Abstract
Although graphene oxide (GO) has recently been considered as a highly attractive nanomaterial for future cancer imaging and therapy, it is still a major challenge to improve its in vivo tumor active targeting efficiency. Here in this full article, we demonstrated the successful and significantly enhanced in vivo tumor vasculature targeting efficacy of well-functionalized GO nanoconjugates by using vascular endothelial growth factor 121 (VEGF121) as the targeting ligand. As-developed GO nanoconjugate exhibits excellent in vivo stability, specific in vitro and in vivo vascular endothelial growth factor receptor (VEGFR) targeting, significantly enhanced tumor accumulation (>8 %ID/g) as well as high tumor-to-muscle contrast, showing great potential for future tumor targeted imaging and therapy.
Keywords: graphene oxide (GO), vasculature targeting, positron emission tomography (PET), VEGF
1. Introduction
Graphene is a well-known material with single-layered carbon atoms packed into a two-dimensional honeycomb lattice [1]. Due to its unique mechanical, electronic, optical, and chemical properties, graphene has attracted tremendous interest over the last several years [2-6]. Among many different subtypes of graphene-based nanomaterials, graphene oxide (GO) with extremely high specific surface area has been recently accepted as an excellent platform for applications in biosensor, drug delivery, gene transfection, to name a few [7-12]. In addition, owing to the intrinsic high near-infrared (NIR) absorbance, functionalized GO has also been employed for photothermal therapy in small animals [13-15].
However, challenges still exist. Most of current studies are focusing on in vivo passive targeted delivery of GO nanoconjugates with only limited tumor accumulation [16, 17]. Developing suitable in vivo active targeting strategies for further improving their targeting efficacy is still one of the major challenges in this field.
It is well accepted that tumor angiogenesis occurs when the tumor reaches a certain size (usually 1-2 mm in diameter), as new blood vessel formation is needed to supply oxygen and nutrients to cancer cells and to remove waste [18]. Tumor angiogenesis targeting (or vasculature targeting) has recently been accepted as a generally applicable in vivo targeting strategy for most of nanoparticles regardless of tumor types [19]. Vascular endothelial growth factor receptor (VEGFR) is a universal target overexpressed on vasculature of multiple solid tumor types and other disease models [20-22]. Being the naturally existing VEGFR ligand, VEGF121 offers several advantages over the synthetic small-molecule VEGFR ligands or anti-VEGFR antibodies, and has much higher binding affinity to VEGFR (nanomolar range) than reported peptidic VEGFR inhibitors (submicromolar to micromolar range) [23].
Although VEGF121 could serve as a promising targeting ligand for cancer diagnosis and treatment in preclinical studies and clinical trials, to date, few examples of positron emission tomography (PET) imaging with VEGF121-conjugated nanoparticle have been reported [24]. Here, we aim for design and synthesis of a new type of GO-based tumor vasculature targeting nanoconjugate by surface engineering of GO with positron emission radioisotopes and VEGF121, forming a novel GO nanoconjugate for non-invasive, quantitative and in vivo vasculature targeted tumor imaging.
2. Materials and methods
2.1. Reagents
VEGF121 was provided by GenScript Corp. (Piscataway, NJ). S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) was purchased from Macrocyclics, Inc. (Dallas, TX). Chelex 100 resin (50-100 mesh) and fluoresce in isothiocyanate (FITC) were purchased from Sigma-Aldrich (St. Louis, MO). Succinimidyl carboxymethyl PEG maleimide (SCM-PEG-Mal; molecular weight: 5 kDa; Creative PEGworks, Winston Salem, NC), rat anti-mouse CD31 primary antibody (BD Biosciences, San Diego, CA), AlexaFluor488- or Cy3-labeled secondary antibodies (Jackson Immunoresearch Laboratories, Inc., West Grove, CA), Bevacizumab (Avastin, Genentech, San Francisco, CA) and PD-10 desalting columns (GE Healthcare, Piscataway, NJ) were all acquired from commercial sources. Water and all buffers were of Millipore grade and pre-treated with Chelex 100 resin to ensure that the aqueous solution was free of heavy metal. All other reaction buffers and chemicals were obtained from Thermo Fisher Scientific (Fair Lawn, NJ).
2.2. Synthesis of GO-PEG-NH2
GO-PEG-NH2 was synthesized by a similar process as detailed in our previous report [25, 26]. Briefly, GO was produced by a modified Hummers method, using flake expandable graphite as the original material. The prepared GO was mixed with 6-arm-polyethylene glycol-amine (10 kDa) at a weight ratio of 1:6 and reacted for ∼ 12 h in the presence of N-(3-dimethylaminopropyl-N'-ethylcarbodiimide) hydrochloride to form GO-PEG-NH2. Excess PEG in the as-synthesized GO-PEG-NH2 solution was removed by centrifuge filtration through 100 kDa MWCO Amicon filters and washed with water for 6 times. Atomic force microscopy and dynamic light scattering were performed to characterize the GO morphology and size distribution of nanoconjugates [27].
2.3. Synthesis of VEGF121-SH
VEGF121 was incubated with Traut's reagent at a molar ratio of 1:15 at pH 8.0 for 2 h. The resulting VEGF121-SH was purified by size exclusion column chromatography with phosphate-buffered saline (PBS, pre-treated with Chelex 100 resin to prevent oxidation of the thiol) as the mobile phase.
2.4. Syntheses of GO-VEGF121 nanoconjugates
GO-PEG-NH2 was first mixed with p-SCN-Bn-NOTA at a molar ratio of 1:10 at pH 9.0, and reacted for 2 h at room temperature. The resulting NOTA-GO was purified by centrifugation with 100 kDa MWCO Amicon filters at 8,000 rpm for 15 min. Subsequently, NOTA-GO was reacted with SCM-PEG-Mal at a molar ratio of 1:30 at pH 8.5 for 2 h. The resulting NOTA-GOPEG-Mal was purified by centrifugation with 100 kDa MWCO Amicon filters at 8,000 rpm for 15min. Afterwards, NOTA-GO-PEG-Mal was reacted with VEGF121-SH at a molar of 1:10 at pH 7.5 in the presence of tris(2-carboxyethyl)phosphine (TCEP). The final products were purified by size exclusion column chromatography and termed as NOTA-GO-VEGF121. Similar strategies were used for the synthesis of FITC-GO-VEGF121 and FITC-GO nanoconjugates.
2.5. Cell lines and animal model
4T1 murine breast cancer, U87MG human glioblastoma, and human umbilical vein endothelial cells (HUVECs) were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured as previously described [28]. Cells were used for in vitro and in vivo experiments when they reached ∼80 % confluence. All animal studies were conducted under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. Four- to five-week-old female nude mice (Harlan, Indianapolis, IN) were each injected with 2×106 U87MG cells in the flank to generate the U87MG glioblastoma model. The mice were used for in vivo experiments when the tumor diameter reached 4-6 mm.
2.6. Flow cytometry
HUVECs (VEGFR positive) [29] and 4T1 (VEGFR negative) [30] cells were harvested and suspended in cold PBS with 2 % bovine serum albumin at a concentration of 5×106 cells/mL. Cells were incubated with FITC-GO-VEGF121 or FITC-GO at a concentration of 5 μg/mL (based on GO) for 30 min at room temperature before washing for three times with cold PBS. Subsequently, the cells were analyzed using a BD FACSCalibur 4-color analysis cytometer equipped with 488 nm and 633 nm lasers (Becton-Dickinson, San Jose, CA) and FlowJo analysis software (Tree Star, Inc., Ashland, OR).
2.7. 64Cu labeling
64Cu was produced with an onsite cyclotron (GE PETrace) in University of Wisconsin - Madison. 64CuCl2 (74 MBq) was diluted in 300 μL of 0.1 M sodium acetate buffer (pH 6.5) and mixed with 50 μg of NOTA-GO-VEGF121 or NOTA-GO. The reaction was conducted at 37 °C for 30 min with constant shaking [31, 32]. The resulting 64Cu-NOTA-GO-VEGF121 or 64Cu-NOTA-GO was purified by size exclusion column chromatography using PBS as the mobile phase. The radioactive fractions containing 64Cu-NOTA-GO-VEGF121 or 64Cu-NOTA-GO were collected for further in vitro and in vivo studies. Since all the GO nanoconjugates will contain the same NOTA and PEG chains, both “NOTA” and “PEG” were omitted from the acronyms of the final nanoconjugates for clarity.
2.8. Serum stability study
Serum stability study was carried out to ensure 64Cu is stable on NOTA-GO-VEGF121 for in vivo PET imaging. 64Cu-NOTA-GO-VEGF121 was incubated in PBS and complete serum at 37 °C for up to 48 h. At different time points, portions of the mixture were sampled and filtered through 100 kDa MWCO filters. The radioactivity that remained on the filter was measured after discarding the filtrate. The retained (i.e., intact) 64Cu on NOTA-GO-VEGF121 was calculated using the equation (radioactivity on filter/total sampled radioactivity × 100%).
2.9. In vivo VEGFR targeted PET imaging and biodistribution studies
U87MG tumor-bearing mice were each intravenously injected with 5-10 MBq of 64Cu-NOTA-GO-VEGF121 or 64Cu-NOTA-GO via tail vein. Serial PET scans were performed at various time points post-injection (p.i.) with using a micro PET/micro CT Inveon rodent model scanner (Siemens Medical Solutions USA, Inc.). Data acquisition, image re-construction, and ROI analysis of the PET data were performed as described previously [26, 31]. Quantitative PET data of the U87MG tumor and major organs was presented as %ID/g. After the last scan at 48 h p.i., biodistribution studies were carried out to confirm that the %ID/g values based on PET imaging truly represented the radioactivity distribution in mice. Mice were euthanized and U87MG tumor, blood and major organs/tissues were collected and wet-weighed. The radioactivity in the tissue was measured using a γ counter (PerkinElmer) and presented as %ID/g (mean ± SD).
2.10. Histology
U87MG tumor-bearing mice were intravenously injected with GO-VEGF121 and GO (5 mg/kg of mouse body weight) and euthanized at 3 h p.i. (when U87MG tumor uptake was at the peak based on PET imaging). Organs including U87MG tumor, liver, spleen and muscle were frozen and cryo-sectioned for histological analysis. Frozen tissue slices of 7 μm thickness were fixed with cold acetone and stained for endothelial marker CD31 by using a rat anti-mouse CD31 antibody and a Cy3-labeled donkey anti-rat IgG [33]. To stain VEGF121, the same tissue slices were also incubated with Avastin (primary antibody) [34] and then AlexaFluor488-labeled goat anti-human IgG (secondary antibody). All images were acquired by using a Nikon Eclipse Ti microscope.
3. Results and discussion
3.1. Synthesis and characterization of GO nanoconjugates
GO was produced by Hummers method and modified with six-armed branched polyethylene glycol (PEG) as previously reported for enhanced in vivo stability and biocompatibility [8, 25]. The presence of amine groups at the terminal end could facilitate the further covalent conjugation of various functional entities. PEGylated GO (i.e. GO-PEG-NH2) was then functionalized with 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) (a well-known chelator for copper-64 (64Cu, t1/2=12.7 h) labeling) and VEGF121 as the targeting ligand for in vivo vasculature targeting.
A schematic structure of final GO nanoconjugate (i.e. 64Cu-NOTA-GO-PEG-VEGF121) after surface engineering is shown in Figure 1A. As synthesized GO-PEG-NH2 existed a size range of 20-50 nm (Figure 1b), based on atomic force microscopy (AFM) measurements (Figure 1B). Dynamic light scattering (DLS) study showed that GO-PEG-NH2 has a hydrodynamic diameter of 27.7 ± 5.8 nm, whereas the diameter of NOTA-GO-VEGF121 was found to be 32.9 ± 3.0 nm (Figure 1C). Since all the GO nanoconjugates will contain the same NOTA and PEG chains, in the following sections both “NOTA” and “PEG” were omitted from the acronyms of the final nanoconjugates for clarity.
Figure 1.

(A) A schematic illustration showing the surface engineering of GO nanoconjugates. (B) AFM image of GO-PEG-NH2. (C) DLS size distribution of the GO-PEG-NH2 (black line) and NOTA-GO-PEG-VEGF121 nanoconjugates (red line).
3.2. In vitro VEGFR targeting
To validate in vitro VEGFR targeting capability of GO-VEGF121 nanoconjugates, flow cytometry was carried out in human umbilical vein endothelial cells (HUVECs, VEGFR positive) [29] and 4T1 murine breast cancer cells (VEGFR negative) [30]. Fluoresce in isothiocyanate (FITC) was further conjugated to GO-VEGF121 to form FITC-GO-VEGF121 (targeted group). FITC-conjugated GO with no VEGF121 (i.e. FITC-GO, non-targeted group) was used as the control. As evidenced in Figure 2A, the fluorescence signal from HUVECs was significantly enhanced (∼20 fold higher than the negative control group) upon incubation with FITC-GO-VEGF121, whereas only slight fluorescence enhancement was observed upon FITC-GO treatment. Note, concentration of GO and in vitro incubation time were all kept the same. No significant fluorescence signal enhancement was observed when using 4T1 cell line for both targeted and non-targeted groups. Taken together, flow cytometry results clearly demonstrated high VEGFR targeting specificity and minimal non-specific binding of GO-VEGF121 nanoconjugates.
Figure 2.

In vitro VEGFR targeting and serum stability studies. (A) Flow cytometry analysis of the GO nanoconjugates in HUVECs (VEGFR+) and 4T1 breast cancer cells (VEGFR-). (B) Serum stability study of 64Cu-GO-VEGF121 at 37 °C for 48 h.
3.3. In vivo VEGFR targeting and ex vivo biodistribution studies
Previously, we reported PET imaging of VEGFR expression level using 64Cu-labeled VEGF121, and demonstrated higher VEGFR expression in smaller (∼60 mm3) U87MG glioblastoma tumors when compared with larger ones (∼1,200 mm3) [35]. In current study, U87MG tumor-bearing mice with tumor volume of ∼60 mm3 were used for in vivo targeted PET imaging studies.
Both GO-VEGF121 and GO nanoconjugates were labeled with 64Cu, and purified by PD-10 column, to form 64Cu-GO-VEGF121 and 64Cu-GO, respectively. In vitro serum stability was later performed by incubating 64Cu-GO-VEGF121 with complete mouse serum at 37 °C for 48 h (Figure 2B). Our results showed that more than 95% of 64Cu remained on the GO-VEGF121 nanoconjugates, indicating high stability of 64Cu-GO-VEGF121 in mouse serum.
As prepared 64Cu-GO-VEGF121 and 64Cu-GO were later intravenously injected to U87MG tumor-bearing mice and imaged using a micro PET/microCT Inveon rodent model scanner at 0.5, 3, 6, 16, 24 and 48 h post-injection (p.i.). Coronal PET images that contain the U87MG tumors are shown in Figure 3, and the quantitative data obtained from region-of-interest (ROI) analysis of the PET data are shown in Figure 4, Table S1 and Table S2.
Figure 3.

In vivo VEGFR targeted PET imaging. Serial coronal PET images of U87MG tumor bearing mice at different time points post-injection of (A) 64Cu-GO-VEGF121 (targeted group) and (B) 64Cu-GO (non-targeted group). Tumors are indicated by yellow arrowheads.
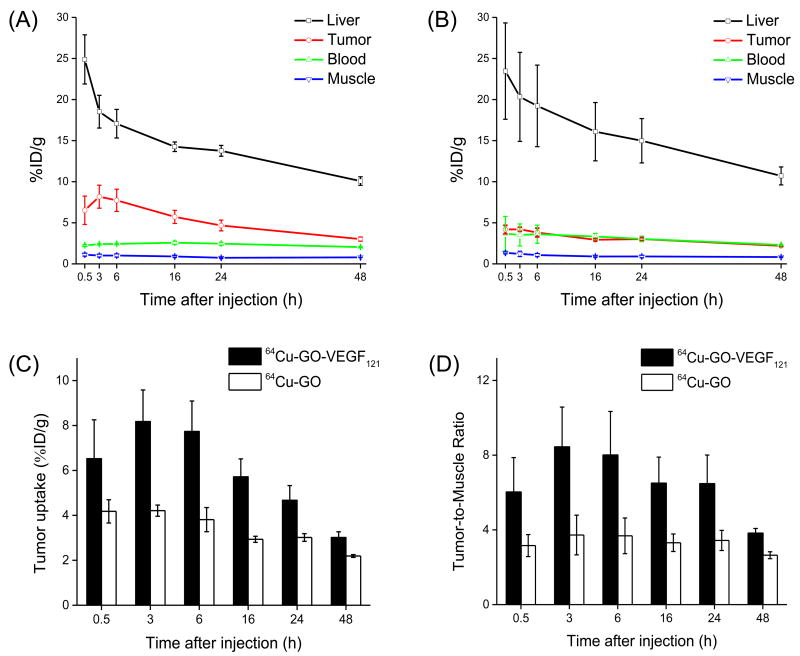
Figure 4.
Quantitative analysis of the PET data. (A) Time activity curves of the liver, U87MG tumor, blood, and muscle upon intravenous injection of 64Cu-GO-VEGF121 (targeted group). (B) Time activity curves of the liver, U87MG tumor, blood, and muscle upon intravenous injection of 64Cu-GO (non-targeted group). (C) Comparison of the U87MG tumor uptake in both targeted and non-targeted groups. (D) Comparison of the tumor-to-muscle ratio in targeted and non-targeted groups. The differences between the tumor uptake and tumor-to-muscle ratio of 64Cu-GO-VEGF121 and 64Cu-GO were statistically significant (P < 0.05) at all time points. All data represent 4 mice per group.
Systematic PET imaging and quantification analysis showed that U87MG tumor uptake of 64Cu-GO-VEGF121 (i.e. targeted group) could be clearly visible at 0.5 h p.i. (6.5 ± 1.7 percentage injected dose per gram of tissue [%ID/g]) and peaked at 3 h p.i. with tumor accumulation found to be 8.2 ± 1.4 %ID/g (Figure 3A, 4A, Table S1). While, without the conjugation of VEGF121, the accumulation of 64Cu-GO was found ∼2 fold lower at all time points examined (Figure 3B, 4B,C, Table S2), clearly indicating that conjugation of VEGF121 to GO could increase tumor uptake through active targeting of VEGFR on the tumor vasculature. Beside higher tumor accumulation, tumor-to-muscle (T/M) ratio was improved as well. The highest T/M ratio in targeted group was found to be 8.4 ± 2.1, which is >2 fold higher than the non-targeted group (Figure 4D, Table S3).
Similar as what we observed in other GO nanoconjugates [26, 31, 36], besides tumor uptake, most of 64Cu-GO-VEGF121 was found in liver with the highest uptake estimated to be 24.9 ± 3.0 %ID/g at 0.5 h p.i. and gradually decreased to 10.1 ± 0.5 %ID/g at 48 h p.i. (n=4, Figure 3A, 4A, Table S1). Similar high liver uptake was also observed in the non-targeted group (n=4, Figure 3B, 4B, Table S2).
To further confirm the accuracy of PET quantification analysis, ex vivo biodistribution studies were carried out at 3 h p.i. (when tumor uptake peaked based on PET imaging in Figure 3A) and 48 h p.i. (after the last PET scan). As shown in Figure 5, the quantitative results based on PET and biodistribution studies matched very well, confirming that serial non-invasive PET imaging accurately reflected the distribution of 64Cu-GO-VEGF121 and 64Cu-GO in U87MG tumor-bearing mice.
Figure 5.
Biodistribution studies in U87MG tumor-bearing mice at (A) 3 h and (B) 48 h post-injection of the GO nanoconjugates. All data represent 4 mice per group.
3.4. Histology
To further confirm that tumor uptake of 64Cu-GO-VEGF121 is VEGFR specific and GO nanoconjugates were indeed delivered to the tumor, histological studies were performed. U87MG tumor-bearing mice were intravenously injected with GO-VEGF121 and GO (dose: 5 mg/kg) and euthanized at 3 h p.i. (when U87MG tumor uptake was at the peak based on PET imaging). Organs including U87MG tumor, liver, spleen and muscle were collected, frozen and cryo-sectioned for histological analysis. Well-established protocols were later used for the staining of CD31 and VEGF121 [33, 34].
As shown in Figure 6A, GO-VEGF121 distribution in the U87MG tumor was found primary on the vasculature with little extravasation, as evidenced by the excellent overlay of the CD31 (red) and GO-VEGF121 (green), while no obvious green signal could be found in U87MG tumor from the non-targeted group (Figure 6B). Strong green fluorescence signal from the liver and spleen slices were observed outside the vasculature, indicating non-specific RES uptake and hepatobiliary clearance of GO-VEGF121 (Figure 6C,D). In addition, little green fluorescence was observed in the muscle, which is consistent with the results of PET and biodistribution studies (Figure 6E). Taken together, our histology study clearly demonstrated the VEGFR targeting specificity of GO-VEGF121.
Figure 6.

Histology study. Immunofluorescence staining of various tissue slices of (A) U87MG tumor (targeted group), (B) U87MG tumor (non-targeted group), (C) Liver, (D) Spleen and (E) Muscle. Red staining represents CD31 (using anti-mouse CD31 primary antibody), while green staining represents GO-VEGF121 (using Avastin as the primary antibody). Scale bar: 100 μm. Note, Slices of liver, spleen and muscle were all from targeted group.
4. Conclusion
In conclusion, we reported the surface engineering and in vivo tumor vasculature targeting of GO nanoconjugates in U87MG tumor-bearing mice, with 64Cu as the radiolabel and VEGF121 as the targeting ligand. Excellent stability and high targeting specificity of GO-VEGF121 were achieved based on systematic in vivo/in vitro/ex vivo studies. More importantly, our newly developed 64Cu-GO-VEGF121 nanoconjugate was able to target vascular VEGFR efficiently in U87MG model with the highest tumor uptake found to be >8 %ID/g, giving an extra boost to tumor uptake based on passive targeting alone (∼4 %ID/g). We believe that GO-VEGF121 with significantly improved tumor targeting efficiency could inspire future design of smart GO-based nanosystems and show great potential for enhanced theranostics in living systems.
Supplementary Material
Acknowledgments
This work is supported, in part, by the University of Wisconsin-Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365 and P30CA014520), the Department of Defense (W81XWH-11-1-0644), the American Cancer Society (125246-RSG-13-099-01-CCE), the National Basic Research Program of China (973 Program, 2012CB932601 & 2011CB911002), and the National Science Foundation of China (51002100 & 51222203).
References
- 1.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–9. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 2.Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6:183–91. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 3.Huang X, Yin Z, Wu S, Qi X, He Q, Zhang Q, et al. Graphene-based materials: synthesis, characterization, properties, and applications. Small. 2011;7:1876–902. doi: 10.1002/smll.201002009. [DOI] [PubMed] [Google Scholar]
- 4.Pumera M. Graphene-based nanomaterials and their electrochemistry. Chem Soc Rev. 2010;39:4146–57. doi: 10.1039/c002690p. [DOI] [PubMed] [Google Scholar]
- 5.Service RF. Materials science. Carbon sheets an atom thick give rise to graphene dreams. Science. 2009;324:875–7. doi: 10.1126/science.324_875. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Wang X, Zhang L, Lee S, Dai H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science. 2008;319:1229–32. doi: 10.1126/science.1150878. [DOI] [PubMed] [Google Scholar]
- 7.Feng L, Zhang S, Liu Z. Graphene based gene transfection. Nanoscale. 2011;3:1252–7. doi: 10.1039/c0nr00680g. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of waterinsoluble cancer drugs. J Am Chem Soc. 2008;130:10876–7. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang LA, Wang J, Loh KP. Graphene-based SELDI probe with ultrahigh extraction and sensitivity for DNA oligomer. J Am Chem Soc. 2010;132:10976–7. doi: 10.1021/ja104017y. [DOI] [PubMed] [Google Scholar]
- 10.Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, et al. A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J Am Chem Soc. 2010;132:15351–8. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Lu Z, Zhao Q, Huang J, Shen H, Zhang Z. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small. 2011;7:460–4. doi: 10.1002/smll.201001522. [DOI] [PubMed] [Google Scholar]
- 12.He S, Song B, Li D, Zhu C, Qi W, Wen Y, et al. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv Funct Mater. 2010;20:453–9. [Google Scholar]
- 13.Yang K, Wan J, Zhang S, Tian B, Zhang Y, Liu Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials. 2012;33:2206–14. doi: 10.1016/j.biomaterials.2011.11.064. [DOI] [PubMed] [Google Scholar]
- 14.Huang P, Xu C, Lin J, Wang C, Wang X, Zhang C, et al. Folic Acid-conjugated Graphene Oxide loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics. 2011;1:240–50. doi: 10.7150/thno/v01p0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L, Wang W, Tang J, Zhou JH, Jiang HJ, Shen J. Graphene oxide noncovalent photosensitizer and its anticancer activity in vitro. Chemistry. 2011;17:12084–91. doi: 10.1002/chem.201003078. [DOI] [PubMed] [Google Scholar]
- 16.Yang K, Hu LL, Ma XX, Ye SQ, Cheng L, Shi XZ, et al. Multimodal Imaging Guided Photothermal Therapy using Functionalized Graphene Nanosheets Anchored with Magnetic Nanoparticles. Adv Mater. 2012;24:1868–72. doi: 10.1002/adma.201104964. [DOI] [PubMed] [Google Scholar]
- 17.Miao W, Shim G, Lee S, Choe YS, Oh YK. Safety and tumor tissue accumulation of pegylated graphene oxide nanosheets for co-delivery of anticancer drug and photosensitizer. Biomaterials. 2013;34:3402–10. doi: 10.1016/j.biomaterials.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J. Tumor angiogenesis: therapeutic implications. New Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 19.Chen F, Cai W. Tumor vasculature targeting: a generally applicable approach for functionalized nanomaterials. Small. 2014;10:1887–93. doi: 10.1002/smll.201303627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005:209–31. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Zhang Y, Cai W. Molecular MRI of VEGFR-2 reveals intra-tumor and inter-tumor heterogeneity. Am J Nucl Med Mol Imaging. 2013;3:312–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Sun ZC, Huang P, Tong G, Lin J, Jin A, Rong PF, et al. VEGF-loaded graphene oxide as theranostics for multi-modality imaging-monitored targeting therapeutic angiogenesis of ischemic muscle. Nanoscale. 2013;5:6857–66. doi: 10.1039/c3nr01573d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Mousawi M, Tchistiakova L, Yurchenko L, Pietrzynski G, Moreno M, Stanimirovic D, et al. A vascular endothelial growth factor high affinity receptor 1-specific peptide with antiangiogenic activity identified using a phage display peptide library. J Biol Chem. 2003;278:46681–91. doi: 10.1074/jbc.M308681200. [DOI] [PubMed] [Google Scholar]
- 24.Chen K, Li ZB, Wang H, Cai W, Chen X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur J Nucl Med Mol Imaging. 2008;35:2235–44. doi: 10.1007/s00259-008-0860-8. [DOI] [PubMed] [Google Scholar]
- 25.Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318–23. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 26.Hong H, Yang K, Zhang Y, Engle JW, Feng L, Yang Y, et al. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano. 2012;6:2361–70. doi: 10.1021/nn204625e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang K, Feng L, Hong H, Cai W, Liu Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat Protoc. 2013;8:2392–403. doi: 10.1038/nprot.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong H, Zhang Y, Severin GW, Yang Y, Engle JW, Niu G, et al. Multimodality Imaging of Breast Cancer Experimental Lung Metastasis with Bioluminescence and a Monoclonal Antibody Dual-Labeled with (89)Zr and IRDye 800CW. Mol Pharm. 2012;9:2339–49. doi: 10.1021/mp300277f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imoukhuede PI, Popel AS. Expression of VEGF receptors on endothelial cells in mouse skeletal muscle. PLoS One. 2012;7:e44791. doi: 10.1371/journal.pone.0044791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willmann JK, Lutz AM, Paulmurugan R, Patel MR, Chu P, Rosenberg J, et al. Dualtargeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology. 2008;248:936–44. doi: 10.1148/radiol.2483072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi S, Yang K, Hong H, Valdovinos HF, Nayak TR, Zhang Y, et al. Tumor vasculature targeting and imaging in living mice with reduced graphene oxide. Biomaterials. 2013;34:3002–9. doi: 10.1016/j.biomaterials.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orbay H, Zhang Y, Valdovinos HF, Song G, Hernandez R, Theuer CP, et al. Positron emission tomography imaging of CD105 expression in a rat myocardial infarction model with (64)Cu-NOTA-TRC105. Am J Nucl Med Mol Imaging. 2013;4:1–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Hong H, Yang Y, Zhang Y, Engle JW, Barnhart TE, Nickles RJ, et al. Positron emission tomography imaging of CD105 expression during tumor angiogenesis. Eur J Nucl Med Mol Imaging. 2011;38:1335–43. doi: 10.1007/s00259-011-1765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31:1219–30. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 35.Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med. 2006;47:2048–56. [PubMed] [Google Scholar]
- 36.Hong H, Zhang Y, Engle JW, Nayak TR, Theuer CP, Nickles RJ, et al. In vivo targeting and positron emission tomography imaging of tumor vasculature with (66)Ga-labeled nano-graphene. Biomaterials. 2012;33:4147–56. doi: 10.1016/j.biomaterials.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.