Abstract
Plasmacytoid dendritic cells (pDC) have been regarded as the “professional type I interferon producing cells” of the immune system following viral recognition that relies on the expression of Toll-like receptor (TLR)7 and TLR9. Furthermore, pDC link the innate and adaptive immune systems via cytokine production and antigen presentation. More recently their ability to induce tolerance and cytotoxicity has been added to their “immune skills”. Such broad range of actions, resembling the diverse functional features of a Swiss army knife, requires strong and prompt molecular regulation to prevent detrimental effects, including autoimmune pathogenesis or tumor escape. Over the last decades, we and others have started to unravel some aspects of the signaling pathways that regulate the various functions of human pDC. Here we review aspects of the molecular regulatory mechanisms to control pDC function in light of their multifaceted roles during immunity, autoimmunity, and cancer.
Introduction
Plasmacytoid dendritic cells (pDC), a subset of the dendritic cell family, develop from hematopoietic stem cells in the bone marrow. The intermediate progenitor cell stages of human pDC are to be defined, but mouse pDC differentiate from either common DC progenitors or lymphoid-primed multipotent progenitors(1). Human and mouse pDC development depend on Fms-like kinase 3 ligand (Flt3L)(2, 3), expression of the transcription factor Spi-B, an Ets-family member controlling expression of the anti-apoptotic gene Bcl2A1(4–7), and the basic helix-loop-helix protein E2-2(8, 9). PDC are key mediators of innate immunity mainly against viruses by sensing their nucleic acids via Toll like receptor (TLR)7 and TLR9. Following TLR7/9 triggering, pDC produce large amounts of type I Interferons (IFNα/3B2) that control viral replication(10). PDC produce also the pro-inflammatory cytokines IL6 and TNFα that regulate T, B, NK cell and conventional (c)-DC responses together with IFNα/β(10). Further, pDC play a role in T cell activation as TLR ligation induces pDC maturation into so-called pDC-derived DC, that exhibit DC morphology and antigen-presentation capacity(11). Over the past years, the molecular pathways involved in controlling pDC activation and maturation are being unraveled, thereby uncovering new aspects of pDC functions, such as cytotoxic and tolerogenic abilities. Such pleiotropic immune abilities, similar to the features of a Swiss army knife (Figure 1), may have detrimental effects when uncontrolled as seen in autoimmune diseases. We review here the main molecular mechanisms that should keep activated pDC “on physiological track” and highlight some aspects of deregulated pathways as observed in disease with a particular focus on human pDC.
Figure 1. The plasmacytoid dendritic cells as the Swiss army knife of the innate immune system.
Illustrated are the multifaceted functions of pDC to produce cytokines, present antigen, induce cytotoxicity and tolerance. Taken together, pDC resemble a Swiss army knife (adapted from clipartist.net) that is equipped with multiple features.
TLR signaling
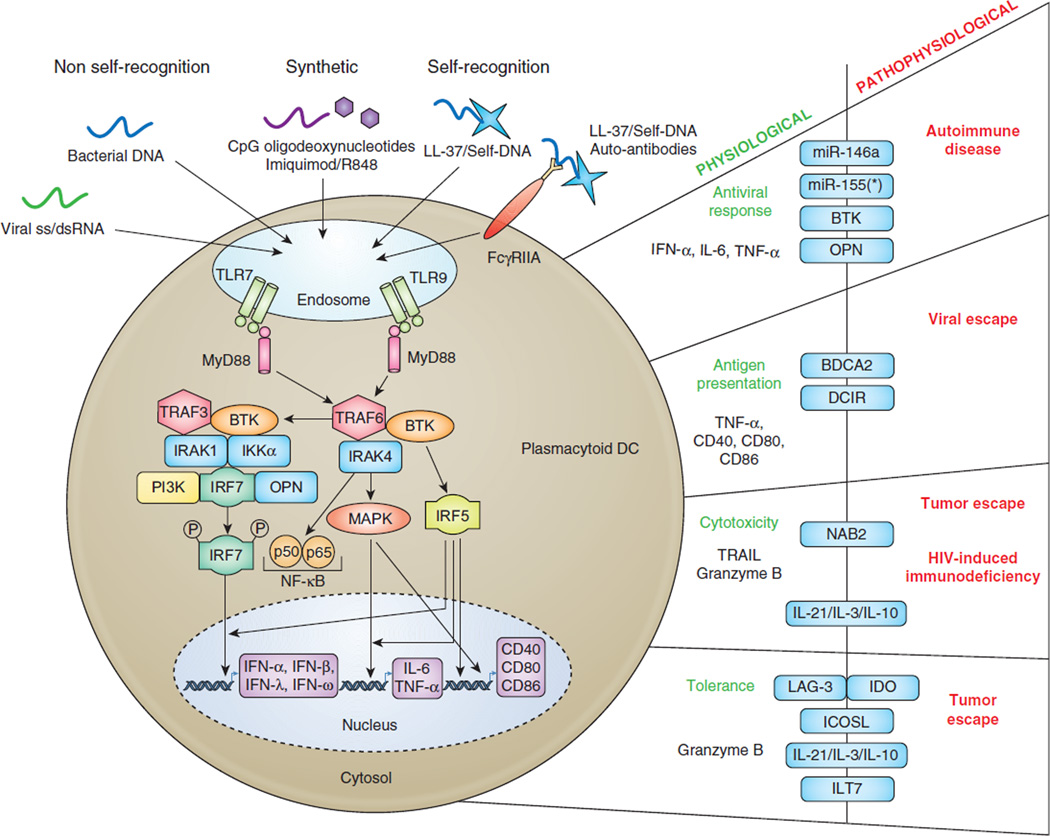
The first 6 hours following TLR7/9 activation, pDC devote up to 60% of their transcriptome to expression of type I IFN genes (IFNα, β, and ω) and type III genes (IFNλ1–3)(12, 13). Such robust secretion capacity requires specific cellular and molecular mechanisms and as such their “plasmacytoid” secretory morphology resembles antibody-secreting plasma cells. The rapid and substantial IFNα/β production by pDC in response to TLR ligation is mediated by constitutive expression of the master regulator Interferon Response Factor (IRF)7 (reviewed in (14)) (Figure 2). The signaling cascades downstream of TLR7/9 depend on the adaptor protein MyD88, that complexes with IL-1 receptor–associated kinase (IRAK)1 and IRAK4, tumor necrosis factor receptor-associated (TRAF)6 and TRAF3, and IRF7 and IRF5 (reviewed in (14)). Both TLR signaling pathways culminate in activation of nuclear factor κB (NFκB) depending on phosphorylation of inhibitory (I)κB proteins by the kinases IκBα and IκBβ and subsequent degradation(15, 16). Known NFκB members are RelA/p65, RelB, cRel, p52, and p50 that form homo- or heterodimers. The RelA/p50 heterodimer is most frequently activated after TLR signaling(15). RelA/p50 dimers are directly responsible for expression of co-stimulatory molecules (i.e. CD40, CD80, CD86), while IRF5 together with NFκB and mitogen-activated protein kinase (MAPK) activation is crucial for the production of IL6 and TNFα (reviewed in (14)). Phosphorylation of IRF7, likely mediated by PI3K activation, leads to IRF7 nuclear translocation with the help of osteopontin (OPN) leading to IFNα/β gene transcription(17, 18). Auto/paracrine production of IFNα/β promotes pDC survival via induction of anti-apoptotic genes, whereas TNFα supports pDC maturation. Currently it is believed that ligation of TLR in the early endosomal/ lysosome-related compartment will preferentially turn on IFN production, whereas late endosomal/lysosomal engagement regulates pro-inflammatory cytokine production and maturation((19) and reviewed in (14)).
Figure 2. TLR activation pathway in plasmacytoid dendritic cells and its regulation in health and disease.
PDC selectively express TLR7 and TLR9, which are expressed in the endosomal compartment. TLR activation is mediated by engagement of viral single strand RNA and bacterial DNA, respectively (non self-recognition). Self-nucleic acids, in complex with the small cationic antimicrobial peptide LL-37 are able to trigger TLR7/9 in pDC. Entry of self-DNA/LL-37 complexes can also be facilitated by plasma cell-derived autoantibodies that engage FcγRIIA. In addition, TLR7 can be activated by synthetic compounds such as Imiquimod or R848, while TLR9 recognizes synthetic CpG oligodeoxynucleotides, including CpG-A and CpG-B. TLR7/9 triggering leads to activation of the myeloid primary-response gene 88 (MyD88) and downstream signaling cascade via NFκB pathway, IRF5/7, and MAPK. This ultimately lead to expression of type I/III IFN, pro-inflammatory cytokines IL6 and TNFα, and costimulatory molecules, such as CD40, CD80, and CD86, that are the key components of pDC-derived antiviral response and antigen presentation. In addition pDC can exert cytotoxic properties via expression of TRAIL, and Granzyme B, which is also involved in the tolerogenic properties of pDC within tumor environment. On the right are listed the different functions of pDC and the different regulators that separate the physiological aspects from the dysfunctional pDC-derived pathophysiology. Antiviral response is mainly controlled via miRNA regulations (miR-146a, miR-155/miR-155*) and failure to do so can lead to autoimmune diseases such as SLE, Sjögren’s syndrome, and psoriasis.
Counter regulation of TLR signaling
TLR7/9 signaling needs to be counter regulated to prevent ongoing cytokine production as this is deleterious for the host. Cell surface receptors on human pDC that dampen TLR-induced responses include the C-type lectin blood dendritic cell antigen 2 (BDCA2), dendritic cell immunoreceptor (DCIR), immunoglobulin-like transcript 7 (ILT7), high-affinity immunoglobulin (Ig)E receptor (FcεRI), natural killer protein 44 (NKp44), adenosine diphosphate P2Y receptors, a nitric oxide-induced cGMP-dependent receptor, and Prostaglandin E2 receptors(20–22). Viruses can highjack the signaling pathways downstream of such receptors and escape from immune recognition (Figure 2). For example, the hepatitis C virus (HCV) envelope glycoprotein E2 binds to BDCA2 and DCIR, which inhibits IFNα production in pDC when exposed to HCV-infected hepatocytes(23). Moreover, exposure of pDC to HCV-infected hepatoma cells prevents NFκB phosphorylation via an endocytosis-dependent mechanism resulting in a lack of cell surface expression of CD40, CCR7, CD86 and TRAIL, and of TNFα and IL6 secretion(24). Another example is HIV, that induces production of IFNα via TLR7 signaling to elicit antiviral activity in acute infection(25). In addition, HIV gp140 binds to DCIR(26) to recruit phosphatases (e.g. SHP1 and SHP2) and tyrosine kinases (e.g. Src, Fyn, Hck, Syk) to the immunoreceptor tyrosine-based inhibitory motif (ITIM) domain of DCIR(27, 28). Recruitment of this signalosome is important for DCIR activity with regard to HIV binding/entry and enhanced HIV replication(26). It is possible that DCIR activation via gp140 inhibits IFNα production in pDC thereby increasing HIV replication. Following HIV-induced IFNα secretion is expression of interferon stimulated genes (ISG), such as MxA and BST2/Tetherin(29) in surrounding cells. While increased expression of BST2 on leukocytes, including CD4+ T cells, may play a role in decreasing HIV virion release from infected cells in acute HIV infection(30), BST2 binding to its inhibitory receptor ILT7 expressed on pDC may dampen IFN production(31) and increase viral replication at least during the acute phase. During chronic HIV infection sustained levels of IFNα return likely as a result of persistent immune activation leading to HIV pathogenesis. During chronic infection, pDC express increased levels of IRF7(32) and lower levels of ILT7(33) that may contribute to persistent IFNα secretion as well. In addition to IFNα, TNFα may be responsible for persistent immune activation as treatment of SIV infected Rhesus Macaques with an antibody to TNFα reduced expression of pro-inflammatory cytokines and immunopathology in lymphoid tissues(40).
A new layer of regulation involved in fine tuning immune responses are microRNAs (miRNA)(34), which are involved in post-transcriptional regulation of protein expression also in pDC. MiR-155 and miR-155* have an opposite role in controlling TLR-induced IFN production by human pDC(35). MiR-155* augments IFNα/β expression by suppressing the negative TLR7 signaling mediator IRAKM(36). MiR-155 inhibits IFN expression by targeting the adaptor TAK1-binding protein 2 (TAB2)(37). We showed that miR-146a is induced in human pDC by TLR7/9 agonists, but not IL3, thereby interfering with cytokine production, maturation and survival(38). Together with similar data in the mouse(39, 40) miR-146a is recognized as a “brake of the immune response” by downregulating IRAK1 and TRAF6 expression and hence dampening of TLR-induced responses.
Cytotoxicity
TLR7/9 stimulation of pDC also induces the expression of TNF-related apoptosis inducing ligand (TRAIL/Apo-2L)(41, 42), which is mediates cell death of TRAIL-sensitive infected cells and tumor cells expressing either TRAIL-R1 or TRAIL-R2(43). As TRAIL-expressing pDC accumulate in Basal Cell Carcinoma lesions topically treated with the TLR7 agonist Imiquimod, this suggests that pDC may be involved in Imiquimod-induced regression of tumor lesions(41, 44). In response to HIV, pDC express TRAIL(45) that are present in peripheral blood and lymph nodes of HIV-infected individuals and may directly kill Death receptor 5 (DR5)+CD4+ T cells via the TRAIL/DR5 pathway(46, 47) although this is debated by others (48). We identified NGFI-A-binding protein 2 (NAB2), which is induced by TLR7/9 signaling in pDC, as a regulator of TRAIL expression(49). Autocrine IFNα/β signaling also regulates TRAIL expression in human and mouse pDC(49–52). PDC may kill target cells via the serine protease granzyme B (GrB) as well, which is constitutively expressed in human pDC(53). PDC-derived GrB lyses the erythroleukemic cell line K562 in a perforin-independent, but caspase-dependent manner(54). This could not be recapitulated, however, when using primary T cells as targets(55).
Antigen uptake
The ability of pDC to induce adaptive immunity through direct antigen presentation to T cells remained controversial for a long time. Most research focused on cDC as they are more efficient as antigen presenting cells (APC). Immature mouse pDC are able to take up soluble antigens, but less efficient than cDC possibly due to a lower macropinocytosis activity(56). Human pDC express several receptors to detect and endocytose pathogens that can be processed and presented to T cells. Antigens coupled to antibodies that target the endocytic receptors DEC-205(57), DCIR(58), Fcγ Receptor IIa (FcγRIIa)(59) and BDCA2(60) efficiently induce antigen-specific CD4+ T cell activation. While BDCA2(61) and DCIR(58) are downregulated after TLR activation, DEC-205 expression is induced after TLR activation and continues to function as antigen internalization receptor(57).
Human pDC can internalize, process and present antigens via MHC Class I and Class II to CD8+ and CD4+ T cells, respectively(11, 62–64) at least in vitro. Whether pDC act as professional APC in cross-presentation of exogenous antigens has been re-evaluated and data show that pDC have an efficient machinery allowing cross-presentation to CD8+ T cells(62, 64–67). Hence, combined with their capacity to produce IFNα/β, pDC are interesting targets for immunotherapy.
Tolerance
In the immature state, pDC have poor ability to support T cell proliferation(68) and even suppress T cell responses indirectly through the induction of regulatory T cells (Treg)(69, 70). PDC contribute to peripheral T cell tolerance in transplantation(71), tumor escape(72), oral-(73) and mucosal tolerance(74). “Tolerogenic” pDC may be present in mouse gut and thymus(75–77). Such pDC may express the chemokine receptor CCR9, that is lost upon TLR triggering correlating with reduced ability to prime tolerance(75). In human, a similar tolerance inducing pDC subset has yet to be identified, but pDC expressing either GrB or indoleamine 2,3-dioxygenase (IDO) impair T cell proliferation(55, 72, 78). GrB is induced in pDC by the cytokines IL21(55), or IL3 plus IL10(78), and inhibition of GrB activity restored pDC-induced T cell activation(55, 78). IL21 may be involved in mediating a negative feed-back loop to terminate adaptive immune responses as human CD4+ and NK-T cells are the main producers in viral or bacterial infections(79).
Melanoma progression in humans may be associated with tumor-infiltrating pDC promoting pro-inflammatory Th2 and Treg through OX40L and ICOSL, respectively(80), although this contradicts with the observation that patients with metastatic melanoma receiving intranodal injections of pDC mount anti-tumor responses(81). In addition, a subset of pDC expressing lymphocyte activation gene 3 (LAG3) negatively regulates T cell activation and positively regulates Treg function by production of IL6(82, 83). Furthermore, ILT7 on pDC engages BST2(31), which is endogenously expressed in tumors(reviewed in (84)), thereby suppressing infiltrating pDC to produce IFN in response to TLR ligands and hence an anti-tumor response.
Autoimmune diseases
Despite the low frequency of pDC in blood and lymphoid tissues, their high potential to produce IFNα also in response to self-nucleic acids raised questions about their putative role in autoimmunity. Unwanted IFNα production by pDC is involved in autoimmune pathogenesis including systemic lupus erythematosus (SLE)(85, 86), Sjögren’s syndrome(87), and psoriasis(88). Blood and tissue cells of these patients have an IFN-signature indicating that Interferon-inducible upregulation of ISG can be used as disease biomarker(89). In addition to deleterious effects of IFN, pDC differentiate into mature pDC with antigen presenting capacity able to steer T cell responses adding to the pathogenesis of autoimmune diseases.
In SLE, auto-antibodies directed to nuclear antigens are aberrantly produced and deposited in tissues causing inflammation. Nucleic acid-containing immune complexes (IC) trigger IFNα release from pDC upon FcγRIIa-mediated uptake into endosomes and local engagement of TLR7/9(90, 91). PDC numbers in blood of SLE patients are reduced, but pDC infiltration is found in skin and renal lesions(92). The IFN-signature correlates with disease activity and severity(85, 93), but is independent of the relative TLR7 gene copy number(94). SLE pathogenesis can be linked to increased IL6 production by activated pDC, which together with IFNα promotes survival and differentiation of auto-reactive B cells into auto-antibody-secreting plasma cells(10). IFNα production by SLE-IC can be inhibited by blocking FcRγ-mediated uptake of IgG(95), by hydroxychloroquine, which increases the intracytoplasmic pH and prevents acidification and maturation of endosomes(96), or by C-reactive protein, which binds apoptotic cells and nucleoprotein auto-antigens(97). Reduced miR-146a expression is found in PBMC of SLE patients and may add to elevated IFNα and IL6 levels(98). Accordingly, SLE is associated with miR-146a polymorphisms(99–101). Lower expression of miR-146a may be linked to a miR-146a promoter variant binding less efficiently to Ets1(100). Not all studies support an association of SLE and miR-146 polymorphisms(102). Other negative regulators of TLR-induced IFNα production in pDC inhibiting SLE pathogenesis are BDCA2 and ILT7, which complex both with FcεRIγ(103, 104). This involves a B cell receptor-like signaling mechanism relying on activation of adaptors such as Syk, B cell linker (BLNK), and B lymphoid tyrosine kinase (BLK). Reducing BLK levels in mouse pDC increased TLR9-induced IFNα production(105). Given that genetic variants in the BLK locus are identified in SLE patients by genome-wide association studies, it is notable that certain polymorphisms correlate with reduced BLK levels(106). Consequently, this may elevate IFNα secretion and hence contribute to SLE predisposition. SLE patients are generally treated with glucocorticoids (GC) that exert an anti-inflammatory effect likely by inhibition of NFκB activation. However, these drugs do not convey maintenance of disease control in the majority of patients due to inefficient NFκB inhibition in pDC(107), thereby preventing GC-induced pDC death and consequently ongoing IFNα production. Improved therapeutic advantage may be gained by treating SLE patients with inhibitors of Syk (108), BTK (109) or TLR(110). Future intervention may aim at altering expression of miR-29b/c, involved in TLR-inhibited GC-induced pDC apoptosis by directly targeting Mcl-1 and Bcl-2(111).
In psoriasis, a disease of chronic skin inflammation, lesions contain activated pDC secreting IFNα/β(88, 112) due to the presence of cathelicidin peptides, including LL-37, produced by activated keratinocytes(113). LL-37 complexes with self-DNA/RNA released by dying cells and engages TLR7/9 leading to chronic IFNα production(113, 114). Psoriatic lesions are effectively treated with Vitamin D (VitD) analogs, which have anti-inflammatory properties(115). PDC may contribute to the tolerance induction, since VitD impairs the ability of pDC to induce T-cell proliferation and secretion of the Th1 cytokine IFNγ(116). It remains unresolved how VitD programs the tolerogenic properties in pDC, but this is not due to altered expression of co-stimulatory molecules, MHC Class II, or production of IFNα. Despite the pathological role of pDC in autoimmune skin diseases, the physiological importance of pDC in initiating skin wound healing is also reported. Following skin injury, pDC are rapidly recruited to the site of tissue damage to sense self-nucleic acids released by dying cells in combination with cathelicidins, and to initiate tissue repair via TLR-induced IFNα production(117).
Conclusions
PDC are major actors of immune responses against viruses and bacteria through TLR7/9 activation. PDC are not only capable of linking the innate and adaptive immune system via rapid and sustained production of cytokines, including type I IFN, IL6 and TNFα, but can also activate T cells through direct antigen presentation in vitro and likely in vivo. In addition, pDC are able to directly kill bystander tumor cells, thereby participating in cancer-induced immune responses. Although the beneficial role of pDC in immunity is undisputable, their recently discovered “tolerogenic” face in different tumors suggests their involvement in tumor escape mechanisms. Such a broad range of action requires tight regulation, both at the transcriptional and post-transcriptional level, to control development, differentiation, function, and survival of pDC. System failures do exist, however, given the existence of type I IFN-mediated autoimmune diseases. More extensive research on pDC is required to unravel the pathways leading to uncontrolled cytokine production and differentiation to enable therapeutic intervention for curing or stabilizing diseases.
Acknowledgments
This work was supported by the Dutch Science Foundation NWO (Grant 917.66.310 to B.B) and the National Institutes of Health (Grant AI080564 to C.H.U.).
References
- 1.Onai N, Kurabayashi K, Hosoi-Amaike M, Toyama-Sorimachi N, Matsushima K, Inaba K, Ohteki T. A clonogenic progenitor with prominent plasmacytoid dendritic cell developmental potential. Immunity. 2013;38:943–957. doi: 10.1016/j.immuni.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Naik SH, O'Keeffe M, Proietto A, Shortman HH, Wu L. CD8+, CD8-, and plasmacytoid dendritic cell generation in vitro using flt3 ligand. Methods Mol Biol. 2010;595:167–176. doi: 10.1007/978-1-60761-421-0_10. [DOI] [PubMed] [Google Scholar]
- 3.Blom B, Ho S, Antonenko S, Liu YJ. Generation of interferon alpha-producing predendritic cell (Pre-DC)2 from human CD34(+) hematopoietic stem cells. J Exp Med. 2000;192:1785–1796. doi: 10.1084/jem.192.12.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schotte R, Rissoan MC, Bendriss-Vermare N, Bridon JM, Duhen T, Weijer K, Briere F, Spits H. The transcription factor Spi-B is expressed in plasmacytoid DC precursors and inhibits T-, B-, and NK-cell development. Blood. 2003;101:1015–1023. doi: 10.1182/blood-2002-02-0438. [DOI] [PubMed] [Google Scholar]
- 5.Schotte R, Nagasawa M, Weijer K, Spits H, Blom B. The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J Exp Med. 2004;200:1503–1509. doi: 10.1084/jem.20041231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki I, Hoshino K, Sugiyama T, Yamazaki C, Yano T, Iizuka A, Hemmi H, Tanaka T, Saito M, Sugiyama M, Fukuda Y, Ohta T, Sato K, Ainai A, Suzuki T, Hasegawa H, Toyama-Sorimachi N, Kohara H, Nagasawa T, Kaisho T. Spi-B is critical for plasmacytoid dendritic cell function and development. Blood. 2012;120:4733–4743. doi: 10.1182/blood-2012-06-436527. [DOI] [PubMed] [Google Scholar]
- 7.Karrich JJ, Balzarolo M, Schmidlin H, Libouban M, Nagasawa M, Gentek R, Kamihira S, Maeda T, Amsen D, Wolkers MC, Blom B. The transcription factor Spi-B regulates human plasmacytoid dendritic cell survival through direct induction of the antiapoptotic gene BCL2-A1. Blood. 2012;119:5191–5200. doi: 10.1182/blood-2011-07-370239. [DOI] [PubMed] [Google Scholar]
- 8.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, Holter W, Rauch A, Zhuang Y, Reizis B. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagasawa M, Schmidlin H, Hazekamp MG, Schotte R, Blom B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur J Immunol. 2008;38:2389–2400. doi: 10.1002/eji.200838470. [DOI] [PubMed] [Google Scholar]
- 10.Gilliet M, Lande R. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr Opin Immunol. 2008;20:401–407. doi: 10.1016/j.coi.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 13.Yin Z, Dai J, Deng J, Sheikh F, Natalia M, Shih T, Lewis-Antes A, Amrute SB, Garrigues U, Doyle S, Donnelly RP, Kotenko SV, Fitzgerald-Bocarsly P. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J Immunol. 2012;189:2735–2745. doi: 10.4049/jimmunol.1102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao M, Liu YJ. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell. 2013;4:40–52. doi: 10.1007/s13238-012-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino K, Sugiyama T, Matsumoto M, Tanaka T, Saito M, Hemmi H, Ohara O, Akira S, Kaisho T. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature. 2006;440:949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 17.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao W. Molecular characterization of human plasmacytoid dendritic cells. J Clin Immunol. 2009;29:257–264. doi: 10.1007/s10875-009-9284-x. [DOI] [PubMed] [Google Scholar]
- 21.Shin A, Toy T, Rothenfusser S, Robson N, Vorac J, Dauer M, Stuplich M, Endres S, Cebon J, Maraskovsky E, Schnurr M. P2Y receptor signaling regulates phenotype and IFN-alpha secretion of human plasmacytoid dendritic cells. Blood. 2008;111:3062–3069. doi: 10.1182/blood-2007-02-071910. [DOI] [PubMed] [Google Scholar]
- 22.Fabricius D, Neubauer M, Mandel B, Schutz C, Viardot A, Vollmer A, Jahrsdorfer B, Debatin KM. Prostaglandin E2 inhibits IFN-alpha secretion and Th1 costimulation by human plasmacytoid dendritic cells via E-prostanoid 2 and E-prostanoid 4 receptor engagement. J Immunol. 2010;184:677–684. doi: 10.4049/jimmunol.0902028. [DOI] [PubMed] [Google Scholar]
- 23.Florentin J, Aouar B, Dental C, Thumann C, Firaguay G, Gondois-Rey F, Soumelis V, Baumert TF, Nunes JA, Olive D, Hirsch I, Stranska R. HCV glycoprotein E2 is a novel BDCA-2 ligand and acts as an inhibitor of IFN production by plasmacytoid dendritic cells. Blood. 2012;120:4544–4551. doi: 10.1182/blood-2012-02-413286. [DOI] [PubMed] [Google Scholar]
- 24.Dental C, Florentin J, Aouar B, Gondois-Rey F, Durantel D, Baumert TF, Nunes JA, Olive D, Hirsch I, Stranska R. Hepatitis C virus fails to activate NF-kappaB signaling in plasmacytoid dendritic cells. J Virol. 2012;86:1090–1096. doi: 10.1128/JVI.05444-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabado RL, O'Brien M, Subedi A, Qin L, Hu N, Taylor E, Dibben O, Stacey A, Fellay J, Shianna KV, Siegal F, Shodell M, Shah K, Larsson M, Lifson J, Nadas A, Marmor M, Hutt R, Margolis D, Garmon D, Markowitz M, Valentine F, Borrow P, Bhardwaj N. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloem K, Vuist IM, van den Berk M, Klaver EJ, van Die I, Knippels LM, Garssen J, Garcia-Vallejo JJ, van Vliet SJ, van Kooyk Y. DCIR interacts with ligands from both endogenous and pathogenic origin. Immunol Lett. 2014;158:33–41. doi: 10.1016/j.imlet.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Lambert AA, Barabe F, Gilbert C, Tremblay MJ. DCIR-mediated enhancement of HIV-1 infection requires the ITIM-associated signal transduction pathway. Blood. 2011;117:6589–6599. doi: 10.1182/blood-2011-01-331363. [DOI] [PubMed] [Google Scholar]
- 28.Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood. 2008;112:1299–1307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 30.Homann S, Smith D, Little S, Richman D, Guatelli J. Upregulation of BST-2/Tetherin by HIV infection in vivo. J Virol. 2011;85:10659–10668. doi: 10.1128/JVI.05524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien M, Manches O, Sabado RL, Baranda SJ, Wang Y, Marie I, Rolnitzky L, Markowitz M, Margolis DM, Levy D, Bhardwaj N. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. J Clin Invest. 2011;121:1088–1101. doi: 10.1172/JCI44960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benlahrech A, Yasmin A, Westrop SJ, Coleman A, Herasimtschuk A, Page E, Kelleher P, Gotch F, Imami N, Patterson S. Dysregulated immunophenotypic attributes of plasmacytoid but not myeloid dendritic cells in HIV-1 infected individuals in the absence of highly active anti-retroviral therapy. Clin Exp Immunol. 2012;170:212–221. doi: 10.1111/j.1365-2249.2012.04647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11:163–175. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- 35.Zhou H, Huang X, Cui H, Luo X, Tang Y, Chen S, Wu L, Shen N. miR-155 and its star-form partner miR-155* cooperatively regulate type I interferon production by human plasmacytoid dendritic cells. Blood. 2010;116:5885–5894. doi: 10.1182/blood-2010-04-280156. [DOI] [PubMed] [Google Scholar]
- 36.Hassan F, Islam S, Tumurkhuu G, Dagvadorj J, Naiki Y, Komatsu T, Koide N, Yoshida T, Yokochi T. Involvement of interleukin-1 receptor-associated kinase (IRAK)-M in toll-like receptor (TLR) 7-mediated tolerance in RAW 264.7 macrophage-like cells. Cell Immunol. 2009;256:99–103. doi: 10.1016/j.cellimm.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Kishida S, Sanjo H, Akira S, Matsumoto K, Ninomiya-Tsuji J. TAK1-binding protein 2 facilitates ubiquitination of TRAF6 and assembly of TRAF6 with IKK in the IL-1 signaling pathway. Genes Cells. 2005;10:447–454. doi: 10.1111/j.1365-2443.2005.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karrich JJ, Jachimowski LC, Libouban M, Iyer A, Brandwijk K, Taanman-Kueter EW, Nagasawa M, de Jong EC, Uittenbogaart CH, Blom B. MicroRNA-146a regulates survival and maturation of human plasmacytoid dendritic cells. Blood. 2013;122:3001–3009. doi: 10.1182/blood-2012-12-475087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao JL, Rao DS, Boldin MP, Taganov KD, O'Connell RM, Baltimore D. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci U S A. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J. Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 42.Matsui T, Connolly JE, Michnevitz M, Chaussabel D, Yu C-I, Glaser C, Tindle S, Pypaert M, Freitas H, Piqueras B, Banchereau J, Palucka AK. CD2 Distinguished Two Subsets of Human Plasmacytoid Dendritic Cells with Distinct Phenotype and Function. J. Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blum A, Chaperot L, Molens J-P, Foissoud V, Plantaz D, Plumas J. Mechanisms of TRAIL-induced apoptosis in leukemic plasmacytoid dendritic cells. Exp. Hemat. 2006;34:1655–1662. doi: 10.1016/j.exphem.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J. Exp. Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barblu L, Machmach K, Gras C, Delfraissy JF, Boufassa F, Leal M, Ruiz-Mateos E, Lambotte O, Herbeuval JP A. E. H. C. S. Group. Plasmacytoid dendritic cells (pDCs) from HIV controllers produce interferon-alpha and differentiate into functional killer pDCs under HIV activation. The Journal of infectious diseases. 2012;206:790–801. doi: 10.1093/infdis/jis384. [DOI] [PubMed] [Google Scholar]
- 46.Stary G, Klein I, Kohlhofer S, Koszik F, Scherzer T, Mullauer L, Quendler H, Kohrgruber N, Stingl G. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–3863. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 47.Hardy AW, Graham DR, Shearer GM, Herbeuval J-P. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulated HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc. Natl. Acad. Sci USA. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chehimi J, Papasavvas E, Tomescu C, Gekonge B, Abdulhaqq S, Raymond A, Hancock A, Vinekar K, Carty C, Reynolds G, Pistilli M, Mounzer K, Kostman J, Montaner LJ. Inability of plasmacytoid dendritic cells to directly lyse HIV-infected autologous CD4+ T cells despite induction of tumor necrosis factor-related apoptosis-inducing ligand. J Virol. 2010;84:2762–2773. doi: 10.1128/JVI.01350-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balzarolo M, Karrich JJ, Engels S, Blom B, Medema JP, Wolkers MC. The transcriptional regulator NAB2 reveals a two-step induction of TRAIL in activated plasmacytoid DCs. Eur J Immunol. 2012;42:3019–3027. doi: 10.1002/eji.201242385. [DOI] [PubMed] [Google Scholar]
- 50.Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 51.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, Colonna M, Sibilia M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalb ML, Glaser A, Stary G, Koszik F, Stingl G. TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-alpha-mediated antitumor reactivity. J Immunol. 2012;188:1583–1591. doi: 10.4049/jimmunol.1102437. [DOI] [PubMed] [Google Scholar]
- 53.Rissoan MC, Duhen T, Bridon JM, Bendriss-Vermare N, Peronne C, de Saint Vis B, Briere F, Bates EE. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood. 2002;100:3295–3303. doi: 10.1182/blood-2002-02-0638. [DOI] [PubMed] [Google Scholar]
- 54.Bratke K, Nielsen J, Manig F, Klein C, Kuepper M, Geyer S, Julius P, Lommatzsch M, Virchow JC. Functional expression of granzyme B in human plasmacytoid dendritic cells: a role in allergic inflammation. Clin Exp Allergy. 2010;40:1015–1024. doi: 10.1111/j.1365-2222.2010.03499.x. [DOI] [PubMed] [Google Scholar]
- 55.Karrich JJ, Jachimowski LC, Nagasawa M, Kamp A, Balzarolo M, Wolkers MC, Uittenbogaart CH, Marieke van Ham S, Blom B. IL-21-stimulated human plasmacytoid dendritic cells secrete granzyme B, which impairs their capacity to induce T-cell proliferation. Blood. 2013;121:3103–3111. doi: 10.1182/blood-2012-08-452995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, Mount AM, Belz GT, O'Keeffe M, Ohmura-Hoshino M, Ishido S, Stoorvogel W, Heath WR, Shortman K, Villadangos JA. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 57.Tel J, Benitez-Ribas D, Hoosemans S, Cambi A, Adema GJ, Figdor CG, Tacken PJ, de Vries IJ. DEC-205 mediates antigen uptake and presentation by both resting and activated human plasmacytoid dendritic cells. Eur J Immunol. 2011;41:1014–1023. doi: 10.1002/eji.201040790. [DOI] [PubMed] [Google Scholar]
- 58.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, Adema GJ. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 59.Benitez-Ribas D, Tacken P, Punt CJ, de Vries IJ, Figdor CG. Activation of human plasmacytoid dendritic cells by TLR9 impairs Fc gammaRII-mediated uptake of immune complexes and presentation by MHC class II. J Immunol. 2008;181:5219–5224. doi: 10.4049/jimmunol.181.8.5219. [DOI] [PubMed] [Google Scholar]
- 60.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu P, Wu J, Liu S, Han X, Lu J, Shi Y, Wang J, Lu L, Cao X. TLR9/TLR7-triggered downregulation of BDCA2 expression on human plasmacytoid dendritic cells from healthy individuals and lupus patients. Clin Immunol. 2008;129:40–48. doi: 10.1016/j.clim.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, Xue Y, Mellman I, Banchereau J, Connolly JE. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guillerme JB, Boisgerault N, Roulois D, Menager J, Combredet C, Tangy F, Fonteneau JF, Gregoire M. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin Cancer Res. 2013;19:1147–1158. doi: 10.1158/1078-0432.CCR-12-2733. [DOI] [PubMed] [Google Scholar]
- 64.Tel J, Schreibelt G, Sittig SP, Mathan TS, Buschow SI, Cruz LJ, Lambeck AJ, Figdor CG, de Vries IJ. Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T cells despite lower Ag uptake than myeloid dendritic cell subsets. Blood. 2013;121:459–467. doi: 10.1182/blood-2012-06-435644. [DOI] [PubMed] [Google Scholar]
- 65.Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, Lebon P, Heshmati F, Guillet JG, Gannage M, Caillat-Zucman S, Casartelli N, Schwartz O, De la Salle H, Hanau D, Hosmalin A, Maranon C. Antigen cross presentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–492. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 66.Lui G, Manches O, Angel J, Molens JP, Chaperot L, Plumas J. Plasmacytoid dendritic cells capture and cross-present viral antigens from influenza-virus exposed cells. PLoS One. 2009;4:e7111. doi: 10.1371/journal.pone.0007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tel J, Sittig SP, Blom RA, Cruz LJ, Schreibelt G, Figdor CG, de Vries IJ. Targeting uptake receptors on human plasmacytoid dendritic cells triggers antigen cross-presentation and robust type I IFN secretion. J Immunol. 2013;191:5005–5012. doi: 10.4049/jimmunol.1300787. [DOI] [PubMed] [Google Scholar]
- 68.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O'Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 69.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, Arima K, Wang YH, Voo KS, Cao W, Liu YJ. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184:2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115:5366–5375. doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- 71.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 72.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mizuno S, Kanai T, Mikami Y, Sujino T, Ono Y, Hayashi A, Handa T, Matsumoto A, Nakamoto N, Matsuoka K, Hisamatsu T, Takaishi H, Hibi T. CCR9(+) plasmacytoid dendritic cells in the small intestine suppress development of intestinal inflammation in mice. Immunol Lett. 2012 doi: 10.1016/j.imlet.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 78.Jahrsdorfer B, Vollmer A, Blackwell SE, Maier J, Sontheimer K, Beyer T, Mandel B, Lunov O, Tron K, Nienhaus GU, Simmet T, Debatin KM, Weiner GJ, Fabricius D. Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion. Blood. 2010;115:1156–1165. doi: 10.1182/blood-2009-07-235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 80.Aspord C, Leccia MT, Charles J, Plumas J. Plasmacytoid Dendritic Cells Support Melanoma Progression by Promoting Th2 and Regulatory Immunity through OX40L and ICOSL. Cancer Immunol Res. 2013;1:402–415. doi: 10.1158/2326-6066.CIR-13-0114-T. [DOI] [PubMed] [Google Scholar]
- 81.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M, Winkels G, Coulie PG, Punt CJ, Figdor CG, de Vries IJ. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 82.Camisaschi C, De Filippo A, Beretta V, Vergani B, Villa A, Vergani E, Santinami M, Cabras AD, Arienti F, Triebel F, Rodolfo M, Rivoltini L, Castelli C. Alternative Activation of Human Plasmacytoid DCs In Vitro and in Melanoma Lesions: Involvement of LAG-3. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.29. [DOI] [PubMed] [Google Scholar]
- 83.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao W, Bover L. Signaling and ligand interaction of ILT7: receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol Rev. 2010;234:163–176. doi: 10.1111/j.0105-2896.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M, Lepajolec C, Labetoulle M, Ardizzone M, Sibilia J, Fournier C, Chiocchia G, Mariette X. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc Natl Acad Sci U S A. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Higgs BW, Liu Z, White B, Zhu W, White WI, Morehouse C, Brohawn P, Kiener PA, Richman L, Fiorentino D, Greenberg SA, Jallal B, Yao Y. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis. 2011;70:2029–2036. doi: 10.1136/ard.2011.150326. [DOI] [PubMed] [Google Scholar]
- 90.Crow MK. Type I Interferon in the Pathogenesis of Lupus. J Immunol. 2014;192:5459–5468. doi: 10.4049/jimmunol.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 92.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bengtsson AA, Sturfelt G, Truedsson L, Blomberg J, Alm G, Vallin H, Ronnblom L. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9:664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 94.Kelley J, Johnson MR, Alarcon GS, Kimberly RP, Edberg JC. Variation in the relative copy number of the TLR7 gene in patients with systemic lupus erythematosus and healthy control subjects. Arthritis Rheum. 2007;56:3375–3378. doi: 10.1002/art.22916. [DOI] [PubMed] [Google Scholar]
- 95.Wiedeman AE, Santer DM, Yan W, Miescher S, Kasermann F, Elkon KB. Contrasting mechanisms of interferon-alpha inhibition by intravenous immunoglobulin after induction by immune complexes versus Toll-like receptor agonists. Arthritis Rheum. 2013;65:2713–2723. doi: 10.1002/art.38082. [DOI] [PubMed] [Google Scholar]
- 96.Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R155. doi: 10.1186/ar3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mold C, Clos TW. C-reactive protein inhibits plasmacytoid dendritic cell interferon responses to autoantibody immune complexes. Arthritis Rheum. 2013;65:1891–1901. doi: 10.1002/art.37968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, Huang X, Zhou H, de Vries N, Tak PP, Chen S, Shen N. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J, Yang B, Ying B, Li D, Shi Y, Song X, Cai B, Huang Z, Wu Y, Wang L. Association of pre-microRNAs genetic variants with susceptibility in systemic lupus erythematosus. Mol Biol Rep. 2011;38:1463–1468. doi: 10.1007/s11033-010-0252-6. [DOI] [PubMed] [Google Scholar]
- 100.Luo X, Yang W, Ye DQ, Cui H, Zhang Y, Hirankarn N, Qian X, Tang Y, Lau YL, de Vries N, Tak PP, Tsao BP, Shen N. A functional variant in microRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS Genet. 2011;7:e1002128. doi: 10.1371/journal.pgen.1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lofgren SE, Frostegard J, Truedsson L, Pons-Estel BA, D'Alfonso S, Witte T, Lauwerys BR, Endreffy E, Kovacs L, Vasconcelos C, Martins da Silva B, Kozyrev SV, Alarcon-Riquelme ME. Genetic association of miRNA-146a with systemic lupus erythematosus in Europeans through decreased expression of the gene. Genes Immun. 2012;13:268–274. doi: 10.1038/gene.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jimenez-Morales S, Gamboa-Becerra R, Baca V, Del Rio-Navarro BE, Lopez-Ley DY, Velazquez-Cruz R, Saldana-Alvarez Y, Salas-Martinez G, Orozco L. MiR-146a polymorphism is associated with asthma but not with systemic lupus erythematosus and juvenile rheumatoid arthritis in Mexican patients. Tissue antigens. 2012;80:317–321. doi: 10.1111/j.1399-0039.2012.01929.x. [DOI] [PubMed] [Google Scholar]
- 103.Cao W, Zhang L, Rosen DB, Bover L, Watanabe G, Bao M, Lanier LL, Liu YJ. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS biology. 2007;5:e248. doi: 10.1371/journal.pbio.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Samuelson EM, Laird RM, Papillion AM, Tatum AH, Princiotta MF, Hayes SM. Reduced B lymphoid kinase (Blk) expression enhances proinflammatory cytokine production and induces nephrosis in C57BL/6-lpr/lpr mice. PLoS One. 2014;9:e92054. doi: 10.1371/journal.pone.0092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Delgado-Vega AM, Dozmorov MG, Quiros MB, Wu YY, Martinez-Garcia B, Kozyrev SV, Frostegard J, Truedsson L, de Ramon E, Gonzalez-Escribano MF, Ortego-Centeno N, Pons-Estel BA, D'Alfonso S, Sebastiani GD, Witte T, Lauwerys BR, Endreffy E, Kovacs L, Vasconcelos C, da Silva BM, Wren JD, Martin J, Castillejo-Lopez C, Alarcon-Riquelme ME. Fine mapping and conditional analysis identify a new mutation in the autoimmunity susceptibility gene BLK that leads to reduced half-life of the BLK protein. Ann Rheum Dis. 2012;71:1219–1226. doi: 10.1136/annrheumdis-2011-200987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, Soumelis V, Banchereau J, Coffman RL, Pascual V, Barrat FJ. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liao C, Hsu J, Kim Y, Hu DQ, Xu D, Zhang J, Pashine A, Menke J, Whittard T, Romero N, Truitt T, Slade M, Lukacs C, Hermann J, Zhou M, Lucas M, Narula S, DeMartino J, Tan SL. Selective inhibition of spleen tyrosine kinase (SYK) with a novel orally bioavailable small molecule inhibitor, RO9021, impinges on various innate and adaptive immune responses: implications for SYK inhibitors in autoimmune disease therapy. Arthritis Res Ther. 2013;15:R146. doi: 10.1186/ar4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Lau KY, Jung J, Ravindran P, Barrat FJ. Bruton's tyrosine kinase regulates TLR9 but not TLR7 signaling in human plasmacytoid dendritic cells. Eur J Immunol. 2014;44:1130–1136. doi: 10.1002/eji.201344030. [DOI] [PubMed] [Google Scholar]
- 110.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 111.Hong Y, Wu J, Zhao J, Wang H, Liu Y, Chen T, Kan X, Tao Q, Shen X, Yan K, Zhai Z. miR-29b and miR-29c are involved in Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid dendritic cells. PLoS One. 2013;8:e69926. doi: 10.1371/journal.pone.0069926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S, Fortugno P, Gonzalvo-Feo S, Franssen JD, Parmentier M, De Pita O, Girolomoni G, Sozzani S. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med. 2009;206:249–258. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 114.Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, Homey B, Barrat FJ, Zal T, Gilliet M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kragballe K. Calcipotriol: a new drug for topical psoriasis treatment. Pharmacol Toxicol. 1995;77:241–246. doi: 10.1111/j.1600-0773.1995.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 116.Karthaus N, van Spriel AB, Looman MW, Chen S, Spilgies LM, Lieben L, Carmeliet G, Ansems M, Adema GJ. Vitamin d controls murine and human plasmacytoid dendritic cell function. J Invest Dermatol. 2014;134:1255–1264. doi: 10.1038/jid.2013.501. [DOI] [PubMed] [Google Scholar]
- 117.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, Digiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]