Abstract
Consistent with evidence of a strong correlation between interferon gamma (IFNγ) production and rabies virus (RABV) clearance from the CNS, we recently demonstrated that engineering a pathogenic RABV to express IFNγ highly attenuates the virus. Reasoning that IFNγ expression by RABV vaccines would enhance their safety and efficacy, we reverse-engineered two proven vaccine vectors, GAS and GASGAS, to express murine IFNγ. Mortality and morbidity were monitored during suckling mice infection, immunize/challenge experiments and mixed intracranial infections. We demonstrate that GASγ and GASγGAS are significantly attenuated in suckling mice compared to the GASGAS vaccine. GASγ better protects mice from lethal DRV4 RABV infection in both pre- and post-exposure experiments compared to GASGAS. Finally, GASγGAS reduces post-infection neurological sequelae, compared to control, during mixed intracranial infection with DRV4. These data show IFNγ expression by a vaccine vector can enhance its safety while increasing its efficacy as pre- and post-exposure treatment.
Keywords: Vaccine, CNS, Rabies, Post-exposure treatment, Interferon gamma
INTRODUCTION
Rabies is an important zoonotic disease that is estimated to kill at least 60 000 people worldwide each year (1). It is caused by the rabies virus, a non-segmented, negative-strand lyssavirus in the Rhabdoviridae family. The majority of rabies deaths occur from rabid dog bites in developing countries where medical resources are limited and/or a lack of awareness about the risk of RABV exposure exists (2).
PEP readily prevents development of clinical rabies and death when given soon after exposure to a rabid animal (3). Unfortunately, because many do not have access to proper medical care, whether for financial or logistical reasons, or may not recognize a potential RABV exposure, prompt administration of rabies PEP to at-risk individuals is not always achieved (4). In these cases RABV enters the brain, clinical rabies develops and the infected individuals almost invariably die. Therefore, there is a great need to decrease RABV infection in dogs (and other reservoirs) and to develop new therapeutics to successfully extend the window of successful post-exposure treatment from days to perhaps weeks or months. Live-attenuated vaccines are promising candidates to fulfill both these need. They can be produced economically and induce long-lasting immunity with a single dose. Furthermore, superinfection with attenuated RABV has successfully protected animals exposed to lethal RABV.
Early superinfection experiments in dogs showed protection from intramuscular (i.m.) injection of a lethal RABV by intrathecal injection of an attenuated RABV (5). Importantly, inactivated RABV could not protect dogs from death in these experiments. Since that time, other animal model experiments have demonstrated the ability of live-attenuated RABV to protect against infection with lethal wild-type RABV (3, 6–8). These data indicate that treatment with attenuated RABV has the potential to protect individuals who would otherwise succumb to rabies.
While questions of reverse mutations and safety persist, the effectiveness of GAS RABV variants, with two attenuating mutations in their glycoproteins, has been well established (6–8). Much of the success of the GAS variants has been attributed to rapid induction of virus neutralizing antibody (VNA) (6, 8). In order to be an effective late PET vector, the virus should initiate an immune response much faster than the typical time that is required to induce a robust VNA response. We recently showed that the addition of IFNγ to a pathogenic RABV backbone highly attenuates the virus and enhances the innate immune response, specifically via induction of type I interferons (D. A. Barkhouse, et al., submitted for publication). Therefore, we constructed two new IFNγ-expressing GAS vectors, GASγ and GASγGAS, to determine if IFNγ expression by a highly attenuated vaccine vector could increase its safety profile and/or its efficacy as pre- and post-exposure treatment.
MATERIALS AND METHODS
Viruses
GAS and GASGAS viruses have been described elsewhere (9, 10) and are fully recombinant vectors based on the prototype recombinant RABV derived from the SADB19 strain (11, 12). The wild type RABV strain DRV4 was isolated from a human rabies victim and propagated in NA cells as previously described (13).
Recombinant virus construction
The recombinant RABVs, SPBNγ, GASγ, GASγGAS and GASoGAS were engineered as described previously (14). As a brief example, for SPBNγ murine IFNγ DNA was PCR-amplified from mRNA extracted from RABV-infected mouse brain tissue using the custom primers (IDT, Coralville, IA) below and DeepVent polymerase (NEB, Ipswich, MA). The forward primer, 5’ATAGAATTCCGTACGAAGATGAACGCTACACACTGCATCTTGGCT3’, contains a BsiWI restriction site, start codon, and the gene specific sequence. The reverse primer, 5’ATTCTCTAGATAGCTAGCTCAGCAGCGACTCCTTTTCCGCTTCCT3’, contains a NheI restriction site, stop codon, and the gene specific sequence. The resultant IFNγ DNA was cloned into the pSPBN plasmid, creating the pSPBNγ plasmid. Standard transformation and transfection methods were used to complete the virus rescue of SPBNγ as outlined above.
Cell lines
BSR cells (a BHK-21 clone) (15) grown in DMEM (Mediatech, Manassas, VA) supplemented with 10% FBS (Atlanta Biologicals, Flowery Ranch, GA) were used to grow virus stocks. Mouse neuroblastoma cells (NA) were grown in RPMI medium 1640 (Mediatech, Manassas, VA) and used for growth curves and virus titers. Mouse astrocytoma (AS) cells and monocyte lineage (MC) cells were grown in DMEM supplemented with 10% FBS and were used for growth curves.
Growth Curves
NA, AS or MC cells were infected at a multiplicity of infection (MOI) of 1 for growth curves. 100 ul supernatant aliquots were taken at 0, 12, 24, and 48 hours post-infection. Virus titers from the supernatants were calculated as described below.
Virus Titers
To determine virus titers, NA cells were seeded into 96 well plates, grown for two days and then infected with virus in serial ten-fold dilutions. Two days post-infection (d.p.i.), the cells were fixed with 80% acetone and stained with FITC-conjugated anti-RABV RNP antibody (Fujirebio Diagnostics, Malvern, PA). Virus titers of quadruplicate samples were determined using a fluorescence microscope.
Mice
Female, six to eight week old and timed pregnant Swiss Webster mice were purchased from Taconic Farms (Germantown, NY). All mouse experiments were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee.
Infection of mice
Groups of ten adult mice were infected under isoflurane (Vedco, St. Joseph, MO) anesthesia: 1) intracranially (i.c.) with 102 or 103 focus-forming units (ffu) of challenge virus in 10 ul PBS, 2) i.c. with a mixture of 102 or 103 ffu of challenge virus with 103–106 ffu of attenuated virus in 10 ul PBS or 3) intramuscularly (i.m.) in each gastrocnemious with 102–105 ffu of attenuated vector in 100 ul PBS. Mice were observed for 28–30 days for appearance of clinical signs of rabies such as limb paralysis, tremors and weight loss. Litters of 10–12 five-day-old suckling mice were infected i.c. with 103 ffu of RABV in 5 ul PBS and observed 40 days for mortality and morbidity. All mice were humanely euthanized when moribund or after losing greater than 30% of starting body weight.
IFNγ Enzyme-Linked Immunosorbent Assay (ELISA)
50 ul aliquots of virus-infected supernatants were assayed for IFNγ using an OptEIA Mouse IFNγ Kit II (BD Biosciences, San Jose, CA) per manufacturer’s instructions. The plates were then scanned in a Synergy H1 plate reader (BioTek, Winooski, VT) and absorbance at 450 nm was recorded.
Behavioral testing
The hanging wire test was used to assess motor neuromuscular impairment in mice that survived RABV infection. The procedure, derived from the taut wire test and SHIRPA phenotypic assessment of mdx mice (16, 17) can found here (http://www.treat-nmd.eu/downloads/file/sops/dmd/MDX/DMD_M.2.1.004.pdf). Briefly, mice are suspended from a taut wire and latency to fall is measured as an indicator of gross motor neuromuscular condition.
Statistical Analysis
All calculations were performed using GraphPad Prism 5.01 (GraphPad Software, San Diego, CA) with the exception of the Habel Test for Potency (18).
RESULTS
Virus constructs
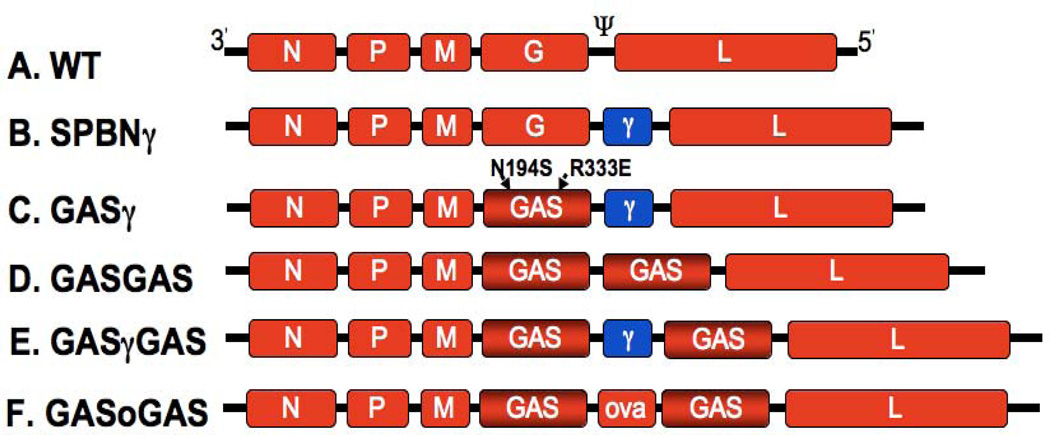
The wild-type RABV genome as well as the engineered viruses SPBNγ, GASγ, GASGAS, GASγGAS and GASoGAS are depicted in Fig. 1A, B, C, D, E and F, respectively. SPBNγ is an attenuated RABV but still retains residual pathogenicity in adult mice. GASγ is the next generation IFNγ-producing RABV constructed using the highly attenuated GAS backbone. The GAS virus has two engineered mutations in its glycoprotein, the R333E mutation renders it attenuated and the N194S mutation increases the safety of this virus by drastically reducing the odds of a revertant mutation (N194K) (9). The GAS glycoprotein is known to act as a dominant negative, capable of preventing the pathogenicity inherent in the wild-type glycoprotein when both are present (10). Therefore, the GASγGAS backbone vector has an improved safety profile over GASγ as reversion to a pathogenic phenotype would require unlikely mutations in both glycoprotein genes. GASGAS is the control vaccine strain to which GASγ is compared and GASoGAS is the control virus for GASγGAS mixed infections.
Figure 1. RABV constructs.
A, The negative-stranded RNA genome of the wild-type (WT) parental vector, SAD B19. B, The IFNγ-expressing RABV, SPBNγ. C, The IFNγ-expressing GASγ, previously attenuated by two mutations in its glycoprotein gene. D, The highly attenuated GASGAS control vaccine vector. E, The IFNγ-expressing GASγGAS. F, The ovalbumin–expressing control vector GASoGAS. N, nucleoprotein; P, phosophoprotein; M, matrix protein; G, glycoprotein; L, large catalytic subunit of the viral polymerase; Ψ, pseudogene region; N194S and R333E, engineered mutations; γ, murine IFNγ gene; ova, chicken ovalbumin gene.
GASγ and GASγGAS in vitro growth varies by cell type
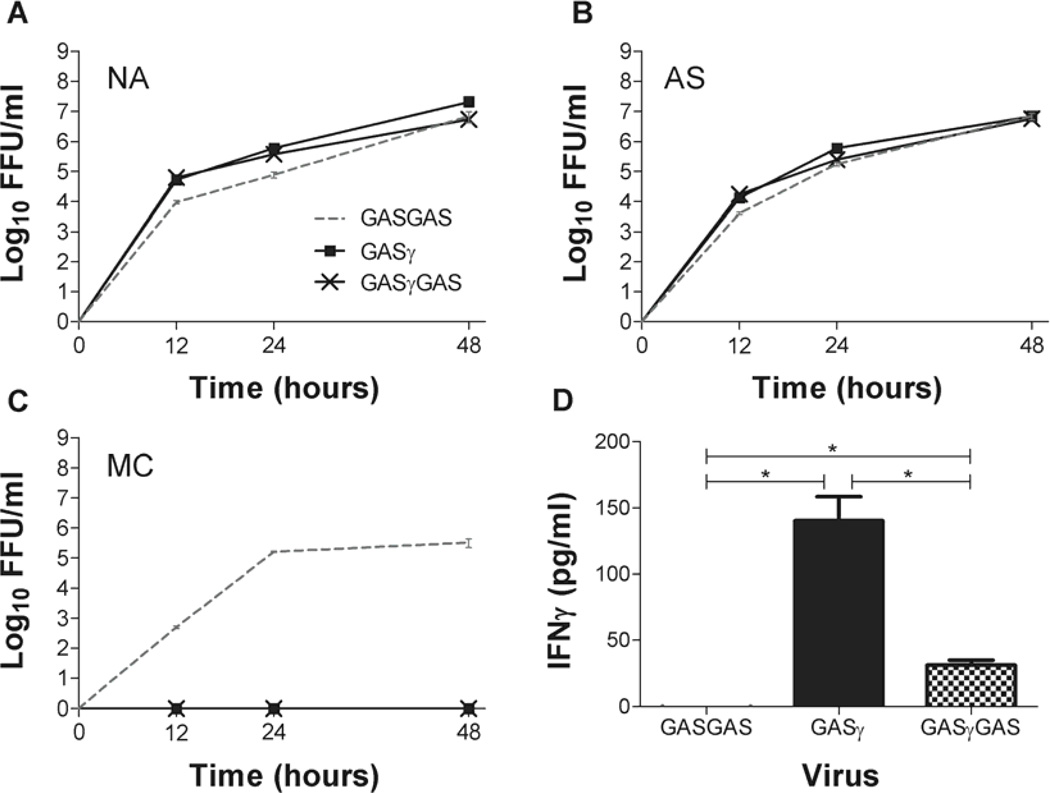
An effective live-attenuated vaccine or PET vector must be able to infect target cells and replicate. To ensure that GASγ and GASγGAS can replicate and produce progeny virions, we created growth curves using GASGAS as the control virus. We infected NA and AS cells at an MOI=1 for all three viruses (Fig. 2A, B). At 12, 24 and 48 hours p.i. we collected supernatants from each of the virus-infected cell cultures and calculated virus titers. At all timepoints there is less than one log10 difference in virus titer between GASγ or GASγGAS and control GASGAS in NA and AS cells, showing that GASγ and GASγGAS can infect and replicate in CNS-relevant cells as well as the GASGAS vaccine strain. The growth characterisitcs of GASoGAS were previously determined and differ by less than one log10 compared to GASγ, GASγGAS or GASGAS (19). We also infected MC cells with the same three GAS variants to ensure that the IFNγ expressed by GASγ and GASγGAS is functional (Fig. 2C). The fact that GASGAS grows well (>105 ffu/ml) in MC cells while GASγ and GASγGAS infected MC cells produced no progeny virus in their supernatants, suggests that the activation of MC cells by IFNγ is preventing virus propagation. Fig. 2D is an IFNγ ELISA showing the presence of murine IFNγ in the 72-hour supernatants of GASγ- and GASγGAS-infected BSR cells while no IFNγ is detected in the GASGAS control supernatant. It should be noted that more IFNγ is present in the GASγ supernatant compared to GASγGAS.
Fig. 2. In vitro growth of GAS variants.
NA, AS and MC cell lines were infected with GASGAS, GASγ or GASγGAS at an MOI=1 (A–C). Supernatant virus titers were measured at the indicated times post-infection. D shows IFNγ present in the supernatants of BSR cells 72 hours p.i. Significant difference was determined by the student’s t-test. *, p≤0.05.
IFNγ reduces pathogenicity of RABV in suckling mice
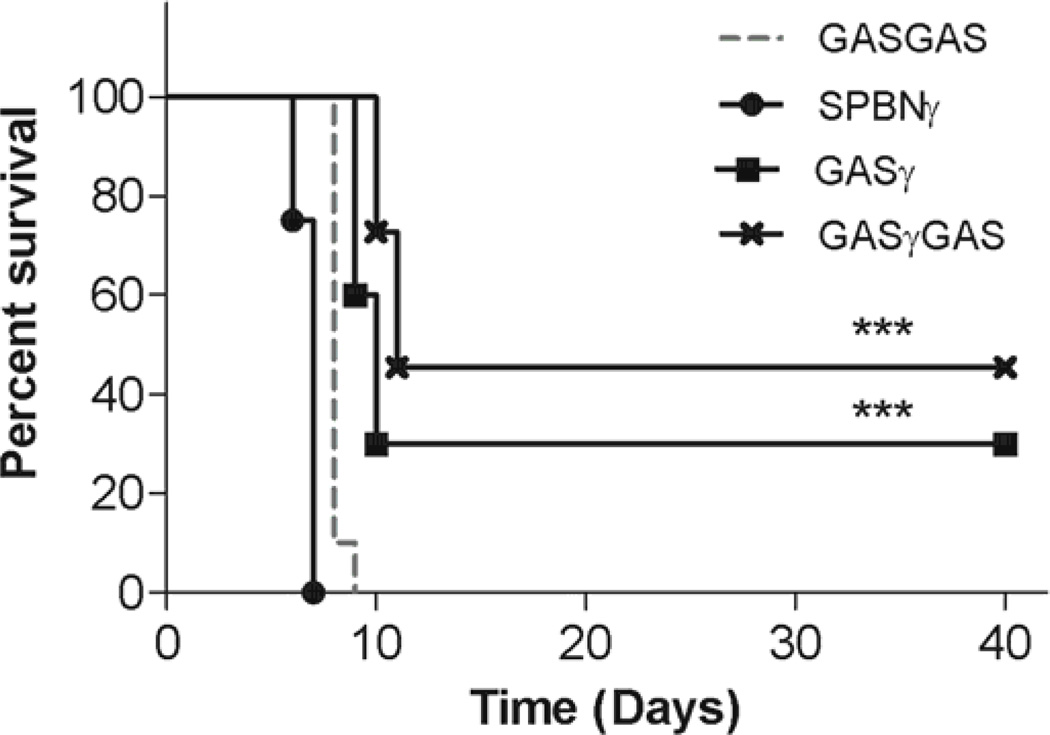
One of the hallmarks of a safe vaccine is its lack of pathogenicity in immunocompromised mice. Having previously demonstrated that virus-encoded IFNγ can strongly attenuate a pathogenic RABV (D. A. Barkhouse, et al., submitted for publication), we hypothesized that a similar strategy could increase the safety of a known vaccine strain. To test this hypothesis we infected, intracranially (i.c.), groups of five-day-old suckling mice with 103 ffu of SPBNγ, GASγ, GASγGAS or GASGAS control vaccine and monitored them for 40 days for mortality and morbidity. The survival curves (Fig. 3) demonstrate that SPBNγ is 100% lethal at this dosage in the suckling mice. This result is not surprising since we have shown that SPBNγ retains residual pathogenicity in adult mice (D. A. Barkhouse, et al., submitted for publication). The pathogenicity of SPBNγ, however, is similar to that of the GASGAS vaccine strain which also kills 100% of mice. As hypothesized, the incorporation of IFNγ into the GAS and GASGAS vectors results in significant attenuation of GASγ and GASγGAS as compared to the GASGAS vaccine with 30% of GASγ-infected and 45% of GASγGAS-infected suckling mice surviving 40 d.p.i.
Fig. 3. Infection of suckling mice.
Five-day-old suckling mice were infected i.c. with 103 ffu of the indicated virus and monitored for mortality and morbidity for 40 days. Significant difference between survival curves was determined by the Mantel-Cox test. ***, p≤0.001 and denotes the difference between the specific curve and the GASGAS control curve.
GASγ is an effective pre-exposure vaccine
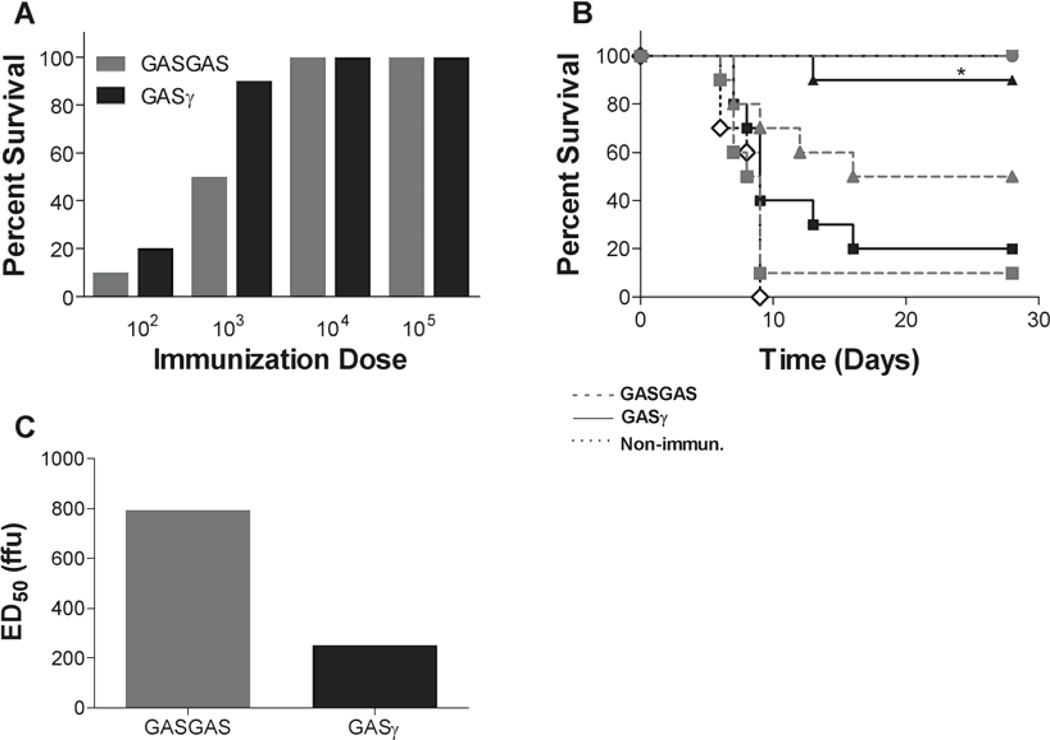
To determine if IFNγ expression by a vaccine vector could affect its ability to protect against lethal challenge, we infected groups of ten mice i.m. in the gastrocnemius with 102–105 ffu of GASγ or control GASGAS and challenged them 13 days post-vaccination with 103 ffu of DRV4 i.c. Fig. 4A shows absolute survival percentages and 4B is the survival curve providing both survival percentages and time to death. There is no significant difference in protection when comparing GASγ to GASGAS vaccination using a dose of 102 ffu, but at 103 ffu GASγ protects significantly more mice than GASGAS. As we increase to high vaccination doses (104 and 105 ffu), the efficacy of GASGAS vaccine is equivalent to GASγ for a 103 ffu DRV4 i.c. challenge, and 100% of mice in these dosage groups survive for 28 days post-challenge. From these survival data we show that the ED50 of GASγ is less than 1/3 that of GASGAS (Fig. 4C).
Fig. 4. Survival after immunization and challenge.
Groups of mice were immunized i.m. in the gastrocnemious with 102–105 ffu of GASGAS or GASγ followed by i.c. challenge with 103 ffu of DRV4 13 days later. They were monitored for mortality and morbidity for 28 days post-challenge. A is a summary of the survival proportions while B shows the survival curves. An ED50 comparison of GASGAS and GASγ is shown in C. Significant difference between survival curves was determined by the Mantel-Cox test. *, p≤0.05 and denotes the difference between the GASγ curve and the GASGAS curve at the same immunization dosage. ■, 102 ffu;  , 103 ffu; ♦, 104 ffu; ●, 105 ffu; ◊, non-immunized control.
, 103 ffu; ♦, 104 ffu; ●, 105 ffu; ◊, non-immunized control.
GASγ protects mice from lethal co-infection
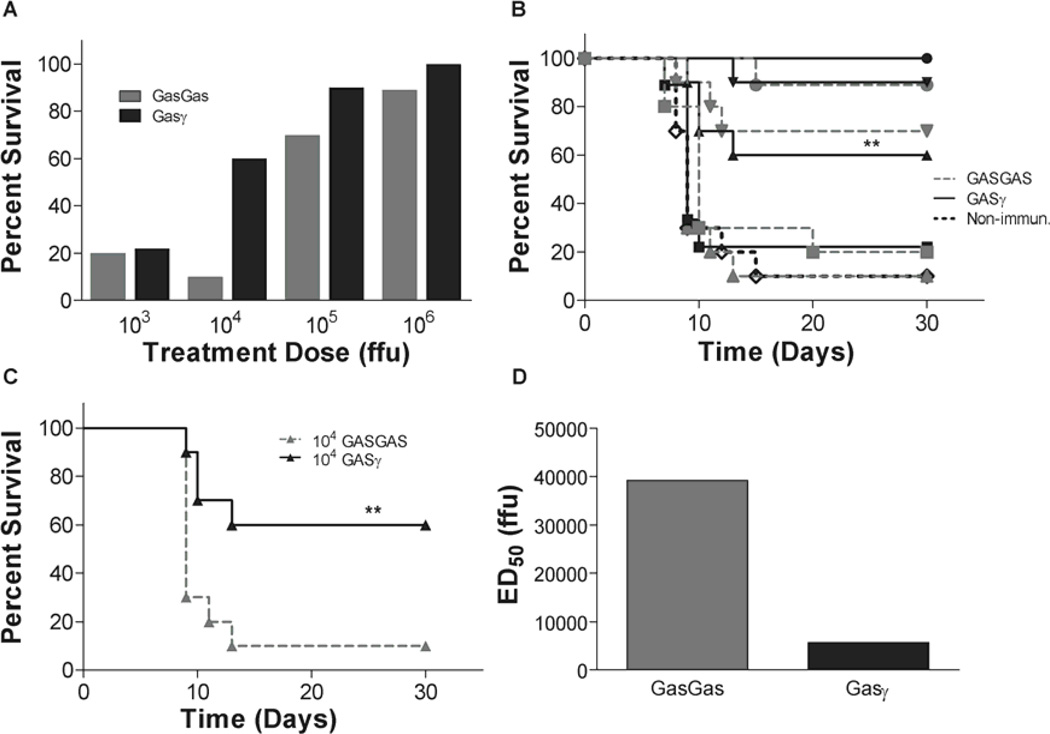
Having proven the increased efficacy of GASγ over GASGAS as a standard pre-exposure, live-attenuated vaccine, we next tested its ability to protect mice from DRV4 during an i.c. mixed infection. Co-infection serves a model of post-exposure treatment since we are administering the treatment vector at the same time as the DRV4 reaches the brain. To determine the efficacy of GASγ to protect mice from lethal DRV4 i.c. infection as compared to GASGAS, we infected groups of mice with a mixture of 102 ffu of DRV4 and 103, 104, 105 or 106 ffu of GASγ or GASGAS. The survival proportions are represented in Fig. 5A and the survival curves are shown in Fig. 5B. At 103 ffu of GASγ or GASGAS there is no significant difference in the ability of the viruses to protect mice from death. As we increase the virus dosage, GASγ is significantly better at protecting against DRV4 infection. 104 ffu of GASγ protects 60% of mice from death while GASGAS prevents death in only 10% of mice. For the sake of clarity, Fig. 5C shows the 104 ffu survival curves from Fig. 5B. For the higher doses of virus (105 and 106 ffu), the ability of GASγ to protect mice from death during mixed infection is not statistically different than GASGAS. Survival proportions were used to calculate the ED50 of GASγ and GASGAS. The ED50 of GASγ is approximately seven fold lower than that of GASGAS (Fig. 5D).
Fig. 5. Survival after mixed infection.
Groups of mice were infected with a mixture of 102 ffu of DRV4 and 103–106 ffu of GASGAS or GASγ and monitored for mortality and morbidity for 30 days. Survival proportions and survival curves for each virus are represented in A and B, respectively. C shows survival curves of the 104 ffu treatment dose only. The ED50 of each treatment virus is found in D. Significant difference between survival curves was determined by the Mantel-Cox test. **, p≤0.01 and denotes the difference between the GASγ curve and the GASGAS curve at the same immunization dosage. ■, 103 ffu;  , 104 ffu; ♦, 105 ffu; ●, 106 ffu; ◊, non-immunized control.
, 104 ffu; ♦, 105 ffu; ●, 106 ffu; ◊, non-immunized control.
Post-exposure treatment with GASγGAS reduces neurological sequelae
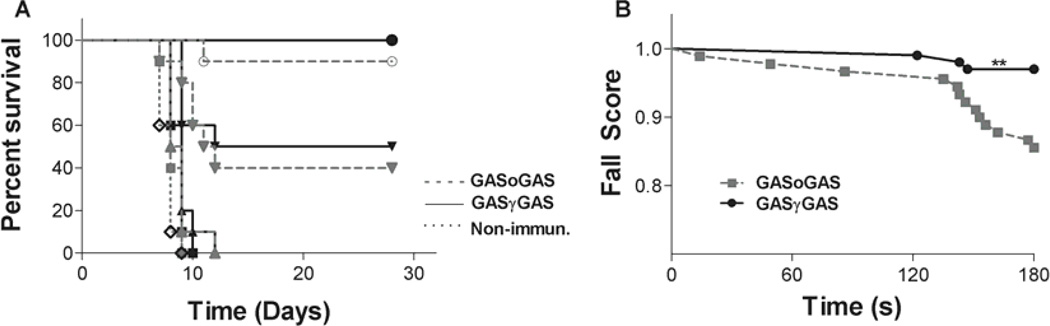
GASγGAS is a potentially more attractive candidate for a PET compared to GASγ. GASγGAS has an improved safety profile due, at least in part, to the presence of the second GAS gene (10). In order to control for the increased genome size and the production of additional virus-encoded proteins (IFNγ and GAS) by the host cellular machinery, we used GASoGAS as a control virus. As a model of post-exposure treatment, we infected groups of mice i.c. with a mixture of 103 ffu of DRV4 and 103, 104, 105 or 106 ffu of GASγGAS or GASoGAS (Fig. 6A). Survival curves show no significant differences in protection from DRV4 infection between GASγGAS and GASoGAS at any treatment dose (Fig. 6A). GASγGAS is effective at preventing death due to DRV4 infection, however, as 50% and 100% of mice treated with 105 or 106 ffu of GASγGAS survive, respectively. Interestingly, we observed qualitative differences in the behaviors of mice treated with GASγGAS versus those treated with GASoGAS. The GASoGAS-treated mice were very aggressive (especially unusual for female mice and a trait not observed prior to infection) and had tremors, whereas the GASγGAS-treated mice showed no unusual behavior. We used the hanging wire test to quantitate specific behavioral differences between mice treated with 106 ffu of GASγGAS or GASoGAS. The results show the GASoGAS-treated group has a significant deficit in motor neuromuscular function compared to the GASγGAS-treated mice as determined by the falls score of each treatment group (Fig. 6B).
Fig. 6. Mortality and morbidity after mixed infection.
Groups of mice were infected with a mixture of 103 ffu of DRV4 and 103–106 ffu of GASoGAS or GASγ and monitored for mortality and morbidity for 28 days. Surviving mice from the 106 ffu treatment groups were then tested for motor neuromuscular function by the hanging wire test. Survival curves are shown in A, and B represents the fall scores of the surviving mice. Significant difference between fall scores was determined by the Mantel-Cox test. **, p≤0.01. ■, 103 ffu;  , 104 ffu; ♦, 105 ffu; ●, 106 ffu; ◊, non-immunized control.
, 104 ffu; ♦, 105 ffu; ●, 106 ffu; ◊, non-immunized control.
DISCUSSION
Previous work demonstrating the importance of IFNγ during RABV clearance from the CNS (20–23) and during in vitro infection of antigen presenting cells (24) led us to hypothesize that overexpression of IFNγ by the RABV itself could attenuate pathogenicity, enhance immunogenicity and aid clearance of pathogenic RABV from the CNS. Furthermore, the clearance of other neurotropic viruses from the CNS is known to be IFNγ-dependent (25, 26) We recently demonstrated the attenuating effects of IFNγ expressed by an otherwise pathogenic RABV (D. A. Barkhouse, et al., submitted for publication), but this virus retains residual pathogenicity in adult mice that precludes its use as a safe PET. Therefore, we constructed next-generation IFNγ-expressing RABVs, GASγ and GASγGAS, using the highly attenuated GAS and GASGAS backbones proven to be non-pathogenic in adult mice (9, 10, 27).
We confirmed previous work showing that the highly attenuated GASGAS virus we used as a control for many experiments is, indeed, lethal for five-day-old suckling mice (6). Interestingly, the addition of IFNγ to the GAS backbone makes GASγ significantly more attenuated in suckling mice compared to the GASGAS control virus. This finding is notable because attenuated RABVs containing two glycoprotein (G) genes are less pathogenic and more immunogenic than their single G counterparts owing to G protein overexpression (27, 28). Therefore, RABV-expressed IFNγ can attenuate the virus to a higher degree than overexpression of G protein alone. We further show that these two attenuating modification are additive in that insertion of the IFNγ gene to the GASGAS backbone virus creates a vaccine strain, GASγGAS, which is even more attenuated than GASγ. While it is evident that IFNγ expression by GASγ strongly attenuates the virus, it is unclear whether the amount of IFNγ produced by the virus is ideal. It is conceivable that the greater attenuation of GASγGAS over GASγ in suckling mice may, at least in part, be a consequence of its more controlled production of IFNγ, as seen in culture. IFNγ expression can contribute to the pathogenesis of certain CNS viral infections but is more commonly found to be neuroprotective (reviewed in 29). Thus we consider that the synergy between increased IFNγ production and overexpression of attenuated G protein account for the decrease in pathogenicity of GASγGAS in suckling mice compared to GASγ.
The effect of IFNγ delivered in cis or in trans with various experimental pre-exposure vaccine vectors has been demonstrated previously. Expression of IFNγ by plasmid transfection at the site of vaccination against very virulent Marek’s Disease Virus (vvMDV) significantly protects chickens from tumor development as compared to control vaccination (30). Additionally, a Simian Immunodeficiency Virus (SIV) expressing IFNγ suppresses viremia after lethal challenge, although it cannot prevent persistent infection with the virulent challenge strain (31). Similarly, we have shown that pre-exposure vaccination with GASγ, despite its lack of G protein overexpression, protects significantly more mice against lethal challenge than the control GASGAS vaccination. More importantly, however, GASγ protects mice from death due to challenge as opposed to simply limiting aspects of morbidity. The significantly greater protection offered by GASγ occurs at low dosage and the effect diminishes as pre-exposure dosage increases. A larger challenge virus dosage may be required to determine if higher doses of GASγ are similarly more efficacious than GASGAS at the lower dose, as there appears to be a threshold level of protection that is attained by both GASγ and GASGAS as we increase the vaccine dose.
While enhancing the safety and efficacy of live-attenuated pre-exposure vaccines will assist in the efforts to eradicate terrestrial rabies and has the potential to make rabies vaccination more affordable and accessible, the primary goal of this research is to determine the feasibility of using a live-attenuated virus as late post-exposure treatment. Currently, there is no universally effective treatment for clinical rabies and even the success of the Milwaukee Protocol (32) is contested (33). We used mixed i.c. infection as a model of late PET since the treatment and challenge viruses reach the brain simultaneously. As with pre-exposure treatment, GASγ protects significantly more mice than GASGAS during i.c. mixed infection with lethal DRV4 at the relatively low treatment dose of 104 ffu. When higher doses of 105 and 106 ffu are used, both treatment viruses protect equally well. As proposed above, a larger dose of challenge virus may be necessary to discriminate between GASγ and GASGAS efficacies at higher treatment doses. Thus we have demonstrated the superior efficacy of an IFNγ-expressing RABV as pre- and post-exposure treatment over a highly attenuated control vaccine.
The lowest tested dose of both GASγ and GASGAS (103 ffu) protects relatively few mice from DRV4 mixed i.c. infection. While 103 ffu of pre-exposure virus has time to replicate, spread and induce sterilizing immunity, this dose is unlikely to have sufficient initial antigenic load to rapidly induce the level of immunity required for PET. Doses as much as 107–109 ffu of live-attenuated virus have previously been used for successful PET (6, 34) while 105 ffu was effective here. These types of experiments often compare the efficacy of live-attenuated RABV to inactivated RABV vaccine as well (28, 34). We show that relatively low doses of IFNγ-expressing RABV, GASγ, used for pre- and post-exposure treatment are highly efficacious and better protect mice than the already established vaccine vector, GASGAS. IFNγ-expressing RABV vectors have many potential advantages over traditional live-attenuated RABV vaccines. First, the lower effective dose equates to less cost per treatment, whether being used as a pre-exposure vaccine or PET. Lowering the cost of RABV vaccines will make vaccinating and treating humans and dogs a more attainable goal in regions of the world with very limited financial resources. PET with a lower effective dose of vaccine also has the potential to limit the chance of the treatment vector contributing to CNS pathology. Finally, the rapid production of IFNγ by a RABV vector induces an innate response earlier than similar vectors not expressing IFNγ, potentially lengthening the window of successful PET.
GASγGAS is potentially a better PET candidate due to overexpression of G protein as well as the dominant negative effect of a second copy of the G gene with respect to reversion. To control for the increased genome size and the potential host cell machinery stress due to additional expression of virus-encoded genes (GAS and IFNγ), we used GASoGAS as the control for i.c. mixed infection with GASγGAS. We found no significant difference in the ability of GASγGAS to protect mice from lethal DRV4 infection at any treatment dose. The additional attenuation of the GASGAS backbone compared to GAS may result in GASγGAS being able to replicate and spread less efficiently in the brain, thereby attenuating the virus too much to be effective PET. For the same reason, the GASγGAS virus may express less IFNγ in the CNS, as seen in BSR cells. Alternatively, a larger challenge dose may be needed to discern any differences in efficacy between GASγGAS and GASoGAS. We did observe neurological differences between GASγGAS and GASoGAS at the106 ffu dose. Neurological sequelae were reduced in the GASγGAS treated group as determined by the hanging wire test. Therefore, GASγGAS shows improved efficacy over its control in terms of reduction in motor neuromuscular deficit incurred during PET.
These data demonstrate that expression of murine IFNγ by an already highly attenuated RABV vector can enhance its safety profile in immunocompromised mice, improve its efficacy as a pre-exposure vaccine, better protect mice from lethal co-infection and reduce neurological sequelae after PET. Therefore, delivery of IFNγ by a live-attenuated vector has the potential to improve pre-exposure vaccine efficacy, but more importantly, may be able to act as late PET for those individuals who would otherwise die from rabies.
IFNγ expression improves attenuated rabies virus safety and immunogenicity
IFNγ expression is safer and more immunogenic than doubling glycoprotein expression
Co-infection with IFNγ-expressing RABV prevents wild-type rabies virus lethality
Vaccine safety and efficacy is additive for IFNγ and double glycoprotein expression
ACKNOWLEDGMENTS
We would like to thank Bernhard Dietzschold for critical review of the manuscript as well as Emily Bongiorno and Samantha Garcia for productive discussions.
This work was supported by the National Institutes of Health grants R01AI093369 and U01AI083045 (to DCH) and R01AI093666 (to MF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Martinez L. Global infectious disease surveillance. Int. J. Infect. Dis. 2000;4:222–228. doi: 10.1016/s1201-9712(00)90114-0. [DOI] [PubMed] [Google Scholar]
- 2.Knobel DL, Cleaveland S, Coleman PG, Fèvre EM, Meltzer MI, Miranda MEG, Shaw A, Zinsstag J, Meslin F-X. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 2005;83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 3.Franka R, Wu X, Jackson FR, Velasco-Villa A, Palmer DP, Henderson H, Hayat W, Green DB, Blanton JD, Greenberg L, Rupprecht CE. Rabies virus pathogenesis in relationship to intervention with inactivated and attenuated rabies vaccines. Vaccine. 2009;27:7149–7155. doi: 10.1016/j.vaccine.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Joseph J, N S, Khan AM, Rajoura OP. Determinants of delay in initiating post-exposure prophylaxis for rabies prevention among animal bite cases: hospital based study. Vaccine. 2013;32:74–77. doi: 10.1016/j.vaccine.2013.10.067. [DOI] [PubMed] [Google Scholar]
- 5.Baer GM, Shaddock JH, Williams LW. Prolonging morbidity in rabid dogs by intrathecal injection of attenuated rabies vaccine. Infect. Immun. 1975;12:98–103. doi: 10.1128/iai.12.1.98-103.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faber M, Li J, Kean RB, Hooper DC, Alugupalli KR, Dietzschold B. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11300–11305. doi: 10.1073/pnas.0905640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Ertel A, Portocarrero C, Barkhouse DA, Dietzschold B, Hooper DC, Faber M. Postexposure Treatment with the Live-Attenuated Rabies Virus (RV) Vaccine TriGAS Triggers the Clearance of Wild-Type RV from the Central Nervous System (CNS) through the Rapid Induction of Genes Relevant to Adaptive Immunity in CNS Tissues. Journal of Virology. 2012;86:3200–3210. doi: 10.1128/JVI.06699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schutsky K, Curtis D, Bongiorno EK, Barkhouse DA, Kean RB, Dietzschold B, Hooper DC, Faber M. Intramuscular inoculation of mice with the live-attenuated recombinant rabies virus TriGAS results in a transient infection of the draining lymph nodes and a robust, long-lasting protective immune response against rabies. J. Virol. 2013;87:1834–1841. doi: 10.1128/JVI.02589-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faber M, Faber M-L, Papaneri A, Bette M, Weihe E, Dietzschold B, Schnell MJ. A Single Amino Acid Change in Rabies Virus Glycoprotein Increases Virus Spread and Enhances Virus Pathogenicity. Journal of Virology. 2005;79:14141–14148. doi: 10.1128/JVI.79.22.14141-14148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faber M, Faber M-L, Li J, Preuss MAR, Schnell MJ, Dietzschold B. Dominance of a Nonpathogenic Glycoprotein Gene over a Pathogenic Glycoprotein Gene in Rabies Virus. Journal of Virology. 2007;81:7041–7047. doi: 10.1128/JVI.00357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology. 1990;175:485–499. doi: 10.1016/0042-6822(90)90433-r. [DOI] [PubMed] [Google Scholar]
- 12.Schnell MJ, Mebatsion T, Conzelmann KK. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietzschold B, Morimoto K, Hooper DC, Smith JS, Rupprecht CE, Koprowski H. Genotypic and phenotypic diversity of rabies virus variants involved in human rabies: implications for postexposure prophylaxis. J. Hum. Virol. 2000;3:50–57. [PubMed] [Google Scholar]
- 14.Faber M, Bette M, Preuss MAR, Pulmanausahakul R, Rehnelt J, Schnell MJ, Dietzschold B, Weihe E. Overexpression of Tumor Necrosis Factor Alpha by a Recombinant Rabies Virus Attenuates Replication in Neurons and Prevents Lethal Infection in Mice. Journal of Virology. 2005;79:15405–15416. doi: 10.1128/JVI.79.24.15405-15416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macpherson I, Stoker M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- 16.Gomez CM, Maselli R, Gundeck JE, Chao M, Day JW, Tamamizu S, Lasalde JA, McNamee M, Wollmann RL. Slow-channel transgenic mice: a model of postsynaptic organellar degeneration at the neuromuscular junction. J. Neurosci. 1997;17:4170–4179. doi: 10.1523/JNEUROSCI.17-11-04170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafael JA, Nitta Y, Peters J, Davies KE. Testing of SHIRPA, a mouse phenotypic assessment protocol, on Dmd(mdx) and Dmd(mdx3cv) dystrophin-deficient mice. Mamm. Genome. 2000;11:725–728. doi: 10.1007/s003350010149. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Laboratory techniques in rabies. 4th ed. Geneva: World Health Organization; 1996. [PubMed] [Google Scholar]
- 19.Badiane FJ. Modification of the immune response to ovalbumin expressed by a rabies virus vector. Master’s thesis. Philadelphia, PA: Thomas Jefferson University; 2011. [Google Scholar]
- 20.Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J. Virol. 1998;72:3711–3719. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J. Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 22.Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-alpha is neither necessary nor sufficient. J. Immunol. 2007;178:7334–7343. doi: 10.4049/jimmunol.178.11.7334. [DOI] [PubMed] [Google Scholar]
- 23.Hooper DC, Roy A, Kean RB, Phares TW, Barkhouse DA. Therapeutic immune clearance of rabies virus from the CNS. Future Virol. 2011;6:387–397. doi: 10.2217/fvl.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, McGettigan JP, Faber M, Schnell MJ, Dietzschold B. Infection of monocytes or immature dendritic cells (DCs) with an attenuated rabies virus results in DC maturation and a strong activation of the NFκB signaling pathway. Vaccine. 2008;26:419–426. doi: 10.1016/j.vaccine.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin DE. Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunologic Research. 2010;47:123–133. doi: 10.1007/s12026-009-8143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson CE, Lawrence DMP, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 2002;76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faber M, Pulmanausahakul R, Hodawadekar SS, Spitsin S, McGettigan JP, Schnell MJ, Dietzschold B. Overexpression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune response. J. Virol. 2002;76:3374–3381. doi: 10.1128/JVI.76.7.3374-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Yang Y, Sun Z, Chen J, Ai J, Dun C, Fu ZF, Niu X, Guo X. A Recombinant Rabies Virus Encoding Two Copies of the Glycoprotein Gene Confers Protection in Dogs against a Virulent Challenge. PLoS ONE. 2014;9:e87105. doi: 10.1371/journal.pone.0087105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rottenberg M, Kristensson K. Effects of Interferon-γ on Neuronal Infections. Viral Immunology. 2002;15:247–260. doi: 10.1089/08828240260066206. [DOI] [PubMed] [Google Scholar]
- 30.Haq K, Elawadli I, Parvizi P, Mallick AI, Behboudi S, Sharif S. Interferon-γ influences immunity elicited by vaccines against very virulent Marek’s disease virus. Antiviral Res. 2011;90:218–226. doi: 10.1016/j.antiviral.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Giavedoni L, Ahmad S, Jones L, Yilma T. Expression of gamma interferon by simian immunodeficiency virus increases attenuation and reduces postchallenge virus load in vaccinated rhesus macaques. J. Virol. 1997;71:866–872. doi: 10.1128/jvi.71.2.866-872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willoughby RE, Jr, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, Chusid MJ, Rupprecht CE. Survival after treatment of rabies with induction of coma. N. Engl. J. Med. 2005;352:2508–2514. doi: 10.1056/NEJMoa050382. [DOI] [PubMed] [Google Scholar]
- 33.Jackson AC. Therapy of human rabies. Adv. Virus Res. 2011;79:365–375. doi: 10.1016/B978-0-12-387040-7.00017-2. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Franka R, Henderson H, Rupprecht CE. Live attenuated rabies virus co-infected with street rabies virus protects animals against rabies. Vaccine. 2011;29:4195–4201. doi: 10.1016/j.vaccine.2011.03.104. [DOI] [PubMed] [Google Scholar]