Summary
Mitochondria generate high levels of reactive oxygen species (ROS) to activate pro-tumorigenic signaling pathways. In parallel, the mitochondria must also increase their antioxidant capacity to lower ROS levels and prevent cancer cell death. In this issue, Ye et al. demonstrate that serine catabolism through one-carbon metabolism within the mitochondrial matrix is necessary to maintain this redox balance.
Cancer cell proliferation requires a robust increase in cellular metabolism to support the massive anabolic requirements for the generation of two daughter cells. Cancer cells engage in multiple glucose- and mitochondrial-dependent anabolic pathways to generate the precursors required for lipid, nucleotide, and protein synthesis as well as to produce NADPH, which provides the reducing equivalents for biosynthesis. Oncogenic signaling and tumor suppressor loss activate these anabolic pathways to support tumor growth. Indeed, as a consequence of mitochondrial metabolism, ROS generated by the mitochondrial electron transport chain (ETC) is essential for cancer cell proliferation, tumorigenesis, and metastasis (1). When rapidly proliferating tumor cells outgrow their available blood supply, regions within a solid tumor become hypoxic (i.e., low oxygen levels). Hypoxia also increases the production of mitochondrial ROS to activate the HIF family of transcription factors and induce the expression of HIF target genes including those involved in metabolism and angiogenesis. Importantly, cancer cells need to maintain a steady state level of ROS, a redox balance, which allows for cell proliferation and HIF activation without allowing ROS to accumulate to levels that would incur cell death or senescence. Thus, mitochondrial ROS levels are tightly regulated in cancer cells. Ye et al. demonstrate that serine catabolism through one-carbon metabolism maintains this mitochondrial redox balance during hypoxia (2).
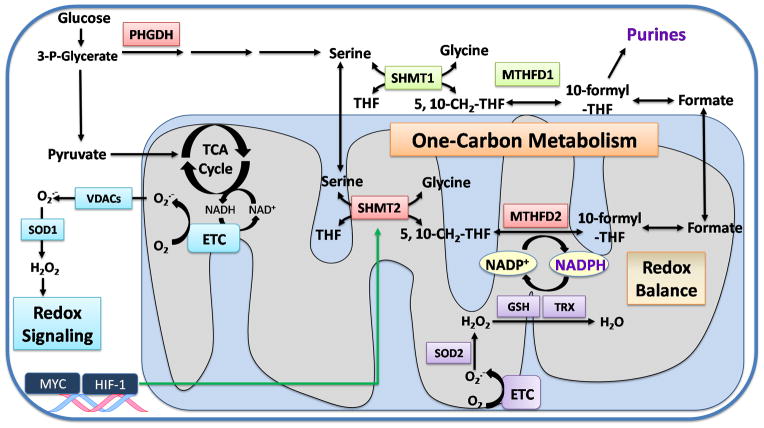
During one-carbon metabolism, serine is converted to glycine in the cytosol and mitochondrial matrix by serine hydroxymethyltransferase 1 and 2 (SHMT1 and 2), respectively. This reaction involves the covalent linkage of tetrahydrofolate (THF), derived from folic acid, to a methylene group (CH2) to form 5,10-methylene-tetrahydrofolate (5,10-CH2-THF). Cytosolic and mitochondrial methylene tetrahydrofolate dehydrogenase (MTHFD1 and 2, respectively) use 5,10-CH2-THF and NADP+ as substrates to produce 5,10-methenyl-tetrahydrofolate (5,10-CH=THF) and NADPH (Figure 1). Subsequently, 5,10-CH=THF is converted into 10-formyl-THF, which is used for purine synthesis. Thus, serine catabolism through one-carbon metabolism supports cancer cell proliferation (3). Recently, several studies have highlighted the role of serine in tumorigenesis. For example, the initial cytosolic enzyme in the de novo serine synthesis pathway, phosphoglycerate dehydrogenase (PHGDH), is upregulated in breast cancer and melanoma (4, 5). Moreover, many tumor cells are highly dependent on the uptake of exogenous serine suggesting that the de novo serine synthesis by itself is not sufficient to meet the requirements for cell proliferation (6).
Figure 1. Serine catabolism maintains redox balance during hypoxia.
Hypoxia triggers the production of superoxide (O2−•) in the mitochondria by the electron transport chain (ETC). O2−• is transported to the cytosol through voltage-dependent anion channels (VDACs) and is converted into hydrogen peroxide (H2O2) by SOD1. H2O2 acts as a signaling molecule to promote cancer cell proliferation and hypoxia-inducible transcription factors (HIFs) activation. O2−• can also be converted to H2O2 in the mitochondrial matrix by SOD2 and further detoxified to water (H2O) by glutathione peroxidases (GPX) and peroxiredoxins (PRX) to maintain the redox balance. The scavenging ability of GPX and PRX is dependent on glutathione (GSH) and thioredoxin (TRX). NADPH is utilized to regenerate the reduced glutathione (GSH) and thioredoxin (TRX) pools. Serine catabolism within the mitochondrial matrix can replenish NADPH. The de novo serine synthesis is initiated in the cytosol by phosphoglycerate dehydrogenase (PHGDH) utilizing the glycolytic intermediate 3-P-Glycerate. Serine is catabolized by the cytosolic (SHMT1) and mitochondrial matrix (SHMT2) enzymes to produce glycine and 5,10-CH2-THF. The MTHFD enzymes further convert 5,10-CH2-THF and NADP+ into 5,10-CH=THF and NADPH. Subsequently, 5,10-CH=THF is converted into 10-formyl-THF, which is utilized for purine synthesis. The transcription factors HIF-1 and MYC cooperate to upregulate SHMT2 in cancer cells during hypoxia to prevent uncontrolled levels of H2O2 in the mitochondrial matrix that would be detrimental for cancer cells.
Given that one-carbon THF units are required for nucleotide synthesis, cancer cells benefit from enhanced serine-dependent one-carbon metabolism. Notably, serine is primarily catabolized through the mitochondrial one-carbon metabolism pathway. If carbon units of THF are needed solely for nucleotide synthesis in the cytosol, why do cells engage in the mitochondrial one-carbon metabolism pathway? A recent elegant study which utilizes a new method for tracing NADPH compartmentalization revealed that serine functions in the mitochondrial one-carbon metabolism pathway to produce NADPH (7). An independent study also demonstrated that serine and glycine catabolism in the mitochondria generates NADPH (8). However, whether this source of NADPH is important for cancer cell proliferation and tumor growth remained unknown.
In this issue, Ye et al. not only describe the importance of the mitochondrial one-carbon metabolism pathway, but provide mechanistic insight into the role of serine in NADPH production for mitochondrial redox homeostasis during hypoxia and tumor growth (2). NADPH plays a critical role in maintaining the cellular antioxidant capacity by regenerating the reduced pools of glutathione (GSH) and thioredoxin (TRX), ROS scavengers which remove excess hydrogen peroxide (H2O2). Ye et al. observed a drastic increase in the amount of mitochondrial ROS produced in SHMT2-knockdown cells under hypoxia compared to normoxia. Moreover, these cells had lower NADPH/NADP+ and glutathione/glutathione disulfide (GSH/GSSG) ratios during hypoxia resulting in increased cell death. Importantly, this effect was rescued when the cells were treated with the antioxidant N-acetylcysteine (NAC) implicating elevated ROS in the increased cell death of SHMT2-knockdown cells. Furthermore, cancer cells with decreased SHMT2 also display impaired tumor growth. Thus, mitochondrial serine catabolism is necessary for NADPH production, ROS detoxification, and cancer cell survival. Importantly, SHMT2 but not SHMT1 is overexpressed in a variety of human cancers and breast cancer patients with low SHMT2 expression survive better compared with high SHTM2 expression. Additionally, there is a positive correlation between mitochondrial SHMT2 (serine catabolism) and cytosolic PHGDH (serine biosynthesis). This correlation is not observed between the two cytosolic proteins, PHGDH and SHMT1. This suggests that cancer cells coordinate the de novo synthesis of serine in the cytosol and the catabolism of serine in the mitochondrial matrix to produce NADPH for detoxification of ROS needed to sustain tumor growth (Figure 1).
In addition, Ye et al. observed that the expression of SHMT2 positively correlated with the expression of HIF-regulated enzymes under hypoxia and confirmed hypoxia-induced upregulation of SHMT2 in a HIF-1 dependent manner in cancer cells (2). Interestingly, hypoxic induction of SHMT2 was only observed in cancer cells with MYC amplification. Since SHMT2 is a reported MYC target gene, Ye et al. further investigated whether MYC was also driving SHMT2 upregulation. Indeed, they confirmed that the induction of SHMT2 under hypoxia was dependent on MYC-driven transcriptional amplification, indicating that both HIF and MYC collaborate to induce SHMT2 expression (Figure 1). This finding was quite unexpected as previous results in clear cell renal cell carcinoma have demonstrated that HIF-1α antagonizes c-MYC function (9). Further studies are necessary to elucidate the circumstances for which HIF-1 and MYC cooperate or antagonize to regulate gene expression.
The degree of hypoxia positively correlates with metastasis; thus, it will be of interest to determine whether enzymes in the one-carbon metabolism pathway predict metastatic progression. It will also be important to assess whether targeting these metabolic enzymes diminishes both primary tumor growth and metastasis. Moreover, given that MYC expression is deregulated in numerous tumor types, the finding that SHMT2 induction occurs only in MYC-amplified cancer cells during hypoxia makes SHMT2 an attractive therapeutic target. Furthermore, p53 null tumors are highly sensitive to exogenous serine depletion (6) suggesting that mitochondrial one-carbon metabolism may prove to be an efficacious cancer therapy in MYC overexpressing and p53 null tumors. Interestingly, anti-folate agents which disrupt one-carbon metabolism (folic acid is the precursor for the one-carbon unit donor THF) have been utilized clinically for decades and thus there is a precedence for targeting this pathway.
A key concern with inhibiting mitochondrial one-carbon metabolism is whether cancer cells exhibit metabolic plasticity to compensate for the loss of NADPH production. It is important to note, that within the mitochondrial matrix, NADPH can also be generated by isocitrate dehydrogenase 2 (IDH2) and malic enzyme 3 (ME3). Another important consideration is whether normal cells require mitochondrial one-carbon metabolism for maintaining redox balance. Although SHMT2-knockout mice have yet to be generated, MTHFD2-knockout mice are embryonic lethal. Given the hypoxic microenvironment of a developing embryo, it is not surprising that mice lacking a key enzyme within the mitochondrial one-carbon metabolism pathway are embryonic lethal. However, normal adult tissues might not require these mitochondrial one-carbon metabolism enzymes since mitochondrial oxidative stress is low and nutrients such as glucose and oxygen are not limiting. Indeed, MTHFD2 was absent in most adult tissues, in stark contrast to developing embryo (10). Meta-analysis data showed that MTHFD2 is the most consistently overexpressed metabolic enzyme across multiple tumor types and correlated with poor survival in breast cancer (10). Thus, targeting mitochondrial one-carbon metabolism for cancer treatment is a potential therapeutic approach. However, a major hurdle for inhibitors targeting this pathway is diffusibility through the lipid membranes, including the cell membrane, outer mitochondrial and inner mitochondrial membranes. To overcome this obstacle, a lipophilic cation can be attached to small molecules to increase their accumulation in the mitochondrial matrix 100 to 500-fold greater in concentration than outside the cell due to the large negative membrane potential generated by the ETC across the mitochondrial inner membrane. Thus, in summary, Ye et al. have unveiled mitochondrial one-carbon metabolism as a promising pathway for targeted cancer therapy, especially in hypoxic tumors where to date the therapeutic options are limited (2).
Acknowledgments
Grant Support
NSC is supported by the NIH (grant R01 CA12306708). IMR was supported by the Ramon Areces Foundation of Spain.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Schieber M, Chandel NS. ROS Function in Redox Signaling and Oxidative Stress. Current biology. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye J, Fan J, Venneti S, Wan Y-W, Pawel BR, Zhang J, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014 doi: 10.1158/2159-8290.CD-14-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labuschagne CF, van den Broek NJ, Mackay GM, Vousden KH, Maddocks OD. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell reports. 2014;7:1248–1258. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 4.Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nature genetics. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maddocks OD, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR, et al. Tracing Compartmentalized NADPH Metabolism in the Cytosol and Mitochondria of Mammalian Cells. Molecular cell. 2014;55:253–263. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nature communications. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]