Abstract
Conventional systemic therapy for disseminated breast cancer is based on the general assumption that the greatest patient benefit is achieved by killing the maximum number of tumor cells. While this strategy often achieves a significant reduction in tumor burden, most patients with metastatic breast cancer ultimately die from their disease as therapy fails because tumor cells evolve resistance. We propose that the conventional maximum dose/maximum cell kill cancer therapy, when viewed from an evolutionary vantage, is suboptimal and likely even harmful as it accelerates evolution and growth of the resistant phenotypes that ultimately cause patient death. As an alternative, we are investigating evolutionary therapeutic strategies that shift the treatment goal from killing the maximum number of cancer cells to maximizing patient survival. Here we introduce two novel approaches for systemic therapy for metastatic breast cancer, considering the evolutionary nature of tumor progression; adaptive therapy and double-bind therapy.
Keywords: adaptive therapy, breast cancer, chemotherapy, double-bind therapy, drug resistance, estrogen, hormonal therapy, tumor evolution
Background
Treatments for metastatic breast cancer, as with most common epithelial tumors, are often effective initially but ultimately fail due to emergence of resistance. Even highly targeted therapies, such as trastuzumab [1] for patients with tumors having increased HER2/neu expression, typically produce only temporary responses. The sequence of response followed by adaptation and progression indicates emergence of resistant phenotypes is governed by Darwinian dynamics in response to the strong selection pressure imposed by anticancer drugs. Thus, evolution of resistance to treatment eventually leads to death of virtually all metastatic breast cancer patients; this will likely remain so even with targeted, personalized therapy.
Generally, the choice of systemic therapy for metastatic breast cancer is dependent on two key molecular factors; estrogen receptor (ER) and HER2 [2]. Briefly, ER-positive (ER+) and HER2-negative (HER2−) cancers have better prognosis than other breast cancer types, and typically respond well to anti-estrogen therapy using selective estrogen receptor modulators (SERMs) or aromatase inhibitors (AIs) [3,4]. By contrast, cancer cells that express a significant level of HER2 (HER2+) or do not express ER (ER−) have a poorer prognosis. The latter does not respond to anti-estrogen therapy. HER2+ tumors usually respond to targeted therapy drugs such as trastuzumab [1] or lapatinib [5], but resistance typically emerges within several months. Triple-negative breast cancers (ER−/PR−/HER2−) are heterogeneous tumors, poorly understood in terms of developing targeted therapies, and therefore chemotherapy with cytotoxic drugs remains the only treatment option.
Unfortunately, despite the large number of available treatments, metastatic breast cancer remains an almost uniformly fatal disease. Regardless of molecular phenotype and choice of therapy, therapeutic resistance almost inevitably emerges and the disease progresses. Although evolution is the direct cause of treatment failure and death in nearly all patients with metastatic breast cancer, most therapeutic strategies make no effort to understand, control, or exploit the underlying Darwinian dynamics. In a recent study, Aktipis et al. found that less than 1% of cancer treatment articles mention evolution and this has not changed over three decades [6].
Here we examine potential therapeutic strategies that use evolutionary principles to delay emergence of resistance and prolong response to therapy. A fundamental assumption of this approach is that, while evolution of resistance due to selection pressures from therapy is uncontrollable and inevitable, proliferation of resistant populations is not. We note that evolving populations can only adapt to local and current environmental selection forces. They can never anticipate selection forces in the future or at a distant site. Importantly, we can, and thus do, use our understanding of Darwinian dynamics to prevent or delay growth of therapy-resistant phenotypes.
Our core hypothesis is that survival of patients with breast cancer can be significantly improved using current drugs more effectively. However, application of therapy using evolutionary principles requires reconsideration of a long-held and intuitively appealing assumption – that a patient gains the most benefit when a therapy kills the maximum possible number of tumor cells. We propose that this conventional approach, when viewed from an evolutionary vantage, is suboptimal and probably even harmful because it accelerates evolution and growth of resistant phenotypes that lead to patient death. From an evolutionary vantage, there are two major flaws with the maximum cell killing strategy: maximum tumor cell death imposes the strongest possible selection pressure for resistance; and by removing all competing populations, it permits unopposed proliferation of resistant cells, a phenomenon known in ecology as ‘competitive release’. Killing of drug-sensitive cells does not promote proliferation of resistant cells per se. However, it can release the resistant phenotype from suppression by the drug-sensitive phenotype when they grow in a resource-limited environment. Owing to technical limitations, these dynamics are not readily monitored in an in vivo setting. However, in vitro data, including ones obtained by our own group, support the idea [7].
As an alternative, we suggest a new paradigm that uses evolutionary principles with the explicit goal of maintaining stable tumor volumes by preventing outgrowth of resistant populations. Our goal shifts from killing the maximum number of tumor cells possible to the minimum necessary, to limit symptoms but also limit evolutionary pressures that result in growth of uncontrollable resistant cells. Here we describe two evolution-based strategies: adaptive therapy and double-bind therapy. The current models are focusing on primary tumor control, and therapy design should be distinctive from adjuvant therapy, for which further studies are required.
Intratumoral heterogeneity & evolution of drug resistance
The importance of intratumoral heterogeneity in determining prognosis is increasingly recognized [8,9]. Most breast cancers are heterogeneous in a wide range of spatial scales from molecular characteristics in cells to tissue-level regions of necrosis and calcification. For example, while breast cancers are typically divided into ER+ and ER−, the former is often a mix of ER+ and ERcells [10]. Even when 1% of cells are ER+ pathologically, the tumor is usually classified as ER+ [4]. Similarly, variations in blood will result in regional hypoxia, acidosis and low concentrations of serum growth factors that select for phenotypes and genotypes different from well-vascularized regions.
A consequence of intratumoral heterogeneity is that resistant phenotypes are almost invariably present within the tumor prior to treatment. These include, as noted above, ER− cells in a tumor that is otherwise ER+ [4,10]. In addition, cells with upregulated xenobiotic pathways (probably due to regional hypoxia) may constitute up to 10% of cancer cells in untreated breast cancers [11]. While de novo resistance may occur, most breast cancers respond at least to first- and second-line therapies, with reduction in tumor volume indicating treatment-induced death in a large number of tumor cells. While tumor regression is a positive outcome, it also has negative significance as the high level of cytotoxicity acts as a very strong selection pressure that promotes the pre-existing resistant phenotypes. Furthermore, by eliminating all of the therapy-sensitive cells, the resistant populations effectively have no intratumoral competition and can proliferate unopposed. In other words, because heterogeneity results in small populations of resistant cell prior to treatment, the tumor response to conventional therapy actually promotes and even accelerates its eventual failure [9,12–14].
The evolutionary dynamics of resistance & its suppression
It is often assumed that evolution of resistance is the result of some mutation that is induced by therapy. However, in considering the Darwinian dynamics that lead to emergence of resistant populations we emphasize three critical points: many molecular mechanisms of resistance do not require a discrete, all-or-none change such as a mutation – often they are due to a graded upregulation of molecular pathways already present in the genome that, for example, extrude cytoplasmic toxins, repair DNA damage, or use a signaling pathway that does not require estrogen; since resistant phenotypes are already present prior to the initiation of therapy, evolution of resistance is uncontrollable and virtually certain; and competition among intratumoral phenotypes imposes selection pressures so that proliferation of resistant phenotypes is not necessarily inevitable.
We have proposed that therapeutic strategies in cancer therapy need to be reconsidered based on the following principles:
Accept the reality that with current drugs, cure of metastatic breast cancer is virtually impossible. The goals of therapy are maximization of survival time and quality of life;
Reconsider the conventional approach that more is better. Achieving the greatest possible reduction in tumor burden is not necessarily the best approach if it also maximally promotes growth of resistant populations. The goal of breast cancer therapy is maximizing time to progression;
Use evolutionary principles. It makes little sense to eliminate all of the cells that can be controlled by therapy leaving only cells that cannot be controlled. Instead, strategies should seek to use the sensitive cells to inhibit proliferation of resistant populations.
These conceptual principles are generally applicable for any cancer developing resistance. However, in particular metastatic breast cancer has many potential systemic therapies that initially produce a response but then fail due to evolution of resistance. The fundamental goal of evolution-based therapy is to prolong the time to progression for each treatment and is, thus, most useful in cancers with several available systemic therapies.
Adaptive therapy: a novel evolutionary approach of systemic cancer therapy
How can the evolutionary dynamics be incorporated into a therapy design? A number of approaches to overcome resistance have been investigated [6]. The most common strategy utilizes a secondary drug to block the drug-resistant machinery or to target the mutation responsible for the resistant mechanism. For example, verapamil, a substrate for the drug extrusion pump that also confers resistance in multiple myeloma patients, can increase sensitivity when administered with the chemotherapy by competitive inhibition of the pump. However, this approach has met with limited clinical success [15].
We have proposed a treatment strategy that represents a trade-off between tumor control and drug-sensitivity management. This approach, termed ‘adaptive therapy’ [7,16], uses lower doses of drugs to maintain a population of sensitive cells within a tumor so that they can suppress the proliferation of resistant populations. The goal is to achieve not the maximum possible cell killing but the minimum necessary to maintain symptomatic control.
This adaptive therapy strategy relies on the fitness difference between drug-sensitive and drug-resistant phenotypes. This fitness difference is based on the ‘cost’ of resistance. That is, any mechanism that confers resistance also requires substrate to synthesize and maintain the necessary macromolecules and to carry out whatever function is used to resist the effects of chemotherapy. For example, many breast cancer cells become resistant by expressing PGP on their membrane [17]. PGP is an ATP-dependent membrane pump that extrudes many substrates from the cell cytoplasm. It is a critical factor in multidrug resistance (MDR) in breast cancer and other clinical malignancies [18]. PGP clearly increases the cells’ fitness in the presence of chemotherapy. However, in the absence of therapy, the cell must devote resources to synthesize and transport PGP. In an environment where resources are limited, the cost for resistance must be diverted from other functions such as proliferation, motility and invasion, which, thus, reduce cell fitness.
By contrast to standard maximum dose-density strategies, adaptive therapy involves adjusting the dose dynamically or periodically withdrawing the treatment from responding tumor growth, allowing the sensitive cells to proliferate and, because of their fitness advantage, reduce the proliferation of the resistant cells. By doing so, the tumor cell population can retain sufficient drug-responding cell populations to prevent competitive release of the resistant cells. Although this approach is not curative (neither is conventional high-dose therapy), it has the potential to allow long-term tumor control.
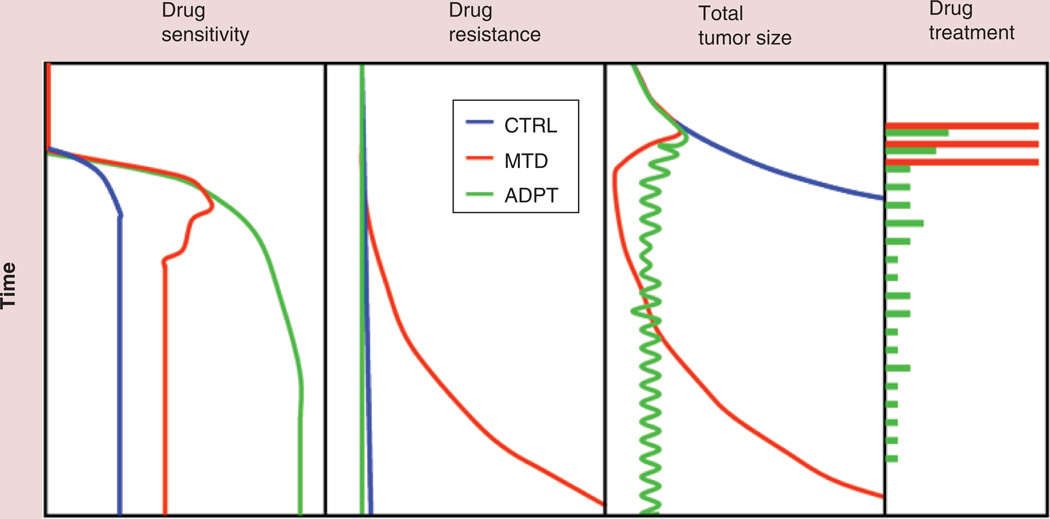
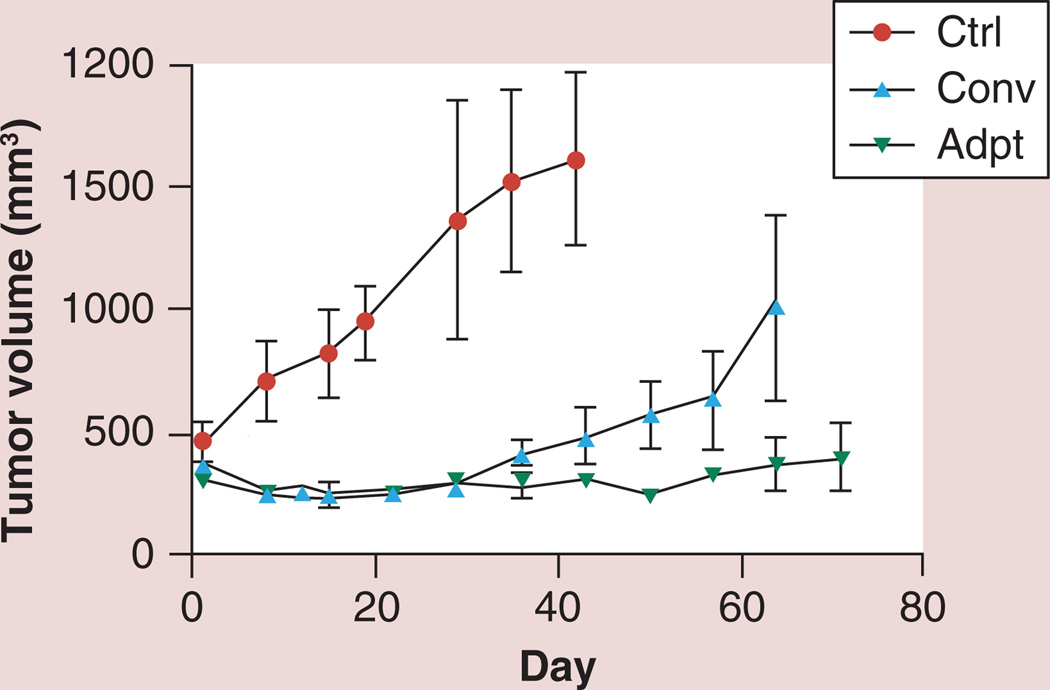
Initially adaptive therapy was designed by mathematical modeling and tested by computational simulation. Figure 1 shows the schematic illustration for adaptive therapy; the low drug doses dynamically adjusted according to tumor size and growth successfully maintain an appropriate size of the drug-sensitive population, effectively suppressing the emergence of the drug-resistant population. In agreement with the theoretical model, in vivo animal experiments using an ovarian cancer cell line (OVCAR) [16], as well as a breast cancer cell line (MDA-MB-231; Figure 2) have demonstrated that adaptive approaches can maintain stable tumors almost indefinitely. Interestingly, the in vivo experiments have consistently demonstrated that over time tumor control is maintained using increasingly small doses of chemotherapeutic drugs [16]. The mechanism for this remains unclear.
Figure 1. Schematic model of adaptive therapy.
The tumor sensitivity persists in ADPT compared with the CTRL without any treatment, and tumor treated at MTD as a high-dose density therapy. Doses in ADPT are adjusted based on tumor response dynamically as shown in the first two panels.
ADPT: Adaptive therapy; CTRL: Control; MTD: Maximum-tolerated dose.
Figure 2. Adaptive therapy outcome from mouse xenograft model.
MDA-MB-231 cells were orthotopically implanted in Nu/Nu mice and the paclitaxel treatment outcome was measured. When the tumor volume reached around 300 mm3 by caliper, mice were divided into three groups; Ctrl, Conv, and Adpt. Ctrl mice were treated with vehicle control by intraperitoneal injections. Conv mice were treated with paclitaxel (20 mg/kg) twice a week for 2.5 weeks. Adpt mice were treated with 15 mg/kg paclitaxel initially and the subsequent treatment doses were adjusted based on the tumor size measured twice a week. The algorithm was adapted from the adaptive therapy strategy previously reported [7]: drug dose was increased by 20% of the previous applied dose if the tumor volume was increased by 10% or more from the previous volume. However, we reduced the drug dose by 20% of the previous dose if tumor size decreased by 10% with respect to previous size. Adaptive therapy could stabilize tumor volume even lower than the initial volume before treatment except in one mouse, which demonstrated continuous tumor growth even with the prolonged treatment. However, even the one exception was still slower than the standard therapy group average. Further investigation seeking the factors affecting the responsiveness of tumors to adaptive therapy is ongoing.
Adpt: Adaptive therapy group; Ctrl: Contol; Conv: Conventional therapy group.
A specific example of adaptive chemotherapy for MDR of breast cancer
As noted above, MDR is a relatively well-described cancer cell strategy to resist the toxic effects of many chemotherapeutic agents [18]. Considering the ‘cost’ of resistance, the increased metabolic energy demand of MDR can be a critical factor that makes this phenotype have lower fitness in the absence of drug. As an ATP-dependent exporting pump, PGP requires two ATPs to export one substrate molecule [17]. The energy demand by the resistant phenotype is apparent in a number of experimental studies in which breast cancer cells with an MDR phenotype showed increased ATP consumption rate [19], glycolysis [7,20] and glucose uptake rate [7,21].
Furthermore, the energy demand increases further when pump substrate it is added to the media [19]. In the presence of PGP substrate, therefore, ATP energy consumption continues in the MDR phenotype regardless of the survival benefit. Importantly, this continued drain of energy requires diversion of substrate from other functions including proliferation and invasion. Thus, when compared with sensitive cells that lack the resistance machinery, drug-resistant cells are less fit in the absence of therapy. When this factor was applied to a computational model for clinical treatment, the time-to-progression for breast cancers treated with chemotherapy could be increased by two- to ten-fold in the presence of a PGP substrate under glucose-limited conditions [7]. This implies that administration of a PGP substrate in the absence of chemotherapy (rather than combined with it as has been previously attempted) could achieve a substantial increase in progression-free survival.
We tested these predictions in both in vitro and in vivo studies. In vitro, we found that the MDR phenotype must expend more energy and decreases proliferation in the presence of PGP substrate and ‘physiological’ glucose concentration [7]. Interestingly, we found that a MDR breast cancer cell line did not demonstrate a growth disadvantage in culture conditions with high concentration of glucose. However, in the presence of physiological or subphysiological (as would be found in many tumors) glucose concentrations, the MDR phenotype proliferated at a significantly lower rate than the wild-type drug-sensitive cells. Thus, the fitness difference between a drug-sensitive phenotype and an MDR phenotype is tightly linked to both the energy cost of the resistance mechanism and the energy resource that are available.
Double-bind therapy & adaptive strategies in anti-estrogen therapy
A second evolution-based approach that we have proposed is ‘double bind therapy’ [22]. This requires a strategic coupling of two or more therapies over time. The first therapy is cytotoxic and so reduces the size of the sensitive cancer cell population. However, the therapy is also administered with the intent of selecting for a specific adaptive strategy. The initial treatment is then followed by a second therapy that is specifically targeted to the evolved. In some ways, this represents the mutual arms race often seen in predator–prey adaptive landscapes.
A clinical example of this approach has been published by Antonia et al. [23]. They treated a cohort of 29 patients with small-cell lung cancer with an antip53 vaccine. This resulted in only one partial response. However, when the cohort was subsequently treated with chemotherapy, the response rate was 67% (compared with historic controls with <5% response). Here the double bind is that the tumor response to the vaccine rendered it far more vulnerable to chemotherapy.
In breast cancer therapy, the potential use of double bind can be suggested particularly for hormonal therapy. The majority of breast cancers contain cells that are dependent on estrogen binding to the ER for their growth [24,25]. Drugs antagonizing ER activation or blocking estrogen production are widely used in clinics to suppress and kill estrogen-dependent breast cancer cells. SERMs (i.e., tamoxifen and fulvestrant) and AIs (i.e., anastrozole, letrozole and exemestane) are agents commonly used in clinic [3,4]. Although responses to anti-estrogen therapies can often be maintained for years, the vast majority of tumors evolve populations that are estrogen-independent and, therefore, resistant to hormonal therapy.
In general, there are clearly many opportunities to apply evolutionary principles to targeted therapies in breast cancer. However, unlike chemotherapy strategies, critical information regarding the cost of resistance and transition from one intracellular pathway to another has not yet been measured. By contrast, upon a hormonal therapy, blocking ER-dependent signaling makes a breast cancer population exploit an alternative growth factor signal path(s) [26–28] and these alternative signal dependencies can be a target of double-bind therapy. In addition, the ER+ phenotype is commonly known to have better prognosis along with hormonal therapy susceptibility, and maintaining a tumor as estrogen-dependent is highly beneficial for patients and for prolonged survival.
The evolution of anti-estrogen therapy resistance has been studied in vitro and in vivo for several decades and several potential adaptive strategies have been identified [29]. For example, the resistance to long-term estrogen deprivation (LTED) has been studied as a counter part of AI or SERM treatment [30]. Some LTED-resistant cells increase ER expression and become hypersensitized allowing them to respond to even trace amounts of estrogen. In other cells, resistance relies on cellular reprogramming, including EGF receptor- and IGF-I-related signaling [26–28]. HER2 overexpression is a clear mechanism through which breast cancer can use other proliferative signaling pathways to adapt to estrogen-limited or SERM existing condition. Abnormally elevated HER2-expression enables breast cancer cells to function in an estrogen-independent fashion.
From these data, a number of potential evolution-based strategies can be proposed, including double-bind therapy. For LTED resistance, it is known to be mediated by hyperexpression of ER, and normal concentration of estrogen becomes toxic to the phenotype [30,31]. Interestingly, there have been several reports of using the estrogen effect to reverse hormone therapy resistance not only to an AI but also to a SERM [32,33]. In addition, estrogen treatment can reverse resistance to SERMs or estrogen-deprivation by inducing apoptosis of the resistant cells [34,35]. The multiple-step evolution to adaptation of breast cancer cells toward the full SERM-resistance and estrogen-independent growth has been suggested [36].
For the HER2+ tumor, trastuzumab can be used to suppress the HER2+ population. However, the therapy is currently applied according to the conventional therapy design only for patients diagnosed as HER2+. However, alternating adaptive therapy design may retain the tumor population longer between ER+/HER− and ER+/HER2+, and may prevent emergence of other phenotypes such as triple-negative breast cancer cells while the tumor burden is stably managed.
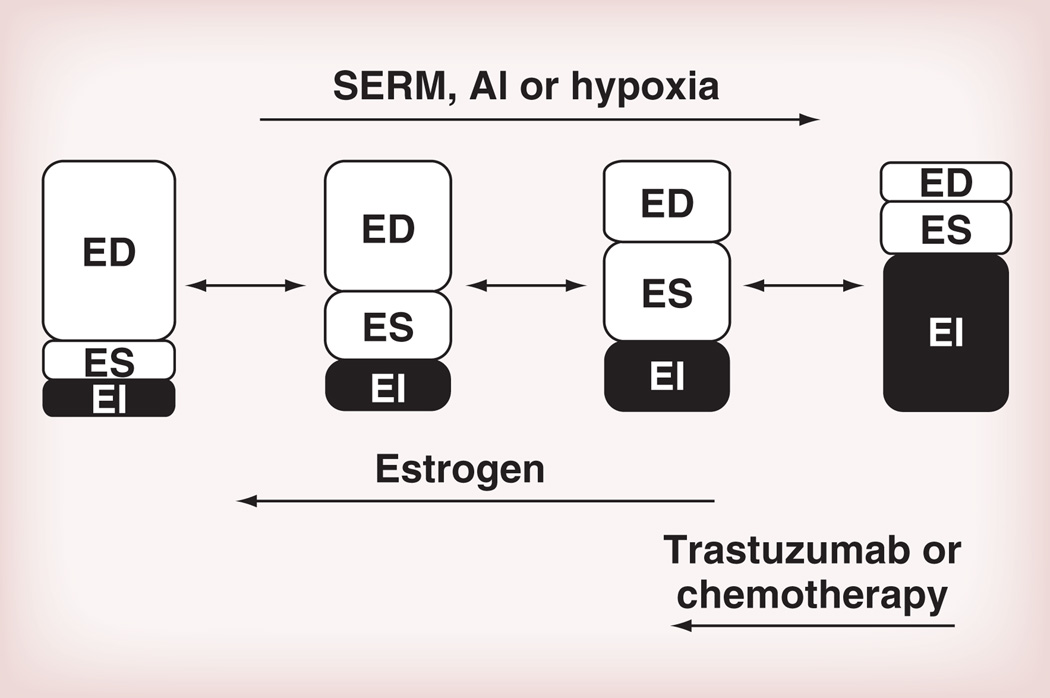
Alternatively, chemotherapy can also be considered as one arm of a double-bind therapy since there has been accumulated evidence that triple-negative tumors tend to be more susceptible to chemotherapy. Again, the conventional therapy design is based on combinational therapy, but the mathematical model has suggested the benefit of double bind over the conventional approach (Figure 3) [22].
Figure 3. Breast cancer evolution model from estrogen-dependent to estrogen-independent, and the potential modulators.
Anti-estrogen therapy and microenvironment change such as hypoxia enforce population evolution toward an EI-dominated population whereas estrogen or EI-targeted therapies may lower the fitness of EI and ES cells.
AI: Aromatase inhibitor; ED: Estrogen dependent; EI: Estrogen independent; ES: Estrogen susceptible; SERM: Selective estrogen receptor modulator.
Conclusion & future perspective
Darwinian dynamics are governed by the fitness differences among heterogeneous, heritable phenotypes competing within specific microenvironmental adaptive landscapes. With the onset of cancer therapy, the administered drugs become strong selection forces that immediately begin promoting adaptive advantages among the tumor cells. It is clear that human cancers are extremely heterogeneous at molecular and tissue scales. As a result, cellular phenotypes that are resistant to therapy are usually present within the tumor prior to treatment.
While tumor evolution begins the instant the first dose of therapy is administered, cancer therapy is typically applied statically so that the same regimen is used repeatedly until the tumor progresses. Furthermore, conventional high-dose therapeutic strategies and metronomic therapies, by killing the maximum possible number of sensitive tumor cells, apply the greatest evolutionary pressure for competitive release of the extant resistant populations. We propose therapeutic strategies that are as adaptive as the tumor being treated can exploit evolutionary dynamics and, in some cases, can achieve prolonged progression-free survival using currently available drugs. However, application of this approach requires reconsideration of conventional treatment strategies designed to kill the maximum number of tumor cells either through fixed, high-dose density chemotherapy or fixed low-dose (metronomic) treatments. Instead, therapy must evolve and adapt more quickly than the tumor with the primary goals of both reducing the tumor burden and suppressing proliferation of resistant populations that will lead to progression.
Although the advantage of adaptive therapy is obvious for the therapy outcome and for the patient’s quality of living, there are still practical concerns and limitations for the clinical study and application remaining. First, most of the direct evidence of nonresistance cell-mediated suppression of the resistance clone relies on in vitro and in silico findings, which make this model limited to primary tumor treatment. The model should be further evaluated for the later stage of tumors such as a metastatic or adjuvant therapy setting. Second, more detailed in silico studies are required as well as in vitro and in vivo validations to ensure that the clinical trial is more feasible, by minimizing the risk factor of residual tumor in patients. Incorporation of clinical data from maintenance therapy for advanced cancer could be one of the ways to improve our understanding of how adaptive therapy works.
Here we present in detail one evolution strategy – termed adaptive therapy – that continuously adjusts drugs and drug doses to maintain a stable population of treatment-sensitive cancer cells and uses them to suppress cell populations that are resistant and thus not controllable. We demonstrate that, in the absence of therapy, resistant cells are less fit due to the diversion of resources to the molecular mechanism(s) of resistance. This fitness difference permits the sensitive cells, spared by reduced treatment, to suppress proliferation of the resistant populations. While much further investigation is necessary, computational models suggest that this approach could substantially prolong survival in patients with metastatic breast cancer.
Supplementary Material
Executive summary.
Tumor evolution & drug resistance
Pre-existing therapy-resistance phenotypes are present in clinical cancers due to underlying heterogeneity. Their prevalence is usually low, indicating they are, in the absence of therapy, less fit than the more abundant treatment-sensitive phenotypes.
Conventional high-dose density therapy, by removing sensitive cancer cell populations, permits unopposed proliferation of resistant cells, a phenomenon known as competitive release in ecology.
Alternative evolution-based therapies use Darwinian principles to delay proliferation of resistant populations.
Adaptive therapy
Instead of eradicating the maximum number of cancer cells, adaptive therapy attempts to maintain a stable population of therapy-responding cells to avoid competitive release and suppress proliferation of the resistant phenotype.
In designing adaptive therapy, the metabolic cost of the resistance mechanisms and the availability of substrate within the tumor microenvironment can be key factors. Investigation of the multidrug-resistant phenotype demonstrates the reduction of fitness caused by diversion of resources to protein extrusion pumps.
Double-bind therapy
Double-bind therapy is a strategic coupling of two or more therapies over time.
The initial therapy is designed to induce cell death and promote a specific adaptive strategy in the resistant populations. The second therapy then specifically targets the mechanism of adaptation.
Acknowledgments
This work was funded by the James S. McDonnell Foundation 21st Century Science Initiative Grant “Cancer Therapy: Perturbing a Complex Adaptive System” as well as NIH/NCI 1U54CA143970–01 and NIH/NCI RO1CA170595.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Hudis CA. Trastuzumab – mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 2.Simpson JF, Gray R, Dressler LG, et al. Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern Cooperative Oncology Group Companion Study, EST 4189. J. Clin. Oncol. 2000;18(10):2059–2069. doi: 10.1200/JCO.2000.18.10.2059. [DOI] [PubMed] [Google Scholar]
- 3. Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69(4):1243–1254. doi: 10.1158/0008-5472.CAN-09-0029. • Informative review for breast cancer therapy development and progress.
- 4.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch. Pathol. Lab. Med. 2010;134(6):907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 6. Aktipis CA, Kwan VS, Johnson KA, Neuberg SL, Maley CC. Overlooking evolution: a systematic analysis of cancer relapse and therapeutic resistance research. PLoS ONE. 2011;6(11):e26100. doi: 10.1371/journal.pone.0026100. • Overview of how the evolutionary concept is used to explain and prevent cancer relapse and therapeutic resistance.
- 7. Silva AS, Kam Y, Khin ZP, Minton SE, Gillies RJ, Gatenby RA. Evolutionary approaches to prolong progression-free survival in breast cancer. Cancer Res. 2012;72(24):6362–6370. doi: 10.1158/0008-5472.CAN-12-2235. •• In vitro and in silico data supporting the adaptive therapy design.
- 8.Gerlinger M, Swanton C. How Darwinian models inform therapeutic failure initiated by clonal heterogeneity in cancer medicine. Br. J. Cancer. 2010;103(8):1139–1143. doi: 10.1038/sj.bjc.6605912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsiao YH, Chou MC, Fowler C, Mason JT, Man YG. Breast cancer heterogeneity: mechanisms, proofs, and implications. Cancer. 2010;1:6–13. doi: 10.7150/jca.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wishart GC, Plumb JA, Going JJ, et al. P-glycoprotein expression in primary breast cancer detected by immunocytochemistry with two monoclonal antibodies. Br. J. Cancer. 1990;62(5):758–761. doi: 10.1038/bjc.1990.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17(1):77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2(2):117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 14.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13(4):e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 15.Gaynor ER, Unger JM, Miller TP, et al. Infusional CHOP chemotherapy (CVAD) with or without chemosensitizers offers no advantage over CHOP therapy in the treatment of lymphoma: a Southwest Oncology Group Study. J. Clin. Oncol. 2001;19:750–755. doi: 10.1200/JCO.2001.19.3.750. [DOI] [PubMed] [Google Scholar]
- 16. Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res. 2009;69(11):4894–4903. doi: 10.1158/0008-5472.CAN-08-3658. •• Primary adpative therapy model described by in silico model and validated by in vivo animal experiment.
- 17.Wind NS, Holen I. Multidrug resistance in breast cancer: from in vitro models to clinical studies. Int. J. Breast Cancer. 2011;2011:967419. doi: 10.4061/2011/967419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat. Rev. Cancer. 2010;10(2):147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 19.Broxterman HJ, Pinedo HM, Kuiper CM, Kaptein LC, Schuurhuis GJ, Lankelma J. Induction by verapamil of a rapid increase in ATP consumption in multidrug-resistant tumor cells. FASEB J. 1988;2(7):2278–2282. doi: 10.1096/fasebj.2.7.3350243. [DOI] [PubMed] [Google Scholar]
- 20.Broxterman HJ, Pinedo HM, Kuiper CM, Schuurhuis GJ, Lankelma J. Glycolysis in P-glycoprotein-overexpressing human tumor cell lines. Effects of resistance-modifying agents. FEBS Lett. 1989;247(2):405–410. doi: 10.1016/0014-5793(89)81380-8. [DOI] [PubMed] [Google Scholar]
- 21.Millon SR, Ostrander JH, Brown JQ, Raheja A, Seewaldt VL, Ramanujam N. Uptake of 2-NBDG as a method to monitor therapy response in breast cancer cell lines. Breast Cancer Res. Treat. 2011;126(1):55–62. doi: 10.1007/s10549-010-0884-1. [DOI] [PubMed] [Google Scholar]
- 22. Cunningham JJ, Gatenby RA, Brown JS. Evolutionary dynamics in cancer therapy. Mol. Pharm. 2011;8(6):2094–2100. doi: 10.1021/mp2002279. •• A double-bind therapy model was proposed.
- 23.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin. Cancer Res. 2006;12(3 Pt 1):878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 24.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 25.Kuukasjarvi T, Kononen J, Helin H, Holli K, Isola J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J. Clin. Oncol. 1996;9:2584–2589. doi: 10.1200/JCO.1996.14.9.2584. [DOI] [PubMed] [Google Scholar]
- 26.Aguilar H, Sole X, Bonifaci N, et al. Biological reprogramming in acquired resistance to endocrine therapy of breast cancer. Oncogene. 2010;29(45):6071–6083. doi: 10.1038/onc.2010.333. [DOI] [PubMed] [Google Scholar]
- 27.Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptorpositive human breast cancer. J. Clin. Invest. 2010;120(7):2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox EM, Miller TW, Balko JM, et al. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 2011;71(21):6773–6784. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JM. Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocr. Relat. Cancer. 2004;11(4):623–641. doi: 10.1677/erc.1.00778. [DOI] [PubMed] [Google Scholar]
- 30.Chan CM, Martin LA, Johnston SR, Ali S, Dowsett M. Molecular changes associated with the acquisition of oestrogen hypersensitivity in MCF-7 breast cancer cells on long-term oestrogen deprivation. J. Steroid Biochem. Mol. Biol. 2002;81(4–5):333–341. doi: 10.1016/s0960-0760(02)00074-2. [DOI] [PubMed] [Google Scholar]
- 31.Santen RJ, Song RX, Zhang Z, et al. Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr. Relat. Cancer. 2005;12(Suppl. 1):S61–S73. doi: 10.1677/erc.1.01018. [DOI] [PubMed] [Google Scholar]
- 32.Mahtani RL, Stein A, Vogel CL. High-dose estrogen as salvage hormonal therapy for highly refractory metastatic breast cancer: a retrospective chart review. Clin. Ther. 2009;31:2371–2378. doi: 10.1016/j.clinthera.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Ingle JN. Estrogen as therapy for breast cancer. Breast Cancer Res. 2002;4(4):133–136. doi: 10.1186/bcr436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osipo C, Gajdos C, Cheng D, Jordan VC. Reversal of tamoxifen resistant breast cancer by low dose estrogen therapy. Steroid Biochem. Mol. Biol. 2005;93(2–5):249–256. doi: 10.1016/j.jsbmb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11(3):206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swaby RF, Jordan VC. Low-dose estrogen therapy to reverse acquired antihormonal resistance in the treatment of breast cancer. Clin. Breast Cancer. 2008;8(2):124–133. doi: 10.3816/CBC.2008.n.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.