993;20:1033–48. doi: 10.1118/1.597000. [DOI] [PubMed] [Google Scholar]
- 2.Held K, Rota Kops, Krause BJ, Wells WM, Kikinis R, Muller-Gartner HW. Markov random field segmentation of brain MR images. IEEE Trans Med Imaging. 1997;16:878–86. doi: 10.1109/42.650883. doi: 10.1109/42.650883. PubMed PMID: 9533587. [DOI] [PubMed] [Google Scholar]
- 3.Liew A, Yan H. An Adaptive Spatial Fuzzy Clustering Algorithm for MR Image Segmentation. IEEE Trans Med Imag. 2003;22:1063–75. doi: 10.1109/TMI.2003.816956. [DOI] [PubMed] [Google Scholar]
- 4.Wells WM, Grimson WL, Kikinis R, Jolesz FA. Adaptive segmentation of MRI data. IEEE Trans Med Imaging. 1996;15:429–42. doi: 10.1109/42.511747. doi: 10.1109/42.511747. PubMed PMID: 18215925. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Kao C, Gore JC, Ding Z. Minimization of region-scalable fitting energy for image segmentation. IEEE Trans Imag Process. 2008 ;17: 1940–9. doi: 10.1109/TIP.2008.2002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahvaran Z, Kazemi K, Helfroush MS, Jafarian N, Noorizadeh N. Variational level set combined with Markov random field modeling for simultaneous intensity non-uniformity correction and segmentation of MR images. J Neurosci Methods. 2012;209:280–9. doi: 10.1016/j.jneumeth.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Chen Y, Pan X, Hong X, Xia D. Level set segmentation of brain magnetic resonance images based on local Gaussian distribution fitting energy. J Neurosci Methods. 2010 ;188:316–25. doi: 10.1016/j.jneumeth.2010.03.004. doi: 10.1016/j.jneumeth.2010.03.004. PubMed PMID: 20230858. [DOI] [PubMed] [Google Scholar]
- 8.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. doi: 10.1016/j.neuroimage.2005.02.018. PubMed PMID: 15955494. [DOI] [PubMed] [Google Scholar]
- 9.Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3D Model-Based Neuroanatomical Segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- 10.Marroquin JL, Vemuri BC, Botello S, Calderon F, Fernandez-Bouzas A. An accurate and efficient bayesian method for automatic segmentation of brain MRI. IEEE Trans Med Imaging. 2002;21:934–45. doi: 10.1109/TMI.2002.803119. doi: 10.1109/tmi.2002.803119. PubMed PMID: 12472266. [DOI] [PubMed] [Google Scholar]
- 11.Van Leemput, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Trans Med Imaging. 1999;18:897–908. doi: 10.1109/42.811270. doi: 10.1109/42.811270. PubMed PMID: 10628949. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Bai J. Atlas-based fuzzy connectedness segmentation and intensity nonuniformity correction applied to brain MRI. IEEE Trans Biomed Eng. 2007;54:122–9. doi: 10.1109/TBME.2006.884645. doi: 10.1109/tbme.2006.884645. PubMed PMID: 17260863. [DOI] [PubMed] [Google Scholar]
- 13.Hall LO, Bensaid AM, Clarke LP, Velthuizen RP, Silbiger MS, Bezdek JC. A comparison of neural network and fuzzy clustering techniques in segmenting magnetic resonance images of the brain. IEEE Trans Neural Netw. 1992;3:672–82. doi: 10.1109/72.159057. doi: 10.1109/72.159057. PubMed PMID: 18276467. [DOI] [PubMed] [Google Scholar]
- 14.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. doi: 10.1016/j.neuroimage.2004.07.051. PubMed PMID: 15501092. [DOI] [PubMed] [Google Scholar]
- 15.Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–42. doi: 10.1016/s1361-8415(02)00054-3. PubMed PMID: 12045000. [DOI] [PubMed] [Google Scholar]
- 16.Tsang O, Gholipour A, Kehtarnavaz N, Gopinath K, Briggs R, Panahi I. Comparison of tissue segmentation algorithms in neuroimage analysis software tools. Conf Proc IEEE Eng Med Biol Soc . 2008;2008:3924–8. doi: 10.1109/IEMBS.2008.4650068. doi: 10.1109/iembs.2008.4650068. PubMed PMID: 19163571. [DOI] [PubMed] [Google Scholar]
- 17.Klauschen F, Goldman A, Barra V, Meyer-Lindenberg A, Lundervold A. Evaluation of automated brain MR image segmentation and volumetry methods. Hum Brain Mapp. 2009;30:1310–27. doi: 10.1002/hbm.20599. doi: 10.1002/hbm.20599. PubMed PMID: 18537111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M, Carmichael O, Lopez-Garcia P, Carter CS, Aizenstein HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Mapp. 2006;27:747–54. doi: 10.1002/hbm.20216. doi: 10.1002/hbm.20216. PubMed PMID: 16463385; PubMed Central PMCID: PMC2886594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging. 2005;24:1548–65. doi: 10.1109/TMI.2005.857652. doi: 10.1109/tmi.2005.857652. PubMed PMID: 16350916. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001 ;20:45–57. doi: 10.1109/42.906424. doi: 10.1109/42.906424. PubMed PMID: 11293691. [DOI] [PubMed] [Google Scholar]
- 21.Dice LR. Measures of the Amount of Ecologic Association between Species. Ecology. 1945;26:297–302. [Google Scholar]
- 22.Jaccard P. The Distribution of Flora in the Alpine Zone. New Phytol. 1912;11:37–50. [Google Scholar]


