Abstract
Background: Sun protection materials have been one of the major concerns in pharmaceutical industry since almost one century ago. Various materials have been found to have such an effect but there are still many unknown substances that have not been discovered.
Objective: To introduce a novel mineral-based sun lotion with considerable UV absorption properties compared to commercially available sunscreens.
Method: UV absorption properties of transparent plastic sheets covered by a uniform cream layer of different mineral-based sun lotions and a commercially available sun lotion were tested.
Results: Sun lotions containing specific proportion of bentonite and zeolite minerals were capable of absorbing the highest level of UV light compared to that of the commercially available sun lotion.
Conclusion: Mineral-based sun lotions can be considered as cost effective alternatives for current commercial sunscreens.
Keywords: UV, Sunscreens, Skin, Mineral, Bentonite, Zeolite
Introduction
The solar ultraviolet spectrum which reaches the earth’s surface consists of two major bands, UVA with a wavelength of 320 to 400 nm and UVB with a wavelength of 280 to 320 nm. In spite of the lower energy in UVA band, it accounts for 95% of the sunlight’s UV exposure [1]. At the cellular level, generation of reactive oxygen species (ROS) due to UVA radiation causes a great deal of oxidative stress and leads to harmful effects on the cell, including cell death through necrosis or apoptosis, DNA damage, and other disorders [1, 2].
Moreover, DNA damage and mutation are specific effects of the exposure of human skin to UVB radiation; i.e. effects of UVB on the DNA molecule such as base changes and formation of cyclobutane-pyrimidine dimers (CPD), pyrimidine-pyrimidone (6-4) photodimers (6-4PP), and 8-hydroxy-deoxyguanosine (8-OHdG) [3-5].
It is widely known that health problems associated with UV exposure are skin cancer, skin aging, and other biological disorders due to physical, chemical, and histological changes in the skin [6-8]. There are many successful methods for treatment of skin damages but sun blocking agents are appropriate methods, because almost all of these adverse effects could be avoided through proper prevention [2, 9, 10].
Based on these facts, sunscreen agents are being widely used to prevent UV radiation damages, and are largely incorporated into products such as creams, lotions and cosmetic products. Evaluation of sunscreen efficacy is expressed by Sun Protection Factor (SPF) which is defined as the UV energy exposed to the protected skin that causes Minimum Erythema Dose (MED). There are many questions about SPF in sunscreens because it is able to prevent sunburn and has no effect on other UV damages such as skin aging and DNA damages. The ability of sunscreens to absorb or reflect UVA and UVB is different and sunburn is believed to be the results of UVB. This means that UVA has been neglected in SPF, but UVA induces free radicals that can affect DNA [11-14].
To date, a wide variety of compounds have used in sunscreen products that absorb or reflect UVA and UVB. However safety and efficacy of these materials are very important. These materials must have no risk and side effects; for example, some of these materials such as PABA (p-aminobenzoic acid) and benzophenone have been banned in Europe because of their toxic effects. [14, 15]
Additionally, one of the important factors in sunscreens is their photostability because some sunscreen’s ingredients lose their protection after UV or light exposure. Hence, it is a challenge to standardize the materials with photostability [16, 17]. Recently, finding materials to protect the skin against both UVA and UVB, has attracted worldwide attention. Therefore, nanoparticles such as ZnO and TiO2 are found to be good inorganic materials for broad beam protection. The spectrum absorbed by ZnO is lower than 380 nm and TiO2 absorbs a spectrum lower than 365 nm. In higher wavelengths, only scattering occurs by ZnO and TiO2, so ZnO is used to provide protection against UVA while TiO2 is used for protection against both UVA and UVB [18]. Sunscreen ingredient studies show that UV radiation exposure on TiO2 can increase photogeneration of reactive oxygen species (ROS), and encapsulation of TiO2 within Zeolite decreases the generation of ROS [19]. Nanotechnology research on UV protection demonstrates that nanoparticles of natural Zeolite applied on textile materials scatter the UV radiation and it has good UV protection [20]. Based on the above facts, it is necessary to find a new sunscreen material for solar UV protection with high protection and photostability.
Material and Methods
XRD and XRF methods were used to determine the structure of zeolite and bentonite minerals. The UV absorption has to be evaluated in all experimental steps; therefore, all the experiments have been done in the peak UV exposure during the midday in a periodic manner (i.e. absorption of series of all specimens were measured consecutively and then the test s were performed again) and the UV meter apparatus was used perpendicularly to the sun beam in order to get the most intensive light beam in each test.
Specimens
The specimens were transparent plastic sheets (10×10 cm2) covered by a uniform cream layer. In order to make a uniform thickness all over the transparent plastic sheets, a silk printing net was used and the average of five different measurements for each sheet was used for evaluation in each experiment.
Experimental groups
There were eight experimental tests as follows:
Test 1. Natural sunlight (No UV absorption)
Test 2. UV absorption by the transparent sheet only
Test 3. UV absorption by control cream (cream without any minerals)
Test 4. UV absorption for cream containing 5% zeolite
Test 5. UV absorption for cream containing 10% zeolite
Test 6. UV absorption for cream containing 5% bentonite
Test 7. UV absorption for cream containing 10% bentonite
Test 8. UV absorption for a commercially available cream (SPF=30)
Results and discussion
Results obtained in this study showed that the bentonite and zeolite minerals were capable of absorbing the highest level of UV light compared to that of the control cream. It was also shown that bentonite and zeolite minerals have UV absorption as much as other conventional sunscreens which are commercially available in the market. The data are shown in figure 1. Our results are partially in line with the findings of a study conducted in 2006 by Perioli et al. who investigated the new sunscreen formulations stabilized by intercalating PABA, within the lamellar structures of two kinds of hydrotalcite. According to these investigators, both intercalated products showed an increased protection range and, in one case, an improved sunscreen photostability. They concluded that the use of these materials resulted in a good strategic technological approach in order to increase the efficacy and safety of solar products [21].
Figure 1.
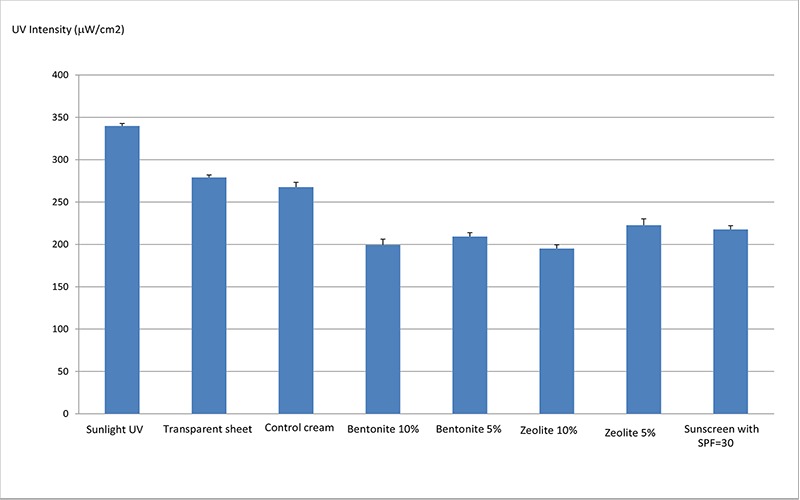
UV absorption of different sun blocking agents
Conclusion
In this study, we demonstrated the UV absorption properties of two minerals, bentonite and zeolite, and their use in sunscreen lotions. Further research is recommended to be conducted in this field of research to determine whether any combination with other materials could enhance the UV absorption features of these materials or not.
Conflict of Interest: None
References
- 1.Bernerd F, Vioux C, Lejeune F, Asselineau D. The sun protection factor (SPF) inadequately defines broad spectrum photoprotection: demonstration using skin reconstructed in vitro exposed to UVA, UVB or UV-solar simulated radiation. Eur J Dermatol. 2003;13:242–9. [PubMed] [Google Scholar]
- 2.Tarozzi A, Marchesl A, Hrelia S, et al. Protective Effects of Cyanidin-3-O-β-glucopyranoside Against UVA-induced Oxidative Stress in Human Keratinocytes. Photochem Photobiol. 2005;81:623–9. doi: 10.1562/2004-06-14-RA-200. doi: 10.1111/j.1751-1097.2005.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 3.Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res. 2005;571:3–17. doi: 10.1016/j.mrfmmm.2004.09.012. doi: 10.1016/j.mrfmmm.2004.09.012. PubMed PMID: 15748634. [DOI] [PubMed] [Google Scholar]
- 4.Ichihashi M, Ueda M, Budiyanto A, et al. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. PubMed PMID: 12821280. [DOI] [PubMed] [Google Scholar]
- 5.Cadet J, Courdavault S, Ravanat JL, Douki T. UVB and UVA radiation mediated damage to isolated and cellular DNA. Pure and Applied Chemistry. 2005;77:947–61. [Google Scholar]
- 6.Thurstan SA, Gibbs NK, Langton AK, et al. Chemical consequences of cutaneous photoageing. Chem Cent J. 2012;6:34. doi: 10.1186/1752-153X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soehnge H, Ouhtit A, Ananthaswamy ON. Mechanisms of induction of skin cancer by UV radiation. Front Biosci. 1997;2:d538–51. doi: 10.2741/a211. PubMed PMID: 9343491. [DOI] [PubMed] [Google Scholar]
- 8.Svobodova A, Walterova D, Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med FacUnivPalacky Olomouc Czech Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. PubMed PMID: 16936899. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf N, Irby C, Katiyar SK, Elmets CA. Photoprotective effects of green tea polyphenols. Photodermatol Photoimmunol Photomed. 2007;23:48–56. doi: 10.1111/j.1600-0781.2007.00262.x. doi: 10.1111/j.1600-0781.2007.00262.x. PubMed PMID: 17254040. [DOI] [PubMed] [Google Scholar]
- 10.Scarlett WL. Ultraviolet radiation: sun exposure, tanning beds, and vitamin D levels. What you need to know and how to decrease the risk of skin cancer. J Am Osteopath Assoc. 2003;103:371–5. PubMed PMID: 12956250. [PubMed] [Google Scholar]
- 11.Dutra EA, Oliveira DA, Kedor-Hackmann ER, Santoro MI. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Braz J Pharma Sci. 2004;40:381–5. [Google Scholar]
- 12.Ho TY. Sunscreens: Is Looking at Sun Protection Factor Enough? Hong Kong Dermatology and Venereology Bulletin. 2001;9:100–8. [Google Scholar]
- 13.Haywood R, Wardman P, Sanders R, Linge C. Sunscreens Inadequately Protect Against Ultraviolet-A-InducedFree Radicals in Skin: Implications for Skin Aging and Melanoma? J Invest Dermatol. 2003;121:862–8. doi: 10.1046/j.1523-1747.2003.12498.x. [DOI] [PubMed] [Google Scholar]
- 14.Beckett A, Mcclure B, Zimmerman K. Benzophenone and Padimate-O Protect Saccharomyces cerevisiae From UV Radiation and Cause Little Harm From UV-Induced Reactive Chemical Species. J Exp Microbiol Immunol. 2004;5:37–43. [Google Scholar]
- 15.Gasparro FP, Mitchnick M, Nash JF. A review of sunscreen safety and efficacy. Photochem Photobiol. 1998;68:243–56. PubMed PMID: 9747581. [PubMed] [Google Scholar]
- 16.Gonzalez H, Tarras-Wahlberg N, Stromdahl B, et al. Photostability of commercial sunscreens upon sun exposure and irradiation by ultraviolet lamps. BMC Dermatology. 2007;7:1–9. doi: 10.1186/1471-5945-7-1. doi: 10.1186/1471-5945-7-1. PubMed PMID: 17324264; PubMed Central PMCID: PMC1831786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maier H, Schauberger G, Brunnhofer K, Honigsmann H. Change of ultraviolet absorbance of sunscreens by exposure to solar-simulated radiation. J Invest Dermatol. 2001;117:256–62. doi: 10.1046/j.0022-202x.2001.01425.x. doi: 10.1046/j.0022-202x.2001.01425.x. PubMed PMID: 11511302. [DOI] [PubMed] [Google Scholar]
- 18.Popov AP, Lademann J, Priezzhev AV, Myllyla R. Effect of size of TiO2 nanoparticles embedded into stratum corneum on ultraviolet-A and ultraviolet-B sun-blocking properties of the skin. J Biomed Opt. 2005;10:064037. doi: 10.1117/1.2138017. doi: 10.1117/1.2138017. PubMed PMID: 16409102. [DOI] [PubMed] [Google Scholar]
- 19.Shen B, Scaiano JC, English AM. Zeolite encapsulation decreases TiO2-photosensitized ROS generation in cultured human skin fibroblasts. Photochem Photobiol. 2006;82:5–12. doi: 10.1562/2005-05-29-RA-551. doi: 10.1562/2005-05-29-ra-551. PubMed PMID: 16149847. [DOI] [PubMed] [Google Scholar]
- 20.Grancaric AM, Tarbuk A, kovacek I. Nanoparticles of Activated Natural Zeolite on Textiles for Protection and Therapy. CI&CEQ. 2009;15:203–10. [Google Scholar]
- 21.Perioli L, Ambrogi V, Bertini B, et al. Anionic clays for sunscreen agent safe use: photoprotection, photostability and prevention of their skin penetration. Eur J Pharm Biopharm. 2006;62:185–93. doi: 10.1016/j.ejpb.2005.08.001. doi: 10.1016/j.ejpb.2005.08.001. PubMed PMID: 16202575. [DOI] [PubMed] [Google Scholar]


