Abstract
Over the past decades there have been great advancements in the survival outcome of patients with cancer. As a consequence, treatment regimens are being extended to patient populations, which would not have qualified in the past based on co-morbidities and age. Furthermore, the anti-cancer regimens, which have been and are being used, can cause considerable morbidity and even mortality. In fact, new drugs such as tyrosine kinase inhibitors have yielded unanticipated side effects in frequency and severity. The cardiovascular disease spectrum is an important element in all of these. In order to optimize the outcome of cancer patients with cardiovascular diseases existing prior to cancer treatment or developing as a consequence of it, a new discipline called “cardio-oncology” has evolved over the past few years. Herein we review the latest developments in this field including cardiotoxicities, vascular toxicities, and arrhythmias. This field is taking on more shape as cardiologists, oncologists, and hematologists are forming alliances, programs, and clinics, supported by the development of expert consensus statements on best management approaches and care of the cancer patient with cardiovascular diseases.
Introduction
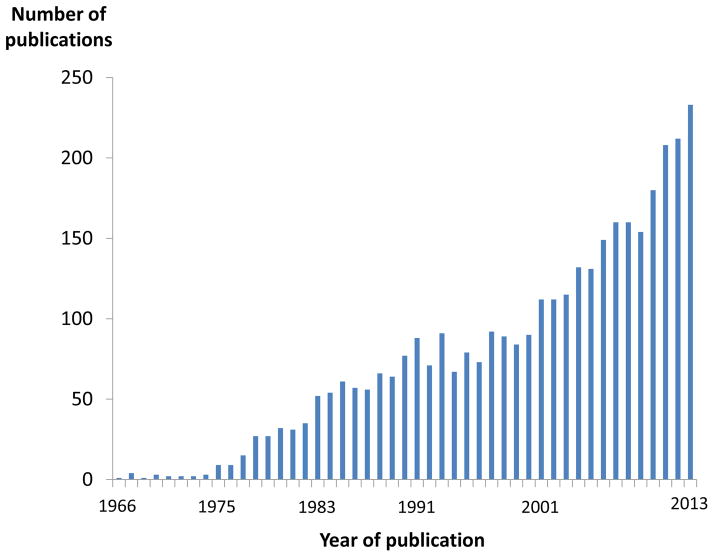
Cardio-Oncology is currently one of the emerging trends in cardiovascular medicine. This is documented in the increasing number of publications (Figure 1) and clinical programs at various institutions in the United States. Cardio-Oncology has less to do with intra-cardiac tumors and more to do with the cardiovascular care of patients with cancer in general. Most importantly, it involves the early assessment and management of any cancer therapy-related cardiovascular complications as well as the evaluation of the risk thereof prior to any initiation of cancer therapy. This evaluation pertains not only to cancer surgery but also, even more so, to radiation therapy and especially chemotherapy.
Figure 1.
Illustration of the number of Pubmed publications on the search terms “cardiotoxicity” and “chemotherapy” pointing out the greatest increase since 2010.
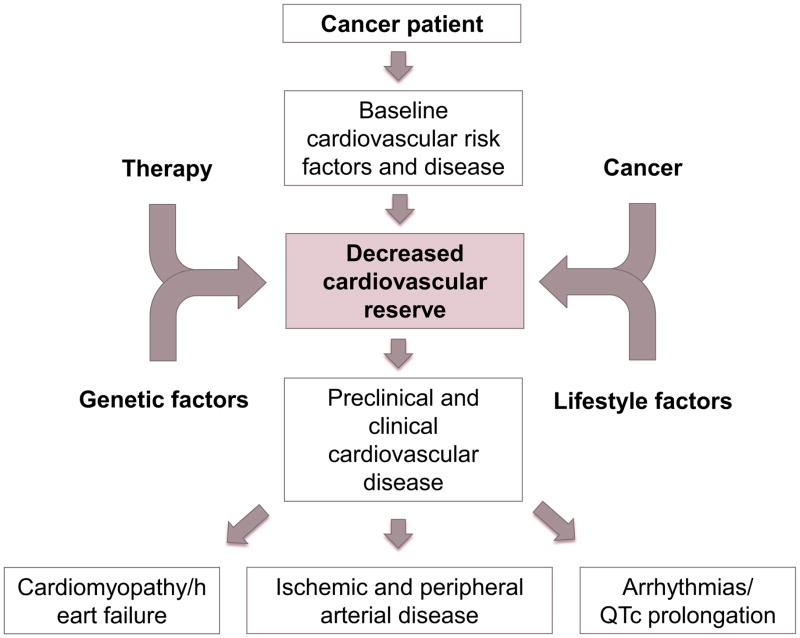
While chemotherapeutics emerged in the years following World War I, cardiac side effects had not been noticed much until the introduction of anthracyclines. In 1973, Lefrak and colleagues were the first to report on the occurrence of heart failure following doxorubicin treatment.(1) This remained the epiphany of chemotherapy-induced cardiotoxicity until the arrival of Trastuzumab (Herceptin), which, in conjunction with anthracyclines, was associated with an unexpected incidence of heart failure of 30% in breast cancer patients. This unexpected trial finding became the wake-up call for the discipline of Cardio-Oncology, which has grown since then, particularly in view of the improved long-term survival outcomes of cancer patients and the need for more comprehensive care. In fact, advances have succeeded to the point of turning cancer into chronic diseases, and the prognosis of cancer therapy-induced cardiomyopathy can be worse than the malignancy for which it was given in the first place. As such, Cardio-Oncology is becoming an important part of cancer survivorship programs. The overall concept and the foundations of this emerging field have been captured as outlined in Figure 2. This review will focus on the most recent developments in this area, capturing cardiac, vascular and heart rhythm aspects.
Figure 2.
Illustration of the Cardio-Oncology concept focusing on the cancer patients with pre-existing or developing cardiovascular disease. In most cases, there seems to be a reduced reserve that is challenged further by the cancer, cancer therapy, and environmental factors to yield the various clinical cardiovascular presentations that can be seen in cancer patients.
Cardiotoxicity
Anthracyclines
Anthracyclines such as doxorubicin and epirubicin remain a central element in treatment protocols for cancers such as breast and gastric cancers as well as leukemias and lymphomas. Their use is limited, however, to a significant degree by their side effect potential, especially cardiotoxicity. Details regarding the underlying mechanisms of the cardiotoxic effects continue to evolve. The “iron and free radical hypothesis” had been the prevailing theory, which entails the transfer of an electron to the quinone moiety of anthracyclines as they enter the cardiomyocytes, which is then passed on to molecular oxygen generating superoxide anions and hydrogen peroxide (so-called “redox cycling”).(2) The preferential binding of anthracyclines to cardiolipin in the inner membrane of the mitochondria brings the outlined dynamics in close proximity to the respiratory chain and low-molecular iron with a marked surge in oxidative stress. This leads to oxidative modification of proteins, lipids, and genomic and mitochondrial DNA damage as well as uncoupling of the electron transport chain, thereby impairment of oxidative phosphorylation and ATP synthesis and thus mitochondrial dysfunction and damage.(2–4) In distinction from this traditional view, inhibition of topoisomerase 2-β in cardiomyocytes has recently been identified as yet another, if not key, mechanisms of anthracycline-induced cardiotoxicity.(5) Accordingly is has become evident that anthracyclines no longer only induce DNA damage but also impair its repair in cardiomyocytes in parallel to its anti-cancer effects, even though, importantly, it is inhibition of topoisomerase 2-α in cancer cells.(6) Further along these lines, based on human samples, there is recent evidence that premature senescence of circulating progenitor cells and their progeny contributes to a decline in cardiac regenerative capacity and thereby to the development of cardiomyopathy in patients undergoing anthracycline-based therapies.(7)
The goal in the care of patients receiving anthracycline-based chemotherapies obviously is to protect the heart without reducing the efficacy of the anti-cancer therapies. This has been the concern for some approaches, e.g. dexrazoxane, a derivative of EDTA and potent iron chelator. Two Cochrane reviews noted this to be the most effective cardio-preventive strategy but concerns regarding reduction of cancer–free survival persist.(8,9) The most recent meta-analysis combining studies in pediatric and adult patient populations found that statins, beta-blocker, and angiotensin antagonists are as effective as dexrazoxane in preventing anthracycline-induced cardiotoxicity (approximately 60% relative risk reduction). (10) In fact, angiotensin antagonism appeared as the most effective strategy for cardioprotection in patients subjected to chemotherapy (90% relative risk reduction), which, however, does not match up to all existing data.(10) Also, there are important distinctions among beta-blockers with a confirmed benefit for carvedilol and nebivolol.(11,12)
An important aspect is the timing of therapy and more potent effects are to be expected the earlier the initiation. It is presently not clear whether combination of these therapies truly yields an additive benefit. The preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies (OVERCOME) trial indicated that combination therapy of enalapril and carvedilol prevented LVEF reduction at 6 months and even outlined a benefit in terms of the combined secondary endpoint of death or heart failure in patients referred for intensive chemotherapy or stem cell transplantation.(13) One may argue that the benefit of this combination therapy was not substantially different from either therapy alone. Differences in study cohorts and endpoints, however, spoil direct comparisons, and thus this comparison study is yet to be done. Most commonly, synergistic effects can be expected with the combination of drugs of different modes of action. All of them, of note, have the capacity to decrease NADPH oxidase, which has important implications for oxidative stress in general but particularly as NADPH-dependent semiquinone formation plays a presumably central role in cardiotoxicity mechanism of anthracyclines.(14) Intriguingly, other general anti-oxidant strategies such as N-acetylcysteine in the EPOCH trial have not been proven useful.(15) Thus, addressing specific molecular targets will remain key for successful cardioprotective efforts including specific topoisomerase II inhibitors and scavengers.
Identification of patient groups that would derive the greatest benefit is important from a risk-benefit and cost-effectiveness standpoint. Models predicting cardiotoxicity risk with anthracycline therapy are currently lacking. Based on multiplicative assumptions of odds ratios, it can be assumed that patients with >= 3 risk factors have a 5- to 6-fold increased risk of cardiotoxicity. Of these, a recent meta-analysis found that the cumulative dose of anthracyclines was the most robust predictor of cardiotoxicity, followed by chest radiotherapy, African-American ethnicity, extremes of the age and body weight spectrum, diabetes, hypertension, and severe co-morbidities.(16) However, even with low to moderate doses of anthracyclines (50 to 375 mg/m2 doxorubicin equivalents), subtle, clinically silent changes in LV function (LV strain) can be noted by MRI as early as 6 months after completion of therapy.(17)
Strain imaging is nowadays mainly obtained by speckle tracking echocardiography and a significant change can be noted as early as after 3 cycles and certainly at the completion of chemotherapy in a subset of patients which then progress to a decline in LVEF.(18–21). A meta-analysis concluded that a greater than 10–15% reduction in global longitudinal strain (GLS) as early as during therapy seems to be the most useful parameter for the prediction of cardiotoxicity. (22) The addition of the assessment of LV twist to GLS might further increase the accuracy of predicting a subsequent decline in LVEF to 93%.(23) On the other hand, the inclusion of LV torsion analysis by 2D speckle tracking imaging may outline significant changes as early as one month after chemotherapy (or 100 g/m2 cumulative doxorubicin dose). (24)
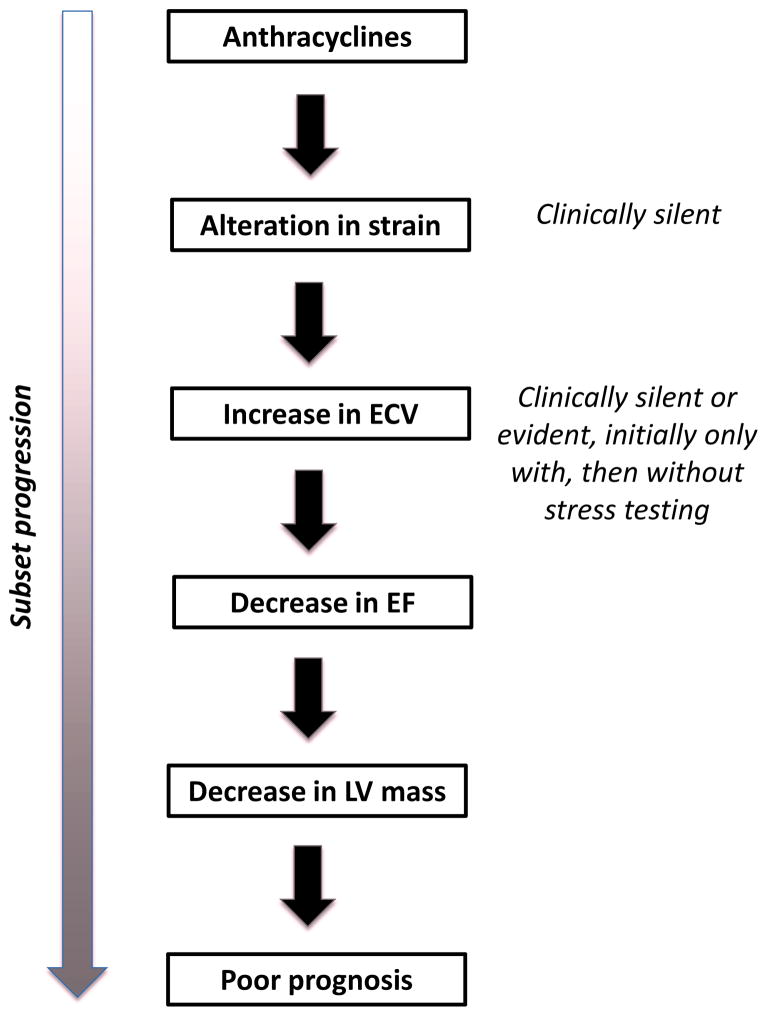
Cardiac MRI has, however, been suggested to be more sensitive than echocardiography over the course of long-term follow-up. Strain imaging abnormalities remain and changes consistent with diffuse myocardial fibrosis can be noted on cardiac MRI before any change in ejection fraction.(25) An increase in extracellular volume (ECV) is of particular interest, correlating positively with cumulative anthracycline dose and negatively with LV mass/volume and LV wall thickness/height ratio, and peak VO2 on exercise testing.(26) Focal fibrosis of the myocardium by positive late gadolinium enhancement is rarely present even in cases of anthracycline cardiomyopathy characterized by a reduction of ejection fraction.(27,28) In pediatric cancer survivors, LV and RV ejection fractions were noted to be abnormal (<45%) in 20–30% of patients and subnormal (45–55%) in another 50–60% of patients approximately 8 years after completion of chemotherapy.(28) A reduction in LV mass has been outlined in this pediatric and adolescent population but can be noted in a subgroup of patients undergoing evaluation for anthracycline-induced cardiomyopathy in mid age as well. (27,29) Intriguingly, echocardiography did not outline reductions in LV mass in these patients; it was only by cardiac MRI with a negative correlation with cumulative lifetime anthracycline dose. A reduction in LV mass was noted in particular and universally in patients who had received cumulative doses >350 mg/m2 and is therefore likely a reflection of more extensive injury.(27) Importantly, LV mass was the strongest independent predictor of future cardiovascular death, appropriate ICD therapy, and heart failure hospitalization. How these data of subset characterization of patients after anthracycline-based chemotherapy by MRI (Figure 3) can be utilized to optimize management and outcome of patients is yet to be outlined, which is an important element in the decision on the utilization of these techniques in an age of resource confinements.
Figure 3.
Illustration of progression of subsets of adult patients undergoing anthracycline therapy. Of those exposed, some will develop abnormalities in myocardial strain early on, and then an increase in extracellular volume (ECV). A subset of patients will progress to a reduction of left-ventricular ejection fraction, and a subset of these will have a reduced left ventricular mass with the worst outcome.
For this reason, 2D echocardiography remains the main mode of assessment of cardiac function. However, the variability of LVEF measurements may exceed 10% and might still be as high as 6% with non-contrast 3D echocardiography (and thus, in asymptomatic patients, a diagnosis of “cardiotoxicity” can be considered only if the decrease exceeds this degree of variability).(30) With cardiac MRI as the reference standard, the sensitivity and specificity of 2D echocardiography to detect a LVEF <50% may be as low as 25%. 3D echocardiography can improve these numbers by 100% but may still be inferior to cardiac MRI.(31) Higher LVEF cutoff values have been suggested to improve the diagnostic performance of echocardiography. As such, cardiac MRI may be considered in patients with an LVEF <60% on standard 2D echocardiography, 75% of these patients may in fact already have an LVEF <50%.(29) Beyond the points raised, the merit of routine screening protocols of the cardiac function of cancer patients has been questioned in general. (32) The current Children’s Oncology Group guidelines, largely endorsed in the recent expert consensus statement by the American Heart Association, recommend echocardiographic surveillance intervals based on cumulative anthracycline dose and radiation exposure.(33) For those 50% of survivors deemed to be at high risk of cardiovascular complications (>300 g/m2 doxorubicin equivalent dose of anthracyclines with or without radiation), yearly echocardiograms are recommended. For the 30% of pediatric cancer survivors considered to be at intermediate risk (200–299 g/m2 anthracyclines without or <300 g/m2 anthracyclines with radiation therapy, or >= 30 Gy radiation therapy), the recommended screening interval is every 2 years. In the remaining 20%, echocardiograms are recommended every 5 years. Two studies, however, found that the most cost-effective approach would be every 2 years, every 5 years, and at most every 10 years in the three risk categories. (34) (35) Similar concerns recently have been raised in adult patients with acute myeloid leukemia, especially as LV function abnormalities were rarely noted on baseline screening evaluation. (36) Moreover, a study in adult lymphoma patients noted that neither decreased LVEF nor higher cumulative doses of anthracyclines had a significant predictive value for cardiovascular prognosis, questioning the merit of routine echocardiographic surveillance protocols. (37) Instead, this study identified previous heart diseases, ECG rhythm abnormalities during chemotherapy, and lack of complete remission as independent predictors for cardiovascular death, which accounted for one third of all deaths during follow-up. (37)
The fact that some patients tolerate high doses of anthracyclines while others develop cardiotoxicity at relatively low doses points to genetic predispositions. Indeed, for instance, functionally relevant NADPH polymorphisms (both protective as well as risk enhancing) have been confirmed. (38) A genome-wide transcript analysis on whole blood RNA samples from women who received doxorubicin-based chemotherapy pointed out >250 transcripts that differed >2-fold between those who did and those who did not develop a drop in LVEF to <40%. One of the most prominent of these was T-cell leukemia/lymphoma 1A, a co-activator for PKB/AKT, which is a major pro-survival and stress pathway for cardiomyocytes, linked and targeted by HER-2 inhibitors. Furthermore, women who developed a depressed LVEF had a lower level of ABCB1 transcript, which encodes the multidrug resistance protein 1, an efflux pump for doxorubicin, which is also inhibited by verapamil and can lead to higher cardiac drug level. (39) This particular gene also has been noted to be of relevance in pediatric patients, and two recent studies indicated that the determination of replicated genetic variants in addition to clinical factors can significantly improve cardiotoxicity risk prediction in this population. (40,41)
With regards to cardiac biomarkers, their role for early detection of chemotherapy-induced cardiotoxicity remains debatable. Highly sensitive cardiac troponin I (hsTnI) by itself is predictive of a future drop in LVEF.(18,42) However, hsTnI does not seem to add much to the predictive value of GLS.(18) The most recent study in this area confirmed that N-terminal pro-B-type natriuretic peptide is not of value and neither are high-sensitivity C-reactive protein, growth differentiation factor-15, placental growth factor, soluble fms-like tyrosine kinase receptor-1, or galectin-3.(42) Yet myeloperoxidase was identified as a putative novel marker but with a low borderline level of significance. Thus, cardiac troponins seem to remain the best biomarkers of chemotherapy-induced cardiotoxicity.
Finally, cardiac technetium-99m sestamibi (MIBI) is being rediscovered as a marker of mitochondrial metabolism and its alteration due to chemotherapy. In a very small-sized study, MIBI uptake was noted to be greater in patients undergoing chemotherapy and to be predictive of cardiac mortality.(43) Further studies are required to confirm these initial proof-of-concept results. In vitro study results, however, do support these observations quite clearly.(44) At last, three different patterns of FDG-PET uptake (increase, decrease, and no change) were observed in lymphoma patients treated with adriamycin-based chemotherapy. (45) This observation is intriguing but its significance is yet still completely to be defined.
Trastuzumab (Herceptin)
Trastuzumab is the anti-cancer agent that has changed the landscape of oncology in terms of new targeted treatment paradigms as well as awareness of the cardiotoxicity that can (unexpectedly) be associated with it. It is a humanized monoclonal antibody that targets the extracellular domain of the epidermal growth factor receptor subunit HER-2/ErbB2 and is FDA approved for the treatment of breast cancers and metastatic gastric or gastroesophageal junction adenocarcinoma overexpressing this receptor. (46) Its cardiotoxicity profile has some aspects that are uniquely different from anthracyclines including lack of histological abnormalities and induction and reversibility of LV function decline (early) after the start and cessation of therapy. This has led to the concept of distinction of two types of chemotherapy-induced cardiotoxicity: a structural injury type, characterized by cardiomyocyte damage (type 1), and a dysfunction type, characterized by functional impairment limited to the time of exposure (type 2).(47)
A new experimental study, however, calls this concept into question, demonstrating increase in tissue oxidative stress, induction of apoptosis, changes of the ultrastructure, and release of cardiac troponin as well as myosin light chain.(48) Another in vitro and in vivo study indicates that Herceptin may impair the regenerative capacities of stem cells.(49) All of these observations are reminiscent of those seen with anthracyclines. A recent biomarker study found cardiac troponin elevation only in patients treated with anthracyclines but not with trastuzumab.(50) Prior clinical studies noted cardiac troponin elevation especially during the early courses of trastuzumab treatment preceding but not uniformly predicting a drop in ejection fraction. (51,52) However, all patients who were found to have cardiac troponin elevation even before receiving any trastuzumab therapy (20% of all patients) developed “trastuzumab-induced cardiomyopathy” without any recovery of cardiac function. Patients with cardiac troponin elevations occurring during trastuzumab therapy were twice as likely to have no recovery of cardiac function in case of induced cardiotoxicity. These observations suggest that at least the baseline (non-ultrasensitive) cardiac troponin elevations are a reflection of underlying and possibly anthracycline-induced cardiac disease and are of significant prognostic value. Possibly, these are the patients with evidence of late gadolinium enhancement on MRI.(53) Finally, increased cardiac uptake on iodine-123-metaiodobenzylguanidine scintigraphy may also identify those patients with low chances of functional recovery of trastuzumab-induced cardiotoxicity.(54)
The most recent study on the incidence of trastuzumab-associated cardiac events showed that the incidence of a decrease of LVEF by at least 10% to less than 50% was 7% at 2 years and 4% at 1 year; NYHA class III or IV heart failure was encountered in 0.8% of the patients only. Discontinuation of trastuzumab was required in 5% and 9% of patients at 1 and 2 years, respectively. Recovery of LV function to >=50% was seen in 80% of the patients with LVEF decline.(55) Importantly, these numbers were derived from a clinical trial with strict inclusion and exclusion criteria whereas clinical practice may expand treatments to a broader patient population. Indeed, a Medicare database-based analysis showed that the adjusted incidence of cardiomyopathy or heart failure is significantly increased in elderly women undergoing treatment with trastuzumab compared with untreated patients (additional 12–13 cases per 100 patients) or patients receiving non-trastuzumab/non-anthracycline-based chemotherapy.(56) Intriguingly, women undergoing treatment with anthracyclines were not at higher risk of developing cardiotoxicity over three years but the combination of anthracyclines and trastuzumab yielded a higher risk than trastuzumab alone over untreated or differently treated patients (22 additional cases per 100 patients), consistent with prior studies.(56,57) Age has consistently been pointed out as a major independent risk factor, increasing the odds of developing cardiotoxicity >11 fold in those >=70 years compared with those <50 years of age. (58) A history of cardiac disease ranked second as a major predictor, increasing the risk >4-fold. (58) Similarly, pre-existing cardiac disease was the most important risk factor in women >70 years of age followed by diabetes. (59). Among pre-existing cardiac disease, coronary artery disease in particular has been noted as a risk factor for trastuzumab cardiotoxicity. (60)
Other studies have noted a history of hypertension as the single most important predictor for the development of heart failure in elderly women undergoing Herceptin treatment (nearly 3-fold elevated risk). (61) The decrease in LV ejection fraction by 2D echocardiography in these patients may not be as profound as once thought and usually does not decease to <50%. (61) Similar to the cardiac troponin study, heart failure (overall incidence 3%) was most commonly (in 44% of the cases) observed in the first 3 months of trastuzumab treatment with decreasing frequency thereafter (25%, 19%, and 12% at 3–6, 6–9, and 9–12 months, respectively). Of further note, despite the development of heart failure, trastuzumab was continued in nearly one third of these patients and most intriguingly with partial recovery in LVEF thereafter; no other adverse events were encountered. (61)
In addition to pre-existing cardiovascular disease and/or its risk factors, previous exposure to anthracyclines remains an important risk factor for trastuzumab cardiotoxicity. In particular, a previous cumulative dose >240 mg/m(2) of doxorubicin or >500 mg/m(2) of epirubicin increases the risk 3-fold compared with lower doses. (62) (63) Diastolic function assessment after completion of the anthracycline-containing elements of current treatment protocols might be an integrative index of the risk for trastuzumab-induced cardiotoxicity based on prior chemotherapy and cardiovascular risk factors. (63) The value of baseline strain imaging has not been studied so far.
Renal dysfunction has been identified as a new independent risk factors for trastuzumab-induced cardiotoxicity with a glomerular filtration rate of <78 ml/min/1.73 m(2) increasing the odds of cardiotoxicity more than 3-fold. (64). Excessive alcohol consumption (>=10 drinks per week) during Herceptin has also been just described as a risk factor and for the first time a specific polymorphisms of HER-2 as well.(65) Finally, an integrative index/score to predict the risk of cardiotoxicity with Herceptin treatment for the individual patient was recently developed and validated (Figure 4).(66) In this model, the main predictors were age, systemic hypertension, prior anthracycline treatment and history of coronary artery disease, all of them individually and independently increasing the risk 2-fold.
Figure 4.
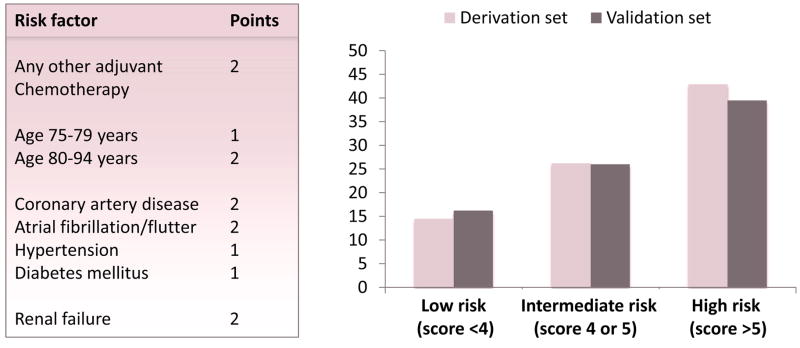
Prediction model for the risk of cardiotoxicity with trastuzumab therapy (generated based on reference 66).
Reproduced with permission:
Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc 2014;3:e000472.
Follow-up protocols have been established, all of which were based on monitoring LVEF. Prior and recent studies, however, confirm the merit of strain imaging as an early marker of evolving trastuzumab cardiotoxicity as well. In fact, one study found a >11% reduction in GLS to be the best predictor and of incremental value, allowing to risk-reclassify 77% of the patients (2x as many into a higher than lower risk). (67)
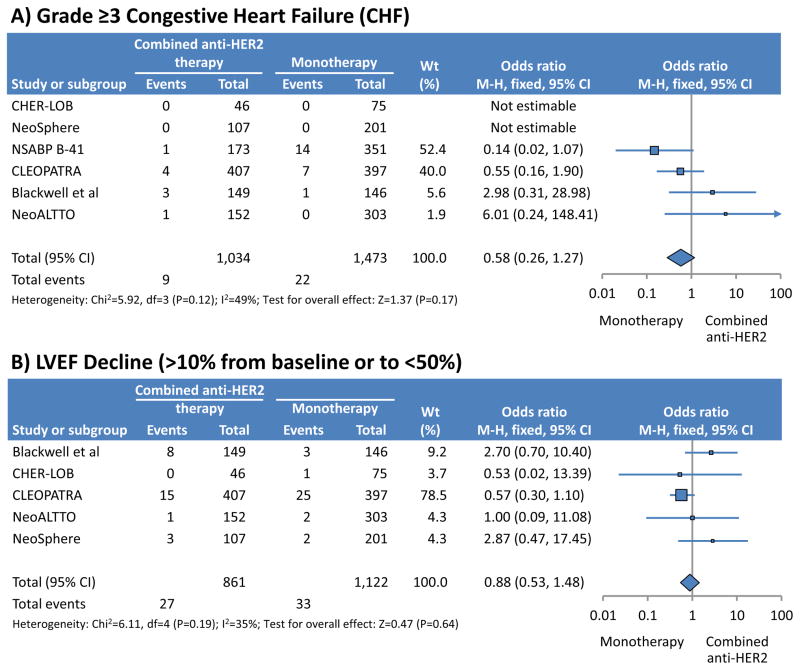
Dual synergistic HER-2 blockade with pertuzumab and trastuzumab has recently been shown to be superior to single blockade with a median gain in progression-free survival of 6 months.(68) Interestingly, the risk of trastuzumab cardiotoxicity was not increased by additional pertuzumab therapy (Figure 5).(69–71) Vice versa, there seems to be no difference in the risk of cardiotoxicity with single pertuzumab or pertuzumab+trastuzumab therapy.(72) The risk is lower in combination with non-anthracycline-based chemotherapy regimens.(72) Thus, HER-2 inhibition has its inherent cardiac risk but once targeted, the risk is not increased further while the anti-cancer effect is more potent. Pending further data, one may conclude that the same cardiac monitoring and management guidelines should be applied for dual as for single HER-2 therapy.
Figure 5.
Comparison of the risk of heart failure and left ventricular function decline with mono- versus dual HER-2 inhibitor therapy (modified from reference 69).
Reproduced with permission:
Valachis A, Nearchou A, Polyzos NP, Lind P. Cardiac toxicity in breast cancer patients treated with dual HER2 blockade. Int J Cancer 2013;133:2245–52.
In addition to anthracyclines and trastuzumab a number of other chemotherapeutics have been noted to induce cardiotoxicity. These include cyclophosphamide in high doses, used, for instance, in stem cell transplantation (type 1 pattern), and the vascular endothelial growth factor (VEGF) signaling pathway inhibitors bevacizumab, sunitinib, and sorafenib, used primarily for the treatment of renal cell carcinoma (type 2 pattern).
Vascular toxicity
One topic that has not received as much attention as the cardiac sides effects of chemotherapeutics are the vascular side effects, including acute vascular side effects. This changed in October 2013 with the release of data from the Ponatinib for CML Evaluation and Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia (PACE) trial. Ponatinib is a multi-targeted tyrosine kinase inhibitor with potent pan-activity against Bcr-abl, which is the oncogenic fusion product of a reciprocal gene translocation (“Philadelphia chromosome”) encoding for a continuously activated tyrosine kinase considered pivotal for the development of chronic myeloid leukemia (CML). Unexpectedly, the PACE trial indicated a nearly 12% incidence of serious arterial thrombotic events (cardiovascular 6.2%, cerebrovascular 4.0% and peripheral vascular 3.6%, with some patients with more than one type of event) at 24 months (8% after 11 months, approximately 10 events/100 patient years). This incidence remains approximately 3 times higher than serious venous occlusive events (2.9% at 2 years, 2.2% at 1 year). While these reports indicate primarily acute thrombotic events resulting in acute myocardial infarction, stroke, and acute limb ischemia, severe vascular narrowing necessitating revascularization procedures and/or amputation in case of peripheral arterial disease (PAD) also have been mentioned. Cases of heart failure have also been reported, likely ischemic in origin. Of note, these adverse events were noted across all age groups and in patients with and without cardiovascular risk factors. After issuing a temporary suspension of marketing and sales, the FDA permitted the prescription of Ponatinib to patients with T315I-positive CML or T315I-positive Philadelphia chromosome positive acute lymphoblastic leukemia (ALL) as of the beginning of 2014 but with new safety measures in place that now lists a black box warning of at least a 27% incidence of arterial and venous thrombosis and occlusions. In the aftermath of these reports, questions and discussions regarding testing and surveillance of cancer drugs have been rekindled. However, with positive malignancy outcomes of trials in patients with leukemia and GIST tumors, there will remain a need for familiarity with Ponatinib and close collaboration between oncologists, hematologists, and cardiologists. The mechanisms underlying the extraordinary high rate of acute thrombotic events are presently unknown as it is the best mode of management.
Prior to these new developments, nilotinib, another tyrosine kinase inhibitor with Bcl-Abl inhibitory properties used for the treatment of patients with CML, had been associated with rapidly progressive PAD, acute myocardial infarction (MI) and spinal infarction, even sudden death in 25% of the 24 patients studies in the initial report.(73) Other series reported a 2-year incidence of 14.8% of progressive PAD and acute limb ischemia, MI, and sudden death. The projected 10-year probability of freedom from PAD was only 67% for patients treated with nilotinib. Intriguingly, such events were very rarely observed (1 in 55) in patients treated with imatinib even though patient characteristics did not differ. (74) In the largest series so far, the incidence of all acute vascular events, which included peripheral and cerebral vasospasms, progressive CAD and PAD and pulmonary embolism was 2% occurring in patients with and without a prior history/diagnosis of atherosclerosis cardiovascular disease or even its risk factors.(75) This series included one patient who developed both CAD and PAD and this coincidence has been recaptured elsewhere to the point of future progression to concomitant cerebrovascular disease and stroke. (76,77) Of note, this pronounced case was an elderly female who was diagnosed with hypertension, hyperlipidemia, and pre-diabetes during her treatment course with nilotinib. Yet, progression of atherosclerotic cardiovascular disease (ASCVD) occurred on this drug with these risk factors being well managed. Most recent reports are clear that atherosclerosis develops and progresses even in patients devoid of significant cardiovascular risk factors, prior atherosclerotic disease, or other cause of arterial damage and persists despite rapid drug withdrawal. The preferential involvement and presentation is PAD, especially lower extremity arteries but renal and mesenteric arteries can be involved as well with subsequent renovascular hypertension, ischemic nephropathy, and mesenteric ischemia.(78,79) Similar to Panotinib, there is currently no understanding of the underlying mechanisms and best modes of management. Even though alterations in ankle-brachial index have been described in 26–36% of patients receiving nilotinib, there are no data on their dynamics (before and after start of therapy) or correlation with angiography results.(80) Thus, whether, for instance, this modality could be used before and serially during the treatment to identify those with developing ASCVD is yet to be seen.
Finally, it is worth mentioning that dasatinib, yet another tyrosine kinase inhibitor used in the treatment for CML, can on occasion (<1%) cause pre-capillary pulmonary hypertension, even of a severe degree to the point of causing right heart failure.(81) While it has been considered to be largely reversible, this may not always be the case and pulmonary pressure may not revert to the prior baseline values.(82) Intriguingly, the pathomechanisms discussed are the same that have been considered as targets for the treatment of pulmonary hypertension.(83) In fact, imatinib, the frontline drug for CML and in the same class of drugs as dasatinib, has been found to be of at least partial benefit for the treatment of patients with pulmonary arterial hypertension.(84,85) Thus, there continues to be a definite future research need in this area.
Arterial thrombotic events have classically been reported for angiogenesis inhibitors. In fact, the drugs mentioned above have recently been considered as “accidental” anti-angiogenic therapies, which may explain some of the vascular events. (86) The so-called “proper” or intended angiogenesis inhibitors including bevacizumab, sunitinib, and sorafenib, have a risk of 1.5 to 4% for acute thromboembolic events, and the overall cardiovascular complication risk can reach 40% (Figure 6).(87–89) Sorafenib has been associated with coronary vasospasm and with progression of CAD recently as well.(90) Their main target is the VEGF signaling pathway, either as a chimeric (capturing) antibody against VEGF or as tyrosine kinase inhibitors with activity against the VEGF receptors (but also c-kit and platelet-derived growth factor receptor). The VEGF signaling pathway is crucial for endothelial cell function and proliferation and thus the very foundation of the formation of new vessels. Even though tumors cannot exceed a certain growth without angiogenesis in general, VEGF signaling pathway inhibitors have been approved only for certain cancers such as renal cell carcinoma and gastro-intestinal malignancies. The fact that endothelial cells are pivotal for overall vascular function, including regulation of vascular tone, thrombosis, and fibrinolysis explains (at least in part) their vascular side effect profile.(86)
Expectedly, patients with pre-existing CAD are at particular high risk for these complications.(91) Thus, it might not be unreasonable to screen patients for CAD prior to starting this type of therapy. In part related to endothelial cell dysfunction, there is high-on-treatment platelet reactivity. Adequate anti-platelet therapy might therefore be a goal for these patients even though this will further enhance the already increased bleeding risk profile in these patients. Interventions shown to improve endothelial function and viability, especially statins, are key.
Vascular disease remains a classical consequence of radiation therapy, at least once a certain threshold dose is exceeded based on traditional concepts. However, a recent study in breast cancer patients undergoing post-mastectomy radiation therapy has changed this view, indicating a linear rather than exponential relationship in the risk of major coronary events with increasing radiation doses to the heart.(92) This risk starts to emerge five years after radiation therapy and continues thereafter. It equally affects women with and without risk factors but those with risk factors start out at a higher level of risk that is then maintained.(92) Thus, determination of risk and presence of CAD prior to initiation of chest radiation therapy might not be unreasonable. Whether radiation-induced atherosclerosis responds in a manner similar to native atherosclerosis to standard medications such as antiplatelet drugs and statins remains to be further investigated. Some preclinical studies may suggest this might not be the case even though they confirm that the disease risk is increased in those with underlying predisposing factors.(93,94) According to the newly published 2013 consensus statement by the European Association of Cardiovascular Imaging and American Society of Echocardiography, following radiation therapy to the chest, patients should undergo appropriate evaluation depending on signs and symptoms. (95) Patients without symptoms are to undergo surveillance echocardiography every 10 years unless deemed at high risk of radiation-induced heart disease, in which case the interval is shortened to every 5 years and should even include a functional non-invasive stress test. It is important to consider that a non-invasive stress test may under-represent the disease burden present. (96) Hence, coronary computed tomography angiography may be considered and might be a very effective way to differentiate intermediate risk patients into low or high risk. The most cost effective screening interval with this technique remains to be defined. Prior to open heart surgery, a chest CT might be of consideration to assess, for instance, for mediastinal fibrosis and porcelain aorta as recently reviewed in detail. (97)
Arrhythmias
Arrhythmias have received even less widespread attention than vascular toxicities in cancer patients. One reason might be that disorders such as atrial fibrillation are not uncommon in the general population, particularly with increasing age, and hence, this might be viewed as not truly related to cardio-oncology. Supraventricular tachycardia and especially atrial fibrillation is reported not infrequently (5%) in patients undergoing bone marrow transplantation and particularly with treatment regimens including Melphalan. Other risk factors include age, renal dysfunction, left atrial enlargement, and cardiac comorbidities. (98) A cohort study of >24,000 all-comer cancer patients found that 2.4% had a prior history of atrial fibrillation and 1.8% developed it. (99) While these numbers are not extraordinarily high, new-onset of atrial fibrillation in these patients was associated with a 2-fold and 6-fold increased risk of thromboembolic events and heart failure independent of other risk factors. (99) The CHADS2 score had a moderate graded association with mortality and heart failure in these patients but not thromboembolism. (99) On the contrary, the CHADS2 showed a graded association with thromboembolism and heart failure in patients with a diagnosis of atrial fibrillation before cancer. Of note, this particular cohort of patients had a higher mortality throughout the entire follow-up period and about two times higher in the multivariate analysis than new-onset atrial fibrillation. The reason for this association is not clear. Taken together, the management of these patients needs to be individualized. As cancer patients can be at a higher bleeding risk as well as a higher thrombotic risk, it is recommended to calculate the CHA2DS2-Vasc risk score and the HAS-BLED score. (100) If the HAS-BLED score is <3 and the CHA2DS2-Vasc risk score is 1 or higher, one may proceed with anticoagulation in the absence of high bleeding risk features. If these are present, then no antithrombotic therapy should be pursued. For those with either a CHA2DS2-Vasc risk score of 0 or a HAS-BLED score <3, antithrombotic therapy may be considered in those who have a high thromboembolic risk based on cancer type or chemotherapy. The potential merit and risks of the novel oral anticoagulants in the cancer patient have recently been reviewed. (101)
Summary
Over the last 3 years, there has been a tremendous increase in the interest and number of publications in Cardio-Oncology. This includes the involvement of professional societies such as the American Heart Association and the American Society of Echocardiography with the publication of expert consensus statements. These as well as other key contributions to the field of Cardio-Oncology were reviewed herein. Beyond doubt, this field is rapidly evolving and in the near future further organization and structure on institutional and societal levels is to be expected.
Acknowledgments
This work was supported by the National Institute of Health/National Heart Lung Blood Institute (HL116952-01A1) and a prospective grant of the Division of Cardiovascular Diseases, Mayo Clinic Rochester.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–14. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Minotti G, Cairo G, Monti E. Role of iron in anthracycline cardiotoxicity: new tunes for an old song? FASEB J. 1999;13:199–212. [PubMed] [Google Scholar]
- 3.Lebrecht D, Walker UA. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7:108–13. doi: 10.1007/s12012-007-0009-1. [DOI] [PubMed] [Google Scholar]
- 4.Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol. 2007;23:15–25. doi: 10.1007/s10565-006-0140-y. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 6.Ky B, Vejpongsa P, Yeh ET, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res. 2013;113:754–64. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piegari E, De Angelis A, Cappetta D, et al. Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res Cardiol. 2013;108:334. doi: 10.1007/s00395-013-0334-4. [DOI] [PubMed] [Google Scholar]
- 8.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane DB Syst Rev. 2008:CD003917. doi: 10.1002/14651858.CD003917.pub3. [DOI] [PubMed] [Google Scholar]
- 9.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane DB Syst Rev. 2011:CD003917. doi: 10.1002/14651858.CD003917.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. EEur J Cancer. 2013;49:2900–9. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 11.El-Shitany NA, Tolba OA, El-Shanshory MR, El-Hawary EE. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Cardiac Fail. 2012;18:607–13. doi: 10.1016/j.cardfail.2012.06.416. [DOI] [PubMed] [Google Scholar]
- 12.Kaya MG, Ozkan M, Gunebakmaz O, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol. 2013;167:2306–10. doi: 10.1016/j.ijcard.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies) J Am Coll Cardiol. 2013;61:2355–62. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, McLaughlin D, Robinson E, et al. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res. 2010;70:9287–97. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jo SH, Kim LS, Kim SA, et al. Evaluation of Short-Term Use of N-Acetylcysteine as a Strategy for Prevention of Anthracycline-Induced Cardiomyopathy: EPOCH Trial - A Prospective Randomized Study. Korean Circ J. 2013;43:174–81. doi: 10.4070/kcj.2013.43.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotrionte M, Biondi-Zoccai G, Abbate A, et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112:1980–4. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Drafts BC, Twomley KM, D’Agostino R, Jr, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–85. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25:733–40. doi: 10.1016/j.echo.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Florescu M, Magda LS, Enescu OA, Jinga D, Vinereanu D. Early Detection of Epirubicin-Induced Cardiotoxicity in Patients with Breast Cancer. J Am Soc Echocardiogr. 2014;27:83–92. doi: 10.1016/j.echo.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Kang Y, Xu X, Cheng L, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail. 2014;16:300–8. doi: 10.1002/ejhf.8. [DOI] [PubMed] [Google Scholar]
- 22.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of Myocardial Strain Imaging by Echocardiography for the Early Detection of Cardiotoxicity in Patients During and After Cancer Chemotherapy - A Systematic Review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 23.Mornos C, Petrescu L. Early detection of anthracycline-mediated cardiotoxicity: the value of considering both global longitudinal left ventricular strain and twist. Can J Physiol Pharmacol. 2013;91:601–7. doi: 10.1139/cjpp-2012-0398. [DOI] [PubMed] [Google Scholar]
- 24.Motoki H, Koyama J, Nakazawa H, et al. Torsion analysis in the early detection of anthracycline-mediated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2012;13:95–103. doi: 10.1093/ejechocard/jer172. [DOI] [PubMed] [Google Scholar]
- 25.Toro-Salazar OH, Gillan E, O’Loughlin MT, et al. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ Cardiovasc Imaging. 2013;6:873–80. doi: 10.1161/CIRCIMAGING.113.000798. [DOI] [PubMed] [Google Scholar]
- 26.Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neilan TG, Coelho-Filho OR, Pena-Herrera D, et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110:1679–86. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ylanen K, Poutanen T, Savikurki-Heikkila P, Rinta-Kiikka I, Eerola A, Vettenranta K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. J Am Coll Cardiol. 2013;61:1539–47. doi: 10.1016/j.jacc.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–84. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 31.Ylanen K, Eerola A, Vettenranta K, Poutanen T. Three-dimensional echocardiography and cardiac magnetic resonance imaging in the screening of long-term survivors of childhood cancer after cardiotoxic therapy. Am J Cardiol. 2014;113:1886–92. doi: 10.1016/j.amjcard.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Watts RG, George M, Johnson WH., Jr Pretreatment and routine echocardiogram monitoring during chemotherapy for anthracycline-induced cardiotoxicity rarely identifies significant cardiac dysfunction or alters treatment decisions: a 5-year review at a single pediatric oncology center. Cancer. 2012;118:1919–24. doi: 10.1002/cncr.26481. [DOI] [PubMed] [Google Scholar]
- 33.Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–95. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 34.Wong FL, Bhatia S, Landier W, et al. Cost-Effectiveness of the Children’s Oncology Group Long-Term Follow-up Screening Guidelines for Childhood Cancer Survivors at Risk for Treatment-Related Heart Failure. Ann Intern Med. 2014;160:672–83. doi: 10.7326/M13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh JM, Nohria A, Diller L. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Ann Intern Med. 2014;160:661–71. doi: 10.7326/M13-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bryant A, Sheppard D, Sabloff M, et al. A single-institution analysis of the utility of pre-induction ejection fraction measurement in patients newly diagnosed with AML. Leuk Lymphoma. 2014:1–17. doi: 10.3109/10428194.2014.883072. [DOI] [PubMed] [Google Scholar]
- 37.Jurczak W, Szmit S, Sobocinski M, et al. Premature cardiovascular mortality in lymphoma patients treated with (R)-CHOP regimen - a national multicenter study. Int J Cardiol. 2013;168:5212–7. doi: 10.1016/j.ijcard.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 38.Cascales A, Pastor-Quirante F, Sanchez-Vega B, et al. Association of anthracycline-related cardiac histological lesions with NADPH oxidase functional polymorphisms. Oncologist. 2013;18:446–53. doi: 10.1634/theoncologist.2012-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCaffrey TA, Tziros C, Lewis J, et al. Genomic profiling reveals the potential role of TCL1A and MDR1 deficiency in chemotherapy-induced cardiotoxicity. Int J Biol Sci. 2013;9:350–60. doi: 10.7150/ijbs.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visscher H, Ross CJ, Rassekh SR, et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer. 2013;60:1375–81. doi: 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]
- 41.Visscher H, Ross CJ, Rassekh SR, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–8. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 42.Ky B, Putt M, Sawaya H, et al. Early Increases in Multiple Biomarkers Predict Subsequent Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. J Am Coll Cardiol. 2014;63:809–16. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carboni GP. A novel clinical indicator using cardiac technetium-99m sestamibi kinetics for evaluating cardiotoxicity in cancer patients treated with multiagent chemotherapy. Am J Cardiovasc Dis. 2012;2:293–300. [PMC free article] [PubMed] [Google Scholar]
- 44.Piwnica-Worms D, Chiu ML, Kronauge JF. Detection of adriamycin-induced cardiotoxicity in cultured heart cells with technetium 99m-SESTAMIBI. Cancer Chemother Pharmacol. 1993;32:385–91. doi: 10.1007/BF00735924. [DOI] [PubMed] [Google Scholar]
- 45.Borde C, Kand P, Basu S. Enhanced myocardial fluorodeoxyglucose uptake following Adriamycin-based therapy: Evidence of early chemotherapeutic cardiotoxicity? World J Radiol. 2012;4:220–3. doi: 10.4329/wjr.v4.i5.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 47.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900–2. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 48.ElZarrad MK, Mukhopadhyay P, Mohan N, et al. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PloS one. 2013;8:e79543. doi: 10.1371/journal.pone.0079543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barth AS, Zhang Y, Li T, et al. Functional impairment of human resident cardiac stem cells by the cardiotoxic antineoplastic agent trastuzumab. Stem Cells Transl Med. 2012;1:289–97. doi: 10.5966/sctm.2011-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mokuyasu S, Suzuki Y, Kawahara E, Seto T, Tokuda Y. High-sensitivity cardiac troponin I detection for 2 types of drug-induced cardiotoxicity in patients with breast cancer. Breast cancer. 2014 doi: 10.1007/s12282-014-0520-8. [DOI] [PubMed] [Google Scholar]
- 51.Morris PG, Chen C, Steingart R, et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17:3490–9. doi: 10.1158/1078-0432.CCR-10-1359. [DOI] [PubMed] [Google Scholar]
- 52.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–6. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 53.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stokkel MP, de Wit-van der Veen LJ, Boekhout A. I-123-MIBG myocardial imaging in trastuzumab-based cardiotoxicity: the first experience. Nucl Med Commun. 2013;34:19–24. doi: 10.1097/MNM.0b013e32835ae523. [DOI] [PubMed] [Google Scholar]
- 55.de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-Associated Cardiac Events at 8 Years of Median Follow-Up in the Herceptin Adjuvant Trial (BIG 1–01) J Clin Oncol. 2014 doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–12. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 57.Chen T, Xu T, Li Y, et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev. 2011;37:312–20. doi: 10.1016/j.ctrv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Bonifazi M, Franchi M, Rossi M, et al. Trastuzumab-related cardiotoxicity in early breast cancer: a cohort study. Oncologist. 2013;18:795–801. doi: 10.1634/theoncologist.2013-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serrano C, Cortes J, De Mattos-Arruda L, et al. Trastuzumab-related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Ann Oncol. 2012;23:897–902. doi: 10.1093/annonc/mdr348. [DOI] [PubMed] [Google Scholar]
- 60.Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–8. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russo G, Cioffi G, Gori S, et al. Role of hypertension on new onset congestive heart failure in patients receiving trastuzumab therapy for breast cancer. J Cardiovasc Med (Hagerstown) 2014;15:141–6. doi: 10.2459/JCM.0b013e328365afb5. [DOI] [PubMed] [Google Scholar]
- 62.Farolfi A, Melegari E, Aquilina M, et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013;99:634–9. doi: 10.1136/heartjnl-2012-303151. [DOI] [PubMed] [Google Scholar]
- 63.Cochet A, Quilichini G, Dygai-Cochet I, et al. Baseline diastolic dysfunction as a predictive factor of trastuzumab-mediated cardiotoxicity after adjuvant anthracycline therapy in breast cancer. Breast Cancer Res Treat. 2011;130:845–54. doi: 10.1007/s10549-011-1714-9. [DOI] [PubMed] [Google Scholar]
- 64.Russo G, Cioffi G, Di Lenarda A, et al. Role of renal function on the development of cardiotoxicity associated with trastuzumab-based adjuvant chemotherapy for early breast cancer. Intern Emerg Med. 2012;7:439–46. doi: 10.1007/s11739-012-0794-9. [DOI] [PubMed] [Google Scholar]
- 65.Lemieux J, Diorio C, Cote MA, et al. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. 2013;33:2569–76. [PubMed] [Google Scholar]
- 66.Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–8. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. New Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valachis A, Nearchou A, Polyzos NP, Lind P. Cardiac toxicity in breast cancer patients treated with dual HER2 blockade. Int J Cancer. 2013;133:2245–52. doi: 10.1002/ijc.28234. [DOI] [PubMed] [Google Scholar]
- 70.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–84. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 71.Swain SM, Ewer MS, Cortes J, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist. 2013;18:257–64. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lenihan D, Suter T, Brammer M, Neate C, Ross G, Baselga J. Pooled analysis of cardiac safety in patients with cancer treated with pertuzumab. Ann Oncol. 2012;23:791–800. doi: 10.1093/annonc/mdr294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aichberger KJ, Herndlhofer S, Schernthaner GH, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86:533–9. doi: 10.1002/ajh.22037. [DOI] [PubMed] [Google Scholar]
- 74.Levato L, Cantaffa R, Kropp MG, Magro D, Piro E, Molica S. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: a single institution study. Eur J Haematol. 2013;90:531–2. doi: 10.1111/ejh.12096. [DOI] [PubMed] [Google Scholar]
- 75.Quintas-Cardama A, Kantarjian H, Cortes J. Nilotinib-associated vascular events. Clin Lymphoma Myeloma Leuk. 2012;12:337–40. doi: 10.1016/j.clml.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Tefferi A, Letendre L. Nilotinib treatment-associated peripheral artery disease and sudden death: yet another reason to stick to imatinib as front-line therapy for chronic myelogenous leukemia. Am J Heamtol. 2011;86:610–1. doi: 10.1002/ajh.22051. [DOI] [PubMed] [Google Scholar]
- 77.Coon EA, Zalewski NL, Hoffman EM, Tefferi A, Flemming KD. Nilotinib treatment-associated cerebrovascular disease and stroke. Am J Heamtol. 2013;88:534–5. doi: 10.1002/ajh.23442. [DOI] [PubMed] [Google Scholar]
- 78.Kristensen T, Randers E, Stentoft J. Bilateral renal artery stenosis in a patient with chronic myeloid leukemia treated with nilotinib. Leuk Res Rep. 2012;1:1–3. doi: 10.1016/j.lrr.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mirault T, Rea D, Azarine A, Messas E. Rapid onset of peripheral artery disease in a chronic myeloid leukemia patient without prior arterial disorder: direct relationship with nilotinib exposure and clinical outcome. Eur J Haematol. 2014 doi: 10.1111/ejh.12367. [DOI] [PubMed] [Google Scholar]
- 80.Kim TD, Rea D, Schwarz M, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27:1316–21. doi: 10.1038/leu.2013.70. [DOI] [PubMed] [Google Scholar]
- 81.Mattei D, Feola M, Orzan F, Mordini N, Rapezzi D, Gallamini A. Reversible dasatinib-induced pulmonary arterial hypertension and right ventricle failure in a previously allografted CML patient. Bone Marrow Transplant. 2009;43:967–8. doi: 10.1038/bmt.2008.415. [DOI] [PubMed] [Google Scholar]
- 82.Montani D, Bergot E, Gunther S, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125:2128–37. doi: 10.1161/CIRCULATIONAHA.111.079921. [DOI] [PubMed] [Google Scholar]
- 83.Pullamsetti SS, Berghausen EM, Dabral S, et al. Role of Src tyrosine kinases in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2012;32:1354–65. doi: 10.1161/ATVBAHA.112.248500. [DOI] [PubMed] [Google Scholar]
- 84.Shah AM, Campbell P, Rocha GQ, et al. Effect of imatinib as add-on therapy on echocardiographic measures of right ventricular function in patients with significant pulmonary arterial hypertension. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. 2013;127:1128–38. doi: 10.1161/CIRCULATIONAHA.112.000765. [DOI] [PubMed] [Google Scholar]
- 86.Conti E, Romiti A, Musumeci MB, et al. Arterial thrombotic events and acute coronary syndromes with cancer drugs: are growth factors the missed link? : what both cardiologist and oncologist should know about novel angiogenesis inhibitors. Int J Cardiol. 2013;167:2421–9. doi: 10.1016/j.ijcard.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 87.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–12. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 88.Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–5. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 89.Schutz FA, Je Y, Azzi GR, Nguyen PL, Choueiri TK. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22:1404–12. doi: 10.1093/annonc/mdq587. [DOI] [PubMed] [Google Scholar]
- 90.Pantaleo MA, Mandrioli A, Saponara M, et al. Development of coronary artery stenosis in a patient with metastatic renal cell carcinoma treated with sorafenib. BMC cancer. 2012;12:231. doi: 10.1186/1471-2407-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Lorenzo G, Autorino R, Bruni G, et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol. 2009;20:1535–42. doi: 10.1093/annonc/mdp025. [DOI] [PubMed] [Google Scholar]
- 92.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New Engl J Med. 2013;368:987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 93.Hoving S, Heeneman S, Gijbels MJ, et al. Anti-inflammatory and anti-thrombotic intervention strategies using atorvastatin, clopidogrel and knock-down of CD40L do not modify radiation-induced atherosclerosis in ApoE null mice. Radiother Oncol. 2011;101:100–8. doi: 10.1016/j.radonc.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 94.Hoving S, Heeneman S, Gijbels MJ, et al. Irradiation induces different inflammatory and thrombotic responses in carotid arteries of wildtype C57BL/6J and atherosclerosis-prone ApoE(−/−) mice. Radiother Oncol. 2012;105:365–70. doi: 10.1016/j.radonc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013–32. doi: 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol. 2007;25:43–9. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 97.Groarke JD, Nguyen PL, Nohria A, Ferrari R, Cheng S, Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2014;35:612–23. doi: 10.1093/eurheartj/eht114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feliz V, Saiyad S, Ramarao SM, Khan H, Leonelli F, Guglin M. Melphalan-induced supraventricular tachycardia: incidence and risk factors. Clin Cardiol. 2011;34:356–9. doi: 10.1002/clc.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu YF, Liu CJ, Chang PM, et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165:355–7. doi: 10.1016/j.ijcard.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 100.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63:945–53. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 101.Short NJ, Connors JM. New oral anticoagulants and the cancer patient. Oncologist. 2014;19:82–93. doi: 10.1634/theoncologist.2013-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]