Abstract
Chemokines are chemotactic cytokines that control the migration of cells between tissues and the positioning and interactions of cells within tissue. The chemokine superfamily consists of approximately 50 endogenous chemokine ligands and 20 G protein–coupled seven-trans membrane spanning signaling receptors. Chemokines mediate the host-response to cancer by directing the trafficking of leukocytes into the tumor microenvironment. This migratory response is complex and consists of diverse leukocyte subsets with both antitumor and pro-tumor activities. Although chemokines were initially appreciated as important mediators of immune cell migration, we now know that they also play important roles in the biology of non-immune cells important for tumor growth and progression. Chemokines can directly modulate the growth of tumors by inducing the proliferation of cancer cells and preventing their apoptosis. They also direct tumor cell movement required for metastasis. Chemokines can also indirectly modulate tumor growth through their effects on tumor stromal cells and by inducing the release of growth and angiogenic factors from cells in the tumor microenvironment. In this Masters of Immunology primer, we focus on recent advances in understanding the complex nature of the chemokine system in tumor biology with a focus on how the chemokine system could be used to augment cancer immunotherapeutic strategies to elicit a more robust and long-lasting host antitumor immune response.
Introduction
The directed movement of cells is tightly regulated by the spatial and temporal expression of chemokines (1). Chemokines are chemotactic cytokines that regulate the trafficking and positioning of cells by activating the seven-transmembrane spanning G protein-coupled chemokine receptors (GPCR). In addition to GPCRs, chemokines also bind non G protein–coupled seven-transmembrane spanning receptors called atypical chemokine receptors (ACKR) that lack the ability to engage conventional chemokine receptor signaling pathways and, instead, act to scavenge chemokines to help maintain chemokine gradients in tissue. Chemokines are basic proteins that also bind to glycosaminoglyans, which play important roles in their biology. Chemokines are divided into four subfamilies based on the position of the first two N-terminal cysteine residues, including the CC, CXC, CX3C and XC subfamilies. To date, nearly 50 chemokines and 20 signaling chemokine receptors and 4 AKCRs have been identified. Differential expression of chemokine receptors on leukocytes results in selective recruitment of specific cell types under particular conditions, providing appropriate and efficient immune responses tailored to the infecting pathogen or foreign insult. Beyond their pivotal role in the coordinated migration of immune cells to the site of inflammation, chemokines are now appreciated to play important roles in the development of lymphoid tissues, in the maturation of immune cells, and in the generation and delivery of adaptive immune responses (1). Dysregulated expression of chemokines and their corresponding receptors is implicated in a broad range of human diseases, including autoimmune and inflammatory diseases and cancer (2, 3).
Tumors are increasingly recognized as a complex microenvironment made up of many different cell types that cohabit and communicate with each other in a complicated signaling network. It is becoming increasingly recognized that cancer can only be fully understood by understanding the function of each cell type in the tumor and how these different cell types interact within the tumor microenvironment (TME). This includes understanding the crosstalk between cells in the tumor in the form of chemokines and cytokines and their effects on the immune response and metastasis.
Chemokines are essential coordinators of cellular migration and cell-cell interactions and therefore have great impact on tumor development. In the TME, tumor-associated host cells and cancer cells release an array of different chemokines, resulting in the recruitment and activation of different cell types that mediate the balance between antitumor and pro-tumor responses. In addition to their primary role as chemoattractants, chemokines are also involved in other tumor-related processes, including tumor cell growth, angiogenesis and metastasis (3). In this Masters of Immunology primer, we focus on the roles of chemokines in cancer biology and tumor immunology and their potential as adjuncts for cancer immunotherapy.
Tumor growth and progression
Unlike normal cells that carefully maintain cellular homeostasis by strictly regulating the spatiotemporal expression of and response to growth factors, tumor cells ‘hijack’ the host cell’s regulatory machinery responsible for growth factor synthesis and signaling to sustain their own growth and proliferation (4). Numerous studies have demonstrated that chemokines and their receptors are involved in tumor cell growth and progression. Chemokines can promote the proliferation and survival of tumor cells in several ways. Ligation of chemokine receptors on tumor cells induces the stimulation of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk) signaling pathway, hence leading to the expression of important growth-stimulating genes, such as cyclins D1 (5), Fos (6) and heparin-binding epidermal growth factor (HB-EGF) (7). Furthermore, chemokines can promote the survival of tumor cells by shifting the balance between pro-apoptotic and anti-apoptotic proteins in tumor cells, including upregulation of the expression of Mdm2 (8) and downregulation of Bcl-2 expression or inhibition of caspase-3 and caspase-9 activation (9).
Tumor cells have been shown to acquire the ability to produce growth-promoting chemokines and to express chemokine receptors. For instance, melanoma has been found to express a number of chemokines, including CXCL1, CXCL2, CXCL3, CXCL8, CCL2 and CCL5, which have been implicated in tumor growth and progression (10). Alternatively, tumor cells may overexpress chemokine receptors, thereby creating a feedback loop in which more cancer cells divide under the influence of growth-promoting chemokines that are available in the TME. For example, CXCR4, which is normally not found on breast epithelial cells, is often expressed on breast cancer cells. The overexpression of CXCR4 renders the tumor cells responsive to its cognate ligand CXCL12 (11, 12). Furthermore, tumor cells can stimulate stromal cells to synthesize and secrete growth-promoting chemokines, establishing a reciprocal tumor-stromal interaction that favors tumor growth. Accessory cells in the TME, such as cancer-associated fibroblasts (CAF) and macrophages have been demonstrated to produce chemokines and promote tumor growth (13-15).
Angiogenesis
Cancer cells within a tumor rely on blood vessels to acquire adequate oxygen and nutrients as well as to eliminate waste (4, 16). Angiogenesis has been described as a rate-limiting step in tumor formation and progression (16). Since a tumor is a rapidly proliferating mass of cells, it requires accelerated neoangiogenesis to provide for the escalating demand in oxygen and nutrient consumption (16). Chemokines and their receptors have been implicated as important regulators of tumor angiogenesis (17), and have been described to have a dual function in tumor blood vessel formation. CXCL12 is the most potent angiogenic chemokine. In fact, mice deficient in CXCL12 or its receptor CXCR4 have vascular abnormalities (18, 19). Other CXC chemokines have also been implicated in angiogenesis. CXC chemokine can be subcategorized into two groups based on the presence of ELR (Glu-Leu-Arg) motif at the N-terminus (17). Generally, ELR+ CXC chemokines (CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7 and CXCL8) that activate CXCR1 and CXCR2 have been described as promoters of angiogenesis while those without the ELR motif (CXCL4, CXCL9, CXCL10, CXCL11 and CXCL14) have been shown to be inhibitors of angiogenesis. However, as mentioned above CXCL12, which is an ELR- chemokine, has angiogenic activity.
Chemokines can affect angiogenesis via binding to chemokine receptors expressed on endothelial cells, leading to increased migration and in some cases proliferation and the inhibition of apoptosis (17). Chemokines can work in concert with other angiogenic factors to promote angiogenesis (17). Some chemokines, such as CXCL8 and CXCL12, upregulate the expression of vascular endothelial growth factor (VEGF), generating a positive feedback loop in which VEGF further enhances the angiogenic chemokine production (20, 21). Furthermore, chemokines can attract angiogenic factor-producing leukocytes, such as macrophages, into the TME, accelerating angiogenesis (22).
As mentioned above, chemokines are also capable of inhibiting angiogenesis. CXCL4 and CXCL10 can suppress basic fibroblast growth factor (bFGF)- and VEGF-induced angiogenesis as well as inhibit endothelial cell chemotaxis and proliferation (23-26). These effects have been shown to be mediated by the ability of CXCL4 and CXCL10 to displace basic endothelial growth factors from their required heparan sulfate proteoglycan coreceptors (23-25). Furthermore, CXCL9, CXCL10 and CXCL11 can attract CXCR3-expressing CD4+ T-helper (Th) 1 cell or CD8+ cytotoxic T cells that may be involved in the angiostatic responses.
Metastasis
Metastasis refers to the process during which malignant tumor cells spread from the site of the primary tumor to distant sites of the body and is the leading cause of death for most solid tumors. Metastasis is a highly complex process, which consists of local invasion, intravasation, circulation, extravasation, proliferation/colonization, angiogenesis and growth (27). A number of studies have found that chemokines play an integral role in metastasis. Tumor cells express selected chemokine receptors, which can help direct tumor cells to specific anatomic sites to form metastases (28). These sites of metastasis produce particular chemokines that attract circulating tumor cells into a ‘premetastatic niche’, which has a supporting microenvironment for the growth of metastatic tumor cells (28).
CXCR4 and its ligand CXCL12 have been implicated in metastasis. Blockade of the CXCR4/CXCL12 axis suppresses the metastasis of breast cancer to the lung (29). Its involvement in metastasis has also been demonstrated in different types of tumors, such as prostate cancer, lung cancer and glioblastoma (28). In human patients, expression of CXCR4 is correlated with increased tumor metastasis (30-33). The entry of tumor cells into lymphatic vessels is critical for the development of metastasis, and CCR7 and its ligand CCL21 are critical for this process for dendritic cells (DC) and T cells and may also play a similar role for tumor cells (28). In addition, it has been demonstrated that CCL1 produced by lymphatic endothelial cells in the subcapsular sinus can attract CCR8+ tumor cells to the lymph node to promote metastasis (34).
Chemokines in tumor immunology
In addition to their intrinsic cellular alterations that allow unchecked proliferation, tumor cells interact with their surroundings to form and sustain a favorable TME by promoting angiogenesis, inflammation, and metastasis as well as modulating the systemic immune response. Although the concept of cancer immunosurveillance was once abandoned, the discovery of the importance of host interferon-γ (IFNγ) in rejecting tumor cells has laid the foundation for renewed interest in cancer immunotherapies (35). Most, if not all, solid tumors have dominant infiltration of immune cells. Chemokines play an important role in leukocyte infiltration into any tissue, including tumors. Hence, they have a critical role shaping the immune cell composition in the TME, which affects tumor development. Immune cells that are responsible for the removal of cancer cells are called “effector cells”, which are able to kill or control the growth of tumor cells. The major effector cells that are involved in the elimination of cancer cells are natural killer (NK) cells, γδ T cells, and effector CD4+ and CD8+ T cells (36). To eradicate a tumor, effector cells must migrate into the TME, and the presence of effector cells within a tumor can be a positive prognostic indicator.
CXCR3 and its ligands CXCL9 and CXCL10 are strongly associated with Th1-biased immune response (37, 38). The quality of the Th1-biased immune response is an important determinant of an effective protective antitumor cellular immune response (36). Recent studies indicate that CXCR3-mediated antitumor responses are achieved by recruiting NK cells, CD4+ Th1 cells, and CD8+ cytotoxic T lymphocytes (CTL) into the tumor (39-42). Since CXCL9 and CXCL10 expression is induced by type I and II IFNs, recruited immune cell-derived IFNs can further upregulate intratumor expression of CXCL9 and CXCL10, generating an amplification loop that limits tumor growth. In addition, an important role of CXCR3 in macrophage polarization was found recently (43). In a murine model of breast cancer, CXCR3 deficiency results in the polarization of macrophages towards an M2 phenotype, which has a role in promoting tumor growth; this result indicates a requirement of CXCR3 for M1 macrophage generation. Macrophages can be polarized into M1 or M2 subtypes, depending on the activation signals they receive from their local cytokine milieu (44). M1 macrophages are induced by IFNγ whereas M2 macrophages are preferentially induced by IL4, IL13 and TGF-β. M1 macrophages exhibit tumoricidal activity and may function as antigen-presenting cells (APC) to activate effector T cells. In contrast, M2 macrophages promote Th2 cell responses and express high level of IL10 and pro-tumorigenic factors that facilitate growth of tumors and metastases. CCR5 and its ligand CCL5 are involved in the recruitment of immune cells with antitumor activity (45). CCR5 deficiency in mice accelerates the growth of transplantable Lewis lung adenocarcinoma (LLC), pancreatic adenocarcinoma (Panc02), and lymphoma (EG7). CCR5 expression on both CD4+ T cells and CD8+ T cells is important for the generation of protective immunity against tumors. Presence of CCR5-expressing CD4+ T cells can improve maturation of APCs via the CD40/CD40L pathway, leading to maximal CD8+ T cell antitumor response.
Paradoxically, different immune cell subsets can suppress the function of effector cells and allow tumor growth, such as tumor-associated macrophages (TAM), myeloid-derived suppressor cells (MDSC), and regulatory T cells (Treg) (36). To gain a growth advantage, tumors can alter the local chemokine expression profile to recruit these suppressive cells. Tregs are frequently present in tumors. They are very potent suppressors of both innate and adaptive immunity via their production of IL10 and TGF-β (46). Selective depletion of Tregs in various experimental and spontaneous tumor models has been shown to potently induce NK cell- and T cell-dependent antitumor immunity (47-50), and the presence of Tregs in cancer patients is associated with a poor prognosis (51). In ovarian cancer, intratumoral CCL22 and CCL28 expression is upregulated and can attract CCR4+ or CCR10+ Tregs, respectively, which can suppress antitumor responses (51, 52) and enhance angiogenesis via the secretion of VEGF (52). Furthermore, in a spontaneous breast cancer metastasis model, CCR4+ Tregs promote the formation of metastases via inducing apoptosis of NK cells (53). Of note, it has been demonstrated that Tregs can coexpress Th-specific transcription factors and chemokine receptors to ensure appropriate specific control of pro-inflammatory effector Th cell responses. For example, T-bet and CXCR3 are not only expressed on Th1 cells, but are also expressed on Tregs in a Th1-type environment (54-56). For tumors to escape from the host protective immunity, which is largely Th1-dependent, it is possible that tumor cells instruct Tregs to express T-bet and CXCR3 in order to suppress Th1-specific immune responses. Interestingly, most of the Tregs in human ovarian carcinoma express CXCR3 (57).
TAMs and MDSCs are heterogeneous populations of immunosuppressive myeloid cells of differing developmental status (58). They can be distinguished by the expression of different surface markers. TAMs and MDSCs infiltration is seen frequently in various models of cancer. They are able to suppress both NK cell- and T cell-mediated antitumor immunity and are potent producer of pro-tumorigenic mediators such as arginase 1, iNOS, and TGF-β (58). Furthermore, they can increase the infiltration of Tregs into tumor bed via releasing different chemokines (59, 60). Various sets of chemokine and chemokine receptor, including CCR2/CCL2, CCR5/CCL5, CXCR2/CXCL5, and CXCR4/CXCL12, have been shown to promote tumor progression by increasing the formation, recruitment and suppressive activity of TAMs and MDSCs (61-63).
Chemokines in cancer therapy
Standard first line cancer therapies, such as chemotherapy and radiotherapy, have been demonstrated to, at least partially, rely on their immunostimulatory effects for their therapeutic efficacy (64). It has been suggested that the specific immune contexture of a tumor can be correlated to clinical outcomes (65). Thus, the presence of specific chemokines in the TME may be a determinant of therapeutic efficacy.
In a murine transplantable tumor model of fibrosarcoma, anthracycline treatment induces the expression of CCL2 in tumor-bearing mice (66). The therapeutic efficacy of anthracycline relies on CCL2-dependent recruitment of CD11b+CD11c+Ly6Chigh cells into the tumor bed. These cells act locally as APCs to stimulate the effector T-cell response. Notably, CD11b+CD11c+Ly6Chigh cells are the major producers of CCL2, thereby generating a positive feedback loop for optimal chemotherapy-induced antitumor responses. In melanoma, chemotherapy can also induce intratumoral expression of CXCR3 ligands and CCL5, which are important for effector T-cell trafficking into the tumor bed (67). There is a positive correlation between the expression of these chemokines and clinical outcome. CXCL10 is also associated with the response to radiotherapy (68). IFNγ-producing CD8+ T cells are important effector cells in radiotherapy. Depletion of these cells abrogated the antitumor effect of radiotherapy. Type I and II IFN-dependent production of CXCL10 by myeloid cells is required for the recruitment of CD8+ effector T cells to achieve the antitumor effect of radiotherapy.
Despite the beneficial effects of chemokines that are induced by chemotherapy, chemokines can also contribute to drug resistance thereby increasing tumor cell survival. For example, in a breast cancer model, chemotherapy with doxorubucin and cyclophosphamide directly kills tumor cells (69). However, these chemotherapeutic agents also induce the expression of CXCL1 and CXCL2 via a TNF-α-stimulated NF-κB pathway. As a result, there is an increased recruitment of myeloid suppressor cells in the TME, which are linked to enhanced tumor survival and metastasis.
Immunotherapy for cancer has come a long way and has just entered a golden age as a result of recent breakthroughs with checkpoint blockade therapies. Immunotherapy targeting T-cell checkpoint molecules, such as CTLA-4 and PD-1, is currently one of the most promising new therapeutic approaches for cancer therapy. Anti-PD-1 and anti-CTLA-4 have been used to attenuate Treg suppressor function and reverse effector T-cell dysfunction to eradicate tumors (70, 71). Adoptive transfer of in vitro expanded autologous T cells or genetically engineered T cells have also been showing clinical promise (72). However, a major obstacle to obtain the maximal therapeutic effect from immunotherapy is the limitation of infiltration of effector T cells into tumor bed due to abnormal tumor vessels, hypoxic conditions and an overall suppressive microenvironment (73). Finding ways to enhance effector T-cell trafficking and fitness has become a major goal in the field since the successful application of immunotherapy in the clinic. In mouse cancer models, monoclonal antibodies that target T-cell inhibitory checkpoint can promote antitumor response by enhancing infiltration, proliferation and activity of CD4+ and CD8+ T cells (74). Differentiation of naïve CD4+ and CD8+ T cells into Th1 and effector T cells, respectively, is accompanied by the sustained upregulation of CXCR3 (37). Results from several studies suggest that PD-L1/PD-1 signaling might induce the expression of chemokines and their corresponding receptors, particularly in the CXCR3 system (41, 75, 76). Recently it has been shown that anti-PD-1 immunotherapy can enhance the therapeutic efficacy of adoptive cell-transfer therapy by increasing CXCL10 expression in the tumor (41).
The aforementioned findings demonstrate the complex role of chemokines in tumor formation and growth. Since cancer type, disease stage as well as intratumor immune cell composition affect the outcome of treatment, great care should be taken when developing chemokine-based therapies for cancer. To date, several strategies have been developed to block chemokine-dependent responses by depleting chemokines and antagonizing their receptor signaling pathways or to augment chemokine-induced effects by increasing the expression of chemokines using different methods, such as naked DNA plasmids, engineered tumor cells and transduced DCs (77). Lack of proper host response in the recognition of immunogenic tumors has remained one of the key challenges in the development of a reliable cancer therapy. Immune recognition of tumor cells can only occur in the presence of APCs and lymphocytes. Hence, there has been great interest in recognizing the chemokine signals that facilitate immune-cell recruitment into tumors, ultimately with the aim of exploring the potential specific enhancement of APC and effector T-cell infiltration and blockade of Treg migration and function.
Concluding remarks
Chemokines are multifunctional mediators that not only affect immune-cell infiltration into tumors, but also have a great impact on the process of tumor growth, angiogenesis and metastasis. Contribution of chemokines to the overall outcome of tumor development depends on the balance between tumor-promoting and tumor-inhibiting factors. It has become increasingly evident that chemokines are potentially bi-functional during tumor development and may display both tumor-promoting and tumor-suppressive capabilities. Therefore, further study of the distinctions between the pro-tumor and antitumor activities of chemokines is warranted in order to develop more effective therapies against cancer. Although the mouse tumor studies are encouraging, there is still a long way to go for utilizing chemokine-targeted therapy for the treatment of cancer patients.
Figure 1. Multifaceted roles of chemokines in tumor development.
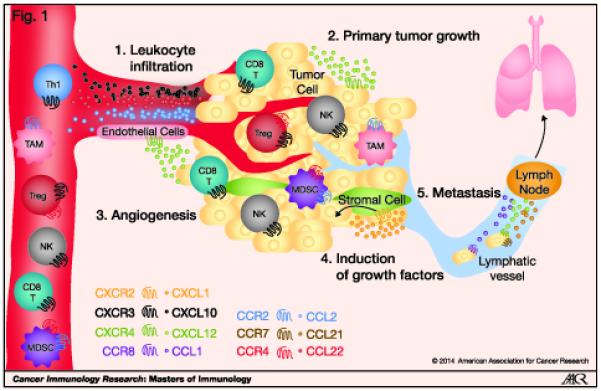
(1) Chemokines produced by tumor cells, intratumor stromal cells, such as fibroblasts, and intratumor leukocytes can attract different immune cell types into the tumor bed. The composition of immune cells in the tumor can affect the outcome of development. (2) Tumor- and stromal cell-derived chemokines can directly support the growth, proliferation and survival of tumor cells. (3) Chemokines released by tumor cells, stromal cells and leukocytes can regulate the process of angiogenesis by their angiogenic or angiostatic activity. (4) Chemokines produced within the tumor can induce the release of tumor-promoting growth factors that can act in a paracrine fashion to promote tumor growth. (5) Chemokines are also involved in the migration of tumor cells to distant sites for the development of metastasis. CD8 T – CD8+ T cell; Th1 – Th1-type CD4+ T cells; NK – natural killer cell; Treg – regulatory T cell; MDSC – myeloid-derived suppressor cell; TAM – tumor–associated macrophage.
Acknowledgements
MTC and ADL were supported by grants from the National Institute of Health R01CA069212; P01AI112521; R37AI040618; U19AI095261; P30DK043351.
References
- 1.Griffith J, Sokol C, Luster A. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 2.Charo I, Ransohoff R. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Wani NA, Nasser MW, Ahirwar DK, Zhao H, Miao Z, Shilo K, et al. CXC motif chemokine 12/CXC chemokine receptor type 7 signaling regulates breast cancer growth and metastasis by modulating the tumor microenvironment. Breast Cancer Res. 2014;16:R54. doi: 10.1186/bcr3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HJ, Edwards R, Tucci S, Bu P, Milsom J, Lee S, et al. Chemokine 25-induced signaling suppresses colon cancer invasion and metastasis. J Clin Invest. 2012;122:3184–96. doi: 10.1172/JCI62110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolitho C, Hahn M, Baxter R, Marsh D. The chemokine CXCL1 induces proliferation in epithelial ovarian cancer cells by transactivation of the epidermal growth factor receptor. Endocr Relat Cancer. 2010;17:929–40. doi: 10.1677/ERC-10-0107. [DOI] [PubMed] [Google Scholar]
- 8.Su H, Sobrino NE, Toth T, Ng C, Lelievre S, Fred M, et al. Chemokine receptor CXCR4–mediated transformation of mammary epithelial cells by enhancing multiple RTKs expression and deregulation of the p53/MDM2 axis. Cancer Lett. 2011;307:132–40. doi: 10.1016/j.canlet.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Song JK, Park MH, Choi D-Y, Yoo HS, Han SB, Yoon DY, et al. Deficiency of CC chemokine receptor 5 suppresses tumor development via inactivation of NF-κB and upregulation of IL-1Ra in melanoma model. PLoS One. 2012;7:e33747. doi: 10.1371/journal.pone.0033747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–22. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 12.Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006;238:30–41. doi: 10.1016/j.canlet.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Lau TS, Chung TKH, Cheung TH, Chan LKY, Cheung LWH, Yim SF, et al. Cancer cell-derived lymphotoxin mediates reciprocal tumour-stromal interactions in human ovarian cancer by inducing CXCL11 in fibroblasts. J Pathol. 2014;232:43–56. doi: 10.1002/path.4258. [DOI] [PubMed] [Google Scholar]
- 14.Mishra P, Banerjee D, Ben-Baruch A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J Leukoc Biol. 2011;89:31–9. doi: 10.1189/jlb.0310182. [DOI] [PubMed] [Google Scholar]
- 15.Rigo A, Gottardi M, Zamò A, Mauri P, Bonifacio M, Krampera M, et al. Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Mol Cancer. 2010;9:273. doi: 10.1186/1476-4598-9-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 17.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 19.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S-i, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 20.Salcedo R, Oppenheim JJ. Role of chemokines in angiogenesis: CXCL12/SDF - 1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation. 2003;10:359–70. doi: 10.1038/sj.mn.7800200. [DOI] [PubMed] [Google Scholar]
- 21.Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFκB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038–42. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–9. [PubMed] [Google Scholar]
- 23.Perollet C, Han ZC, Savona C, Caen JP, Bikfalvi A. Platelet Factor 4 Modulates Fibroblast Growth Factor 2 (FGF-2) Activity and Inhibits FGF-2 Dimerization. Blood. 1998;91:3289–99. [PubMed] [Google Scholar]
- 24.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219–31. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campanella GS, Colvin RA, Luster AD. CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS One. 2010;5:e12700. doi: 10.1371/journal.pone.0012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimberg A. The Chemokine System in Experimental and Clinical Hematology. Springer; 2010. Chemokines in angiogenesis; pp. 59–80. [Google Scholar]
- 27.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 28.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 29.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 30.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18:1240–2. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 31.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–7. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 32.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gönner U, Wilsberg V, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–50. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 33.Kang H, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The elevated level of CXCR4 is correlated with nodal metastasis of human breast cancer. The Breast. 2005;14:360–7. doi: 10.1016/j.breast.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210:1509–28. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFNγ receptors. Immunity. 1994;1:447–56. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 36.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 37.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317:620–31. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groom J, Richmond J, Murooka T, Sorensen E, Sung J, Bankert K, et al. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity. 2012;37:1091–103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-γ and CXCR3 ligands. Cancer Res. 2008;68:8437–45. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- 40.Hensbergen PJ, Wijnands PGB, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother. 2005;28:343–51. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 41.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209–18. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson Å , Yang S-C, Huang M, Zhu L, Kar UK, Batra RK, et al. IL-7 promotes CXCR3 ligand-dependent T cell antitumor reactivity in lung cancer. J Immunol. 2009;182:6951–8. doi: 10.4049/jimmunol.0803340. [DOI] [PubMed] [Google Scholar]
- 43.Oghumu S, Varikuti S, Terrazas C, Kotov D, Nasser MW, Powell CA, et al. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology. 2014;143:109–19. doi: 10.1111/imm.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 45.González-Martín A, Gómez L, Lustgarten J, Mira E, Mañes S. Maximal T cell-mediated antitumor responses rely upon CCR5 expression in both CD4+ and CD8+ T cells. Cancer Res. 2011;71:5455–66. doi: 10.1158/0008-5472.CAN-11-1687. [DOI] [PubMed] [Google Scholar]
- 46.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 2010;70:7800–9. doi: 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- 48.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–99. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Kostareli E, Suffner J, Garbi N, Hämmerling GJ. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol. 2010;40:3325–35. doi: 10.1002/eji.201041093. [DOI] [PubMed] [Google Scholar]
- 50.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210:2435–66. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 52.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang L-P, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature. 2011;475:226–30. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 53.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koch MA, Tucker-Heard Gs, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoerning A, Koss K, Datta D, Boneschansker L, Jones CN, Wong IY, et al. Subsets of human CD4+ regulatory T cells express the peripheral homing receptor CXCR3. Eur J Immunol. 2011;41:2291–302. doi: 10.1002/eji.201041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suga H, Sugaya M, Miyagaki T, Ohmatsu H, Okochi H, Sato S. CXCR3 deficiency prolongs Th1-type contact hypersensitivity. J Immunol. 2013;190:6059–70. doi: 10.4049/jimmunol.1201606. [DOI] [PubMed] [Google Scholar]
- 57.Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, et al. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res. 2012;72:4351–60. doi: 10.1158/0008-5472.CAN-12-0579. [DOI] [PubMed] [Google Scholar]
- 58.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602–11. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, et al. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One. 2011;6:e19495. doi: 10.1371/journal.pone.0019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:292376. doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 65.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 66.Ma Y, Mattarollo SR, Adjemian S, Yang H, Aymeric L, Hannani D, et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res. 2014;74:436–45. doi: 10.1158/0008-5472.CAN-13-1265. [DOI] [PubMed] [Google Scholar]
- 67.Hong M, Puaux A-L, Huang C, Loumagne L, Tow C, Mackay C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71:6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 68.Lim JY, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8+ T cells. Cancer Immunol Immunother. 2014;63:259–71. doi: 10.1007/s00262-013-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–78. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti–CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. 2013;3:231. doi: 10.3389/fonc.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 75.El Annan J, Goyal S, Zhang Q, Freeman GJ, Sharpe AH, Dana R. Regulation of T-cell chemotaxis by programmed death-ligand 1 (PD-L1) in dry eye-associated corneal inflammation. Invest Ophthalmol Vis Sci. 2010;51:3418–23. doi: 10.1167/iovs.09-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during Type 1 Diabetes. Diabetes. 2013;62:2859–69. doi: 10.2337/db12-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schall TJ, Proudfoot AE. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol. 2011;11:355–63. doi: 10.1038/nri2972. [DOI] [PubMed] [Google Scholar]


