Summary
Reduced susceptibility to sporadic colorectal cancer in native Africans (NA) is correlated with low consumption of animal products and greater microbial production of colonic methane. In this context, two hydrogenotrophic microbial groups are of interest, methanogenic Archaea (MA) utilizing H2 to produce methane and sulfate-reducing bacteria (SRB) generating hydrogen sulfide, which has been linked with chronic inflammatory disorders of the colon. In the present study, stool samples from NA, consuming a diet high in resistant starch and low in animal products, and from African Americans (AA) and European Americans (EA), both consuming a typical Western diet, were examined for genetic diversity and structure of Archaea, MA and SRB communities. In general, a greater proportion of NA than AA and EA harbored the full range of targeted hydrogenotrophic groups. Terminal restriction fragment length polymorphism analysis of 16S rRNA genes and specific functional genes, combined with multivariate statistical analyses, revealed that NA harbored more diverse and different Archaea and MA populations than AA and EA. Also, NA harbored significantly distinct SRB populations compared to AA and EA. Taken together, these data are consistent with diet selecting for distinct hydrogenotrophic microbiota.
Keywords: Hydrogenotrophs, methanogenic Archaea, sulfate-reducing bacteria, TRFLP
Introduction
In the human colon, the main products of microbial fermentation include short chain fatty acids (SCFA; acetate, propionate and butyrate) and gases (CO2 and H2). The H2 produced during this metabolic process is either excreted or used in situ by hydrogenotrophic microbes, including methanogenic Archaea (MA), sulfate-reducing bacteria (SRB), and reductive acetogens (Smith and Bryant, 1979; Strocchi et al., 1994; Bernalier et al., 1996). Hydrogenotrophic organisms are typically present at much lower densities than are fermentative bacteria (Eckburg et al., 2005). However, in the absence of H2-consuming organisms, the H2 partial pressure rapidly reaches a level that thermodynamically inhibits further fermentation (Schink, 1997; Adams et al., 2006). In humans, methane and hydrogen sulfide production have been recognized as the main pathways for H2 disposal in the colon, and a variety of intestinal factors such as colonic pH, sulfate availability, digesta transit time, and microbial composition may influence which of these pathways predominates (Gibson et al., 1990; Strocchi et al., 1991; Christl et al., 1992; El Oufir et al., 1996; Nakamura et al., 2010).
For example, it has long been recognized that NA excrete more methane in their breath than do individuals in westernized populations (O’Keefe et al., 1999). For example, Segal et al (1988) reported that 84% of rural black Africans excreted breath methane while only 52% of European subjects were methane excreters. Also, it was found that 70% of stool samples from European subjects exhibited sulfate reduction activity and the remaining 30% produced methane. The opposite trend was observed in stool samples from rural black South Africans, where only 15% of the samples contained measurable hydrogen sulfide and the remaining 85% generated methane (Gibson et al., 1988). A similar trend was observed in a study by Christl and coworkers, who reported that approximately 50% of healthy human adults from European and North American populations and 90% of rural black Africans were predominantly methane excreters (Christl et al., 1995). Correspondingly, native Africans (NA) are rarely diagnosed with colorectal cancer (CRC) (O’Keefe et al., 2007). In Western countries, however, sporadic CRC is the second leading cause of cancer death (Horner et al., 2009), and compared to other U.S. racial and ethnic groups, African Americans (AA) have the highest incidence and mortality from sporadic CRC (Horner et al., 2009; Altekruse et al., 2010).
The structure of colonic hydrogenotrophic microbiota may explain some of the differences in predisposition to sporadic CRC. Hydrogen sulfide, produced by SRB, has been linked in clinical studies with chronic inflammatory disorders of the colon (Roediger et al., 1997; Levine et al., 1998) and is also a potent genotoxin (Attene-Ramos et al., 2006). Evidence for resource competition between SRB and MA has been shown in natural environments (Purdy et al., 2003), and a similar competition has been suggested to occur in the colon (Pochart et al., 1992; Dore et al., 1995), although this hypothesis remains controversial (Leclerc et al., 1980). A Western diet is characterized by a higher intake of dietary proteins (meat, milk and eggs) that provide sulfate and sulfite to colonic bacteria. An increase in stool sulfide has been demonstrated in subjects fed a diet high in meat compared to a vegetarian diet (Magee et al., 2000). Thus, it can be hypothesized that differences in SRB and MA communities may be related to the low incidence of CRC in NA as a result of their apparent propensity for methanogenic rather than sulfidogenic H2 disposal. Little is known about the effects of diet or the host’s genetic background on the composition of the intestinal microbiota in general, and the structure of hydrogenotroph populations have been overlooked in recent microbiome studies.
The aim of the present study was to characterize the diversity of SRB and MA populations in stool samples from healthy NA, AA and EA and, thereby, examine possible correlations between hydrogenotrophic microbiota and diet.
Results and discussion
Amplification of Archaea, MA and SRB 16S rRNA and functional genes
The samples evaluated for hydrogenotrophic microbes in the present work were collected in a study that compared dietary, metabolic and physiological parameters in NA recruited from the rural Limpopo Province of South Africa consuming a diet low in animal products versus AA and EA consuming a typical Western diet (O’Keefe et al., 2007). In that study, clear differences were observed in dietary intakes and breath methane concentrations in NA relative to the other two groups. Specifically, NA consumed significantly fewer calories and less total and animal protein, total and saturated fat, and cholesterol than the AA or EA groups. Fasting breath methane was significantly higher in NA than in AA or EA subjects. In contrast, breath hydrogen was highest in AA. Thus, methanogens were expected to be more ubiquitous among NA subjects.
The proportion of stool samples positive for gene amplification in NA, AA and EA were 17/19, 15/18 and 14/17 for Archaea 16S rRNA gene and 17/19, 16/18 and 12/17 for mcrA, respectively. Intriguingly, Archaea 16S rRNA gene amplification did not always correlate with the detection of mcrA gene sequences. Two EA subjects were positive only for Archaea 16S rRNA gene, and one AA and one NA was positive for mcrA but not the Archaea 16S rRNA gene. We suspect these differences reflect amplification biases relating to the relative low abundance of MA in stool and potential DNA degradation or modification, but that presumably all subjects were colonized by MA. Based on data collected from mucosal biopsies of the colon from twenty-five subjects undergoing a screening colonoscopy, we have previously confirmed the prevalence of MA in the colonic mucosa of American (mostly EA) subjects, despite difficulties with detection in stool (Nava et al, 2011). The observation that almost all NA were colonized by MA, in contrast with other groups for which a significant number of subjects were negative is possibly linked to differences in methane excretion, although quantitative data would be needed to directly confirm (or refute) this hypothesis. This would indirectly confirm greater microbial fermentation in NA driven by a diet high in resistant starch.
The SRB functional gene dsrA was detected in 19/19 NA, 15/18 AA and 16/17 EA samples, whereas the Desulfovibrio 16S rRNA gene was detected in all stool samples from NA and AA and 16 of 17 from the EA group. This observation confirms other reports on the relative prevalence of SRB in the human colon (Pitcher et al., 2000; Zinkevich and Beech, 2000; Fite et al., 2004; Scanlan et al., 2009; Nava et al., 2011).
Diversity and distribution of Archaea 16S rRNA gene and mcrA TRFs among subjects
Archaea and mcrA profiles were moderately diverse (compared to the SRB TRF profiles) in stool from the three groups (Table 1). Twelve Archaea TRF were identified with BfaI, and nine with Hpy188I. Twenty-four mcrA TRF were identified with Hpy188III, and six with DdeI. Figure 1 shows the relative abundance of TRF from the most diverse TRFLP profiles among subjects of the three groups. In the most diverse profile (BfaI), EA harbored one specific Archaea and seven specific mcrA TRF, AA had two specific mcrA TRF and NA harbored one specific mcrA TRF.
Table 1.
Diversity indices for the four taxonomic targets*
| TRF number | Shannon index | Evenness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Arch | mcrA | dsrA | DSV | Arch | mcrA | dsrA | DSV | Arch | mcrA | dsrA | DSV |
| NA | 4.29a | 2.82 | 4.87 | 5.18 | 0.91 | 0.28 | 0.85a | 0.89 | 0.63 | 0.53 | 0.52a | 0.53 |
| AA | 3.77b | 2.63 | 5.2 | 4.91 | 0.85 | 0.22 | 0.79a | 0.83 | 0.69 | 0.56 | 0.45b | 0.57 |
| EA | 3.04c | 2.67 | 4.88 | 5.46 | 0.73 | 0.27 | 0.72b | 0.94 | 0.73 | 0.57 | 0.46b | 0.58 |
Averages for the two restriction enzymes based on non-singleton TRF
Letters indicate significant differences (t-test)
Figure 1. Intergroup interindividual variation in the diversity and relative abundance of Archaea 16S rRNA gene and mcrA TRF.
A nested PCR-TRFLP approach was used for the amplification of Archaea 16S rRNA gene and mcrA from stool samples from healthy native Africans (NA; n = 19), African Americans (AA; n = 18) and European Americans (EA; n = 17) taken from fresh feces voided during the first defecation of the morning and immediately stored under anaerobic conditions at −80°C until DNA extraction was performed. Genomic DNA was extracted from 200 mg of stool sample using a commercial kit (QIAamp DNA Stool Mini Kit, Qiagen, Valencia, CA). Methodological details for the nested PCR-TRFLP analyses are described in Nava et al. (2011). All primer pairs used for nested PCR are listed in Table S1.
Relative abundance of (A) Archaea 16S rRNA gene and (B) mcrA TRF in stool samples from NA, AA and EA using BfaI endonuclease for Archaea 16S rRNA gene and Hpy188III endonuclease for mcrA. Each color represents a TRF and its relative size within each chart represents relative abundance; the black bars represent the TRF that were represented more often in NA.
A higher diversity of Archaea TRF was observed among NA subjects relative to EA and AA (Table 1), but interindividual variability was also lowest for NA. The greatest interindividual variation was detected in EA with members of this group harboring the greatest number of singletons. For statistical analyses, TRF that were present in less than two different subjects among one or more groups were excluded. With both enzymes, three representative TRF were ubiquitous among subjects and groups, and thus constituted a “core profile.” One additional specific TRF was detected only in NA subjects (10/15, MANOVA P < 0.05).
After removal of singletons, seven representative Archaea 16S rRNA gene TRF were selected with BfaI and Hpy188I, and seven representative mcrA TRF were selected with Hpy188III and three with DdeI. With both enzymes, two representative TRF were ubiquitous among subjects and groups. One additional specific TRF was observed only in NA subjects (11/17). Overall, comparable diversity in mcrA TRF was observed among the three groups (Table 1). The discordance between Archaea and mcrA TRF numbers indicate either the potential presence of mrtA-harboring methanogens such as Msp. stadmaneae (Fricke et al., 2006) or non-methanogenic Archaea, which were documented recently with 16S rRNA gene rRNA sequences phylogenetically related to the Crenarchaeota phylum (Rieu-Lesme et al., 2005) and to Halorubrum alimentarium, H. saccharovorum, H. koreense strain and Halococcus morrhuae amplified from stool of Korean subjects (Nam et al., 2008).
Multivariate cluster analysis derived from the Kulczynski similarity index and the non-parametric MANOVA (P < 0.05) of Archaea populations revealed that NA differ significantly from AA, and that AA and EA harbor comparable Archaea populations (Figure 2A). Principal component analysis confirmed that slight differences exist in TRF content between NA, AA and EA. Similar statistical outcomes were observed for mcrA genes (Figure 2B). Thus, all subjects appear to share a core group of 2–3 Archaea/MA, with NA distinguished by the presence of an additional archaeal/MA genotype. While primer specificity may have contributed to the differential amplification efficacy between the two gene targets, consistent detection of additional genotypes in NA with the two primer sets supports the hypothesis that diet or environmental factors shape the composition of archaeal/MA communities. Native Africans were more homogeneous in Archaea 16S rRNA gene and mcrA TRF content as a group as illustrated by the NA clusters containing a majority of NA subjects (Figure 2A), whereas AA and EA were more dispersed among the clusters. Although individual diet intake data were not available for this study, the greater heterogeneity in archaeal/MA genotypes in AA and EA subjects may reflect a greater range of food choices in the Western diet.
Figure 2. Diversity of Archaea 16S rRNA gene and mcrA sequences.
A nested PCR-TRFLP approach was used for the amplification of Archaea 16S rRNA gene and mcrA from stool samples from healthy NA, AA and EA. Sample preparation and data analyses were executed as described in Fig. 1. Differences in the structure and composition of Archaea and mcrA sequences between the three groups were examined by multivariate analysis. (A) Cluster analysis of Archaea 16S rRNA gene TRF derived from the Kulczynski similarity index (presence-absence data) and B) Cluster analysis of mcrA TRF derived from the Kulczynski similarity index. Green symbols represent NA; blue symbols, AA and red symbols, EA. Each symbol designates a single subject. Intergroup differences among NA, AA and EA were estimated by means of non-parametric MANOVA. Differences were considered significant if P < 0.05.
Diversity and distribution of dsrA and Desulfovibrio 16S rRNA gene TRF among subjects
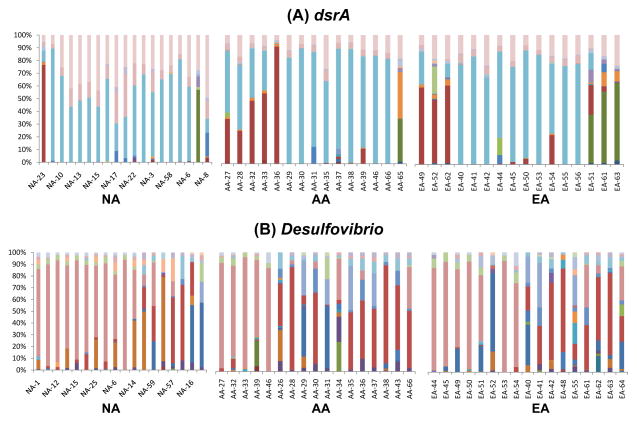
The dsrA and Desulfovibrio 16S rRNA gene TRF were substantially more diverse than Archaea and mcrA TRF (Table 1). Among all subjects, 50 dsrA TRF were identified with BstUI, and 23 with Sau96I (Figure 3A). Forty-three Desulfovibrio 16S rRNA gene TRF were identified with ScrfI and 32 with HpyCh4IV (Figure 3B).
Figure 3. Intergroup interindividual variation in the diversity and relative abundance of dsrA and Desulfovibrio 16S rRNA gene TRF.
A nested PCR-TRFLP approach was used to examine diversity in (A) dissimilatory sulfite reductase (dsrA) and (B) Desulfovibrio spp. 16S rRNA genes in stool samples from NA, AA and EA. Sample preparation and data analyses were executed as described in Fig. 1. Each color represents a TRF and its relative size within each chart represents relative abundance using BstUI endonuclease for dsrA and ScrfI endonuclease for Desulfovibrio 16S rRNA gene.
A higher diversity of dsrA TRF was observed among NA subjects relative to EA and AA, whereas the three groups harbored similar diversity in Desulfovibrio TRF (Table 1). Overall, the extent of interindividual variation in dsrA and Desulfovibrio genotypes was also substantially greater than for archaeal/MA genotypes. For statistical analyses, TRF that were present in less than two different subjects among one or more groups were excluded. Thus, 16 representative dsrA TRF were selected with BstUI and 10 with Sau96I. Twenty-three representative Desulfovibrio 16S rRNA gene TRF were selected with ScrfI and 12 with HpyCh4IV.
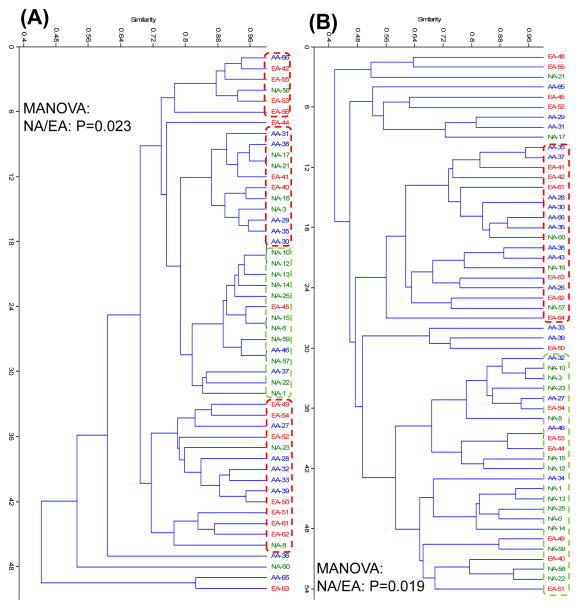
Multivariate cluster analysis derived from the Kulczynski similarity index and the non-parametric MANOVA of dsrA TRF revealed that NA differ significantly from EA, whereas dsrA TRF content was indistinguishable for AA and EA (Figure 4A). Principal component analysis confirmed that subtle differences exist in TRF content between NA, AA and EA. For Desulfovibrio 16S rRNA gene TRF, similar statistical outcomes were observed (Figure 4B). Native Africans were more homogeneous in dsrA and Desulfovibrio 16S rRNA gene TRF content as a group as illustrated by the NA clusters containing a majority of NA subjects and only a few AA and EA, whereas AA and EA were more dispersed among the clusters. Thus, both hydrogenotrophic groups appear to be different in NA compared to AA and EA, again possibly reflective of the marked differences in diet or environment among the groups.
Figure 4. Diversity of dsrA and Desulfovibrio 16S rRNA gene sequences.
A nested PCR-TRFLP approach was used for the amplification of dsrA and Desulfovibrio spp. 16S rRNA genes from stool samples. Sample preparation and data analyses were executed as described in Fig. 1. Differences in the structure and composition of dsrA and Desulfovibrio spp. 16S rRNA gene sequences between the three groups were examined by multivariate analysis. (A) Cluster analysis of dsrA TRF derived from the Kulczynski similarity index (presence-absence data) and B) Cluster analysis of Desulfovibrio spp. 16S TRF derived from the Kulczynski similarity index. Green symbols represent NA; blue symbols, AA and red symbols, EA. Each symbol designates a single subject. Intergroup differences among NA, AA and EA were estimated by means of non-parametric MANOVA. Differences were considered significant if P < 0.05.
A range of nutritionally and physiologically distinct SRB have been cultured from stool (Gibson et al., 1991; 1993; Willis et al., 1997) with Desulfovibrio being the most predominant SRB genus in the human colon (Gibson et al., 1991). Here, the multivariate analyses revealed that differences between NA and EA in the diversity of SRB populations were likely associated with the presence or absence of particular dsrA TRF. These analyses suggest that non-Desulfovibrio SRB are probably more specific to the three racial groups and, thus, could have been selected primarily by diet. These taxa must be identified and described, as they could significantly influence the rate and production of hydrogen sulfide in the colon. Moreover, the present data indicate the importance of understanding how diet impacts hydrogen metabolism and its potential contribution to colonic health and disease.
Supplementary Material
A nested PCR-TRFLP approach was used for the amplification of Archaea 16S rRNA, mcrA, dsrA and Desulfovibrio spp. 16S rRNA genes from stool samples. Sample preparation and data analyses were executed as described in Fig. 1. Differences in the structure and composition of Archaea 16S rRNA, mcrA, dsrA and Desulfovibrio spp. 16S rRNA gene sequences between the three groups were examined by multivariate analysis. Principal component analysis of (A) Archaea 16S rRNA gene TRF, (B) mcrA TRF, (C) dsrA TRF and (D) Desulfovibrio spp. 16S rRNA gene TRF. Green symbols represent NA; blue symbols, AA and red symbols, EA. Each symbol designates a single subject. Samples are plotted along the first two principal component axes. The ellipses of PCA correspond to the joint 95% confidence limits.
Acknowledgments
This work was supported by a grant from the American Institute for Cancer Research (SJOK) and NIH RO1 CA135379 (SJOK & HRG). The authors thank Roderick I. Mackie and Tony Yannarell (University of Illinois) for constructive review of the manuscript.
References
- Adams CJ, Redmond MC, Valentine DL. Pure-culture growth of fermentative bacteria, facilitated by H2 removal: bioenergetics and H2 production. Appl Environ Microbiol. 2006;72:1079–1085. doi: 10.1128/AEM.72.2.1079-1085.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2007. 2010 URL http://seer.cancer.gov/csr/1975_2007/
- Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- Bernalier A, Rochet V, Leclerc M, Dore J, Pochart P. Diversity of H2/CO2-utilizing acetogenic bacteria from feces of non-methane-producing humans. Curr Microbiol. 1996;33:94–99. doi: 10.1007/s002849900081. [DOI] [PubMed] [Google Scholar]
- Christl SU, Gibson GR, Cummings JH. Role of dietary sulphate in the regulation of methanogenesis in the human large intestine. Gut. 1992;33:1234–1238. doi: 10.1136/gut.33.9.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christl SU, Scheppach W, Kasper H. Hydrogen metabolism in the large intestine--physiology and clinical implications. Z Gastroenterol. 1995;33:408–413. [PubMed] [Google Scholar]
- Dore J, Pochart P, Bernalier A, Goderel I, Morvan B, Rambaud JC. Enumeration of H2-utilizing methanogenic archaea, acetogenic and sulfate-reducing bacteria from human feces. FEMS Microbiol Ecol. 1995;17:279–284. [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oufir L, Flourie B, Bruley des Varannes S, Barry JL, Cloarec D, Bornet F, Galmiche JP. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996;38:870–877. doi: 10.1136/gut.38.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fite A, Macfarlane GT, Cummings JH, Hopkins MJ, Kong SC, Furrie E, Macfarlane S. Identification and quantitation of mucosal and faecal desulfovibrios using real time polymerase chain reaction. Gut. 2004;53:523–529. doi: 10.1136/gut.2003.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke WF, Seedorf H, Henne A, Kruer M, Liesegang H, Hedderich R, et al. The genome sequence of Methanosphaera stadtmanae reveals why this human intestinal archaeon is restricted to methanol and H2 for methane formation and ATP synthesis. J Bacteriol. 2006;188:642–658. doi: 10.1128/JB.188.2.642-658.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Macfarlane GT, Cummings JH. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol. 1988;65:103–111. doi: 10.1111/j.1365-2672.1988.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Cummings JH, Macfarlane GT. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiology Letters. 1991;86:103–111. [Google Scholar]
- Gibson GR, MacFarlane S, MacFarlane GT. Metabolic interactions involving sulphate-reducing bacteria and methanogenic bacteria in the human large intestine. FEMS Microbiol Ecol. 1993;12:117–125. [Google Scholar]
- Gibson GR, Cummings JH, Macfarlane GT, Allison C, Segal I, Vorster HH, Walker AR. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990;31:679–683. doi: 10.1136/gut.31.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975–2006. 2009 URL http://seer.cancer.gov/csr/1975_2006/
- Leclerc H, Oger C, Beerens H, Mossel DAA. Occurrence of sulfate reducing bacteria in the human intestinal flora and in the aquatic environment. Water Research. 1980;14:253–256. [Google Scholar]
- Levine J, Ellis CJ, Furne JK, Springfield J, Levitt MD. Fecal hydrogen sulfide production in ulcerative colitis. Am J Gastroenterol. 1998;93:83–87. doi: 10.1111/j.1572-0241.1998.083_c.x. [DOI] [PubMed] [Google Scholar]
- Magee EA, Richardson CJ, Hughes R, Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. American Journal of Clinical Nutrition. 2000;72:1488–1494. doi: 10.1093/ajcn/72.6.1488. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Lin HC, McSweeney CS, Mackie RI, Gaskins HR. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Ann Rev Food Sci Technol. 2010;1:363–395. doi: 10.1146/annurev.food.102308.124101. [DOI] [PubMed] [Google Scholar]
- Nam YD, Chang HW, Kim KH, Roh SW, Kim MS, Jung MJ, et al. Bacterial, archaeal, and eukaryal diversity in the intestines of Korean people. J Microbiol. 2008;46:491–501. doi: 10.1007/s12275-008-0199-7. [DOI] [PubMed] [Google Scholar]
- Nava GM, Carbonero F, Croix JA, Greenberg E, Gaskins HR. Abundance and diversity of mucosa-associated hydrogenotrophic microbes in the healthy human colon. ISME J. 2011 doi: 10.1038/ismej.2011.90. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe SJ, Kidd M, Espitalier-Noel G, Owira P. Rarity of colon cancer in Africans is associated with low animal product consumption, not fiber. Am J Gastroenterol. 1999;94:1373–1380. doi: 10.1111/j.1572-0241.1999.01089.x. [DOI] [PubMed] [Google Scholar]
- O’Keefe SJ, Chung D, Mahmoud N, Sepulveda AR, Manafe M, Arch J, et al. Why do African Americans get more colon cancer than Native Africans? J Nutr. 2007;137:175S–182S. doi: 10.1093/jn/137.1.175S. [DOI] [PubMed] [Google Scholar]
- Pitcher MC, Beatty ER, Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000;46:64–72. doi: 10.1136/gut.46.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochart P, Dore J, Lemann F, Goderel I, Rambaud JC. Interrelations between populations of methanogenic archaea and sulfate-reducing bacteria in the human colon. FEMS Microbiol Lett. 1992;77:225–228. doi: 10.1016/0378-1097(92)90160-p. [DOI] [PubMed] [Google Scholar]
- Purdy KJ, Munson MA, Cresswell-Maynard T, Nedwell DB, Embley TM. Use of 16S rRNA-targeted oligonucleotide probes to investigate function and phylogeny of sulphate-reducing bacteria and methanogenic archaea in a UK estuary. FEMS Microbiol Ecol. 2003;44:361–371. doi: 10.1016/S0168-6496(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Rieu-Lesme F, Delbes C, Sollelis L. Recovery of partial 16S rDNA sequences suggests the presence of Crenarchaeota in the human digestive ecosystem. Curr Microbiol. 2005;51:317–321. doi: 10.1007/s00284-005-0036-8. [DOI] [PubMed] [Google Scholar]
- Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci. 1997;42:1571–1579. doi: 10.1023/a:1018851723920. [DOI] [PubMed] [Google Scholar]
- Scanlan PD, Shanahan F, Marchesi JR. Culture-independent analysis of Desulfovibrios in the human distal colon of healthy, colorectal cancer and polypectomized individuals. FEMS Microbiol Ecol. 2009;69:213–221. doi: 10.1111/j.1574-6941.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal I, Walker AR, Lord S, Cummings JH. Breath methane and large bowel cancer risk in contrasting African populations. Gut. 1988;29:608–613. doi: 10.1136/gut.29.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Bryant MP. Introduction to metabolic activities of intestinal bacteria. Am J Clin Nutr. 1979;32:149–157. doi: 10.1093/ajcn/32.1.149. [DOI] [PubMed] [Google Scholar]
- Strocchi A, Furne JK, Ellis CJ, Levitt MD. Competition for hydrogen by human faecal bacteria: evidence for the predominance of methane producing bacteria. Gut. 1991;32:1498–1501. doi: 10.1136/gut.32.12.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strocchi A, Furne J, Ellis C, Levitt MD. Methanogens outcompete sulphate reducing bacteria for H2 in the human colon. Gut. 1994;35:1098–1101. doi: 10.1136/gut.35.8.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CL, Cummings JH, Neale G, Gibson GR. Nutritional aspects of dissimilatory sulfate reduction in the human large intestine. Curr Microbiol. 1997;35:294–298. doi: 10.1007/s002849900257. [DOI] [PubMed] [Google Scholar]
- Zinkevich VV, Beech IB. Screening of sulfate-reducing bacteria in colonoscopy samples from healthy and colitic human gut mucosa. FEMS Microbiol Ecol. 2000;34:147–155. doi: 10.1111/j.1574-6941.2000.tb00764.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A nested PCR-TRFLP approach was used for the amplification of Archaea 16S rRNA, mcrA, dsrA and Desulfovibrio spp. 16S rRNA genes from stool samples. Sample preparation and data analyses were executed as described in Fig. 1. Differences in the structure and composition of Archaea 16S rRNA, mcrA, dsrA and Desulfovibrio spp. 16S rRNA gene sequences between the three groups were examined by multivariate analysis. Principal component analysis of (A) Archaea 16S rRNA gene TRF, (B) mcrA TRF, (C) dsrA TRF and (D) Desulfovibrio spp. 16S rRNA gene TRF. Green symbols represent NA; blue symbols, AA and red symbols, EA. Each symbol designates a single subject. Samples are plotted along the first two principal component axes. The ellipses of PCA correspond to the joint 95% confidence limits.