Abstract
The care for patients with cancer has advanced greatly over the past decades. A combination of earlier cancer diagnosis and greater use of traditional and novel systemic treatments has decreased cancer-related mortality. Effective cancer therapies, however, can result in short- and long-term co-morbidities that can decrease the net clinical gain by impacting quality of life and survival. In particular, cardiovascular complications of cancer treatments can have a profound impact on the health of cancer patients and are more common among those with recognized or unrecognized underlying cardiovascular diseases. A new discipline termed “cardio-oncology” has thus evolved to address the cardiovascular needs of cancer patients and optimize their care in a multidisciplinary approach. This review provides a brief introduction and background on this emerging field and then focuses on its practical aspects including: cardiovascular risk assessment and prevention before cancer treatment, cardiovascular surveillance and therapy during cancer treatment, and cardiovascular monitoring and management after cancer therapy. The content of this review is based on a literature search of PubMed between January 1, 1960, and February 1, 2014 using the search terms cancer, cardiomyopathy, cardiotoxicity, cardio-oncology, chemotherapy, heart failure, and radiation.
Introduction
Over the past decades, there has been a tremendous improvement in the survival rates of a number of cancers and a steady increase in the number of cancer survivors (Supplemental Figure 1 and Supplemental Table 1). As a result, an increasing number of cancer patients are now being followed not only by oncologists or hematologists but also by general practitioners. Cardiovascular complications are not uncommonly encountered in these patients with potentially profound impact on morbidity and mortality, and thus their recognition and management has become an important element in the overall care for cancer patients.1,2 Furthermore, there is an intriguing geographic overlap in the prevalence of cancer and cardiovascular disease (Supplemental Figure 2) and expansion of cancer therapies to more elderly individuals with a greater burden of co-morbidities.3-5 Hence, an increasing number of patients with pre-existing cardiovascular diseases are now being considered for cancer therapy, which adds another level of complexity. Involvement of cardiologists has thus become more and more advisable not only to most optimally manage cardiovascular complications of cancer therapy but also to assist in the overall care of cancer patients from the initial assessment to survivorship. This integrative approach has been termed “Cardio-Oncology”,6,7 and herein we will reflect on this emerging field. An overview of cancer therapy-induced cardiotoxicity is provided in the first part and practical steps to its evaluation, management, and prevention in the following parts. The content is based on a literature search of PubMed between January 1, 1960, and February 1, 2014 using the search terms cancer, cardiomyopathy, cardiotoxicity, cardio-oncology, chemotherapy, heart failure, and radiation.
Part 1: Chemotherapy and radiation therapy-induced cardiotoxicity
The armamentarium for the treatment of a variety of cancers has increased substantially over the past decades with a gradual change from a cell cycle kinetics-based approach to more specific targeting of crucial signaling pathway(s). In most cases, these are cell proliferation pathways, which are regulated by receptor and non-receptor tyrosine kinases, leading to the development of a wide range of inhibitors. The extent to which this would interfere with normal cardiovascular function has often not been well anticipated, but such “off target” effects have become clinically relevant and revealing with regards to the functional role of signaling pathways in the cardiovascular system. A comprehensive list of currently used cancer drugs with a propensity for cardiovascular toxicities is provided in Table 1, along with their, FDA-approved cancer indications. 8-22
Table 1.
Most commonly used chemotherapeutics with cardiotoxicity potential
| Chemotherapeutic class and agents | Cardiomyopathy incidence | Other types of cardio-vascular toxicity | Clinical use in cancer therapy |
|---|---|---|---|
| Anthracyclines | |||
| Doxorubicin8 | 3-26% | Myo-pericarditis, Cardiac arrhythmias, ECG abnormalities | Acute myeloid leukemia; Acute myelogenous leukemia, Chronic lymphocytic leukemia; Hodgkin's and Non-Hodgkin's lymphoma; Kaposi's sarcoma; Mycoises Fungoides; Thyroid cancer; Breast cancer; Ewing's sarcoma; transitional cell bladder cancer; multiple myeloma; Gastric cancer; prostate cancer; Lung cancer; Nephroblastoma |
| Epirubicin | 0.9-3.3% | Cardiac arrhythmias, ECG abnormalities, arterial embolism | Breast, esophageal, and gastric cancer |
| Idarubicin | 5-18% | ECG abnormalities | Acute myeloid leukemia |
| Mitoxantrone9 | 0.2-30% | Cardiac arrhythmias, ECG abnormalities, myocardial ischemia, hypertension | Acute nonlymphocytic leukemias, prostate cancer (multiple sclerosis) |
| Alkylating agents | |||
| Cyclophosphamide (high dose)8 | 7-28% | Peri-/myocarditis, cardiac tamponade, arrhythmias | Bone marrow transplant, bladder cancer; lung cancer; sarcomas, anal cancer; Myeloproliferative disorders Chronic myelogenous leukemias; |
| Ifosfamide8 | 17% | Arrhythmias, cardiac arrest, myocardial hemorrhage, myocardial infarction | Testicular cancer, cervical cancer, Hodgkin's and non-Hodgkin's lymphoma, Ewing sarcoma, osteosarcoma, soft tissue sarcoma |
| Busulfan10 | rare | Endomyocardial fibrosis, Pericardial effusion and tamponade, ECG changes, chest pain, hyper-/hypotension, thrombosis, arrhythmias | Chronic myelogenous leukemia, Hematopoietic stem cell (HSCT) conditioning regimen, Polycythemia vera and essential thrombocythemia |
| Mitomycin10 | 10% | Stomach or pancreas adenocarcinoma, anal carcinoma, bladder cancer | |
| Antimetabolites | |||
| Clofarabine8 | 27% | Arrhythmias, hypo-/hypertension, pericarditis/pericardial effusion | Acute lymphocytic leukemia |
| 5-Fluorouracil11 | 2-20% | Coronary vasospasm, Myocardial ischemia and infarction, arrhythmias, ECG changes incl. ventricular ectopy, hypotension | Advanced colon cancer, Anal cancer; Gastro-Intestinal cancers; Pancreatic cancer; Hepato-biliary cancers; Breast cancer; bladder cancer; Head and Neck cancers and as a radiation sensitizer in several tumors |
| Capecitabine11 | 2-7% | Coronary vasospasm, Myocardial ischemia and infarction, arrhythmias, ECG changes, thrombosis | Breast cancer; Advanced colon cancer, Anal cancer; Gastro-Intestinal cancers; Pancreatic cancer; Hepato-biliary cancers; |
| Cytarabine10 | Unknown | Pericarditis, chest pain (incl. angina) | Hodgkin's and Non-Hodgkin's lymphoma; Acute leukemia (myeloid and lymphocytic) |
| Platinum agents | |||
| Cisplatin10 | Rare | Arterial vasospasm, cardiac/cerebral/mesenteric/limb ischemia, hypo-/hypertension, arrhythmias | Lung cancer; bladder cancer; sarcomas; testicular cancer; ovarian cancer; head and neck cancer; metastatic breast cancer; cancer of unknown origin; esophageal cancer |
| Antimicrotubule agents | |||
| Vincristine10 | 25% | Hyper/-hypotension, myocardial ischemia and infarction, arrhythmias | Acute lymphocytic leukemia, Central nervous system tumors, Hodgkin's and Non-Hodgkin's lymphoma, Multiple myeloma, Ewing's sarcoma, ovarian cancer, small cell lung cancer, thymoma |
| Monoclonal antibody-based tyrosine kinase inhibitors (TKIs) | |||
| Bevacizumab | 1.7-3% | Hypertension, arterial and venous thromboembolism | Renal cancer, colorectal cancer, lung cancer |
| Trastuzumab8 | 2-28% | Hyper-/hypotension, arrhythmia, vascular thrombosis | HER2+ breast cancer, HER2+ gastric cancer |
| Pertuzumab12 | 3-7% | HER2+ breast cancer | |
| Alemtuzumab13 | Rare | Hypo-/hypertension, arrhythmia | Chronic lymphocytic leukemia; cutaneous T-cell lymphoma (CTCL); bone marrow transplantation |
| Small molecule TKIs | |||
| Dasatinib8 | 2-4% | Pericardial effusion, hypertension, arrhythmia, QT interval prolongation | Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia |
| Imatinib mesylate | 0.5-1.7% | Pericardial effusion and tamponade, anasarca, arrhythmias, hypertension, Raynaud's | Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia, gastro-intestinal stromal tumors, dermatofibrosarcoma protuberans, hypereosinophilic syndrome |
| Lapatinib14 | 1.5-2.2% | QTc prolongation, myocardial ischemia (Prinzmetal's) | Her2+ Breast cancer |
| Sunitinib16,17,15 | 3-15% | Hypertension, arterial and venous thrombosis, arrhythmias, aortic dissection, QTc prolongation | Renal cell cancer; pancreatic neuro-endocrine tumors (pNET); gastro-intestinal stromal tumors |
| Sorafenib18 | 4-28% | Hypertension, thrombosis, coronary vasospasm, myocardial ischemia/infarction | Renal cell cancer, hepatocellular carcinoma, differentiated thyroid carcinoma |
| Pazopanib18,19 | 7-13% | Hypertension, thrombosis, myocardial ischemia/infarction, bradycardia, QTc prolongation | Renal cell cancer, soft tissue sarcoma |
| Proteasome inhibitor | |||
| Bortezomib8 | 2-5% | Ischemia, bradycardia | Multiple myeloma; mantle cell lymphoma |
| Miscellaneous | |||
| All trans-retnoic acid10 | 6% | Hypotension, Pericardial effusion | Acute myeloid leukemia (Promyelocytic leukemia) |
| Pentostatin10 | 3-10% | Myocardial ischemia and infarction, acute arrhythmias | Hairy cell lymphoma, Chronic lymphocytic leukemia, Cutaneous T-cell lymphoma |
| Interferon alpha-2b20 | 25% | Hypotension, myocardial ischemia and infarction, ECG changes, sudden cardiac death | Metastatic melanoma; renal cell carcinoma |
| Aflibercept21,22 | 1-6.8% | Hypertension, myocardial ischemia/ infarction, stroke | Metastatic colorectal cancer |
Considering the spectrum of cardiovascular effects, a distinction can be made between those agents that primarily affect cardiac function (e.g. anthracyclines and trastuzumab), vascular function (e.g. 5-fluorouracil and capacitabine), or both (e.g. bevacizumab and sunitinib). Radiation therapy leads to an all-encompassing form of injury to the myocardium, the pericardium, the valvular apparatus, and the coronary vasculature from epicardial to microvascular level, though modern approaches appear to reduce cardiovascular damage compared to older techniques. The focus herein will be on cardiotoxicity, and vascular toxicities will be discussed only as much as they relate to this topic.
Chemotherapy-related cardiotoxicity
In order to organize the broad spectrum of cardiotoxicity due to chemotherapy, an operational classification system was introduced by Ewer and Lippman (Supplemental Table 2).23 This system is based on the presence of structural abnormalities and extent of functional reversibility. Accordingly, a distinction can be made between an injury type (type 1 chemotherapy-induced cardiotoxicity) and a dysfunction type (type 2 chemotherapy-induced cardiotoxicity). Given that cardiac magnetic resonance imaging (MRI) has provided evidence for scar formation in patients with presumed type 2 cardiotoxicity, and appropriate heart failure therapy led to improvement of presumed type 1 cardiotoxicity, the outlined classification pattern may not be as much of an absolute as perceived.24,25 Also, one has to be cognisant of the fact that there is no consensus definition of cardiotoxicity at present.26,27 The one used in recent time was developed by the Cardiac Review and Evaluation Committee of trastuzumab-associated cardiotoxicity (CREC) and defines chemotherapy-related cardiotoxicity as a reduction of left ventricular ejection fraction (LVEF) ≥5% to <55% in the presence of symptoms of heart failure (diagnosed by a cardiologist), or an asymptomatic reduction of LVEF ≥10% to <55%.28
Chemotherapy-related cardiotoxicity type 1
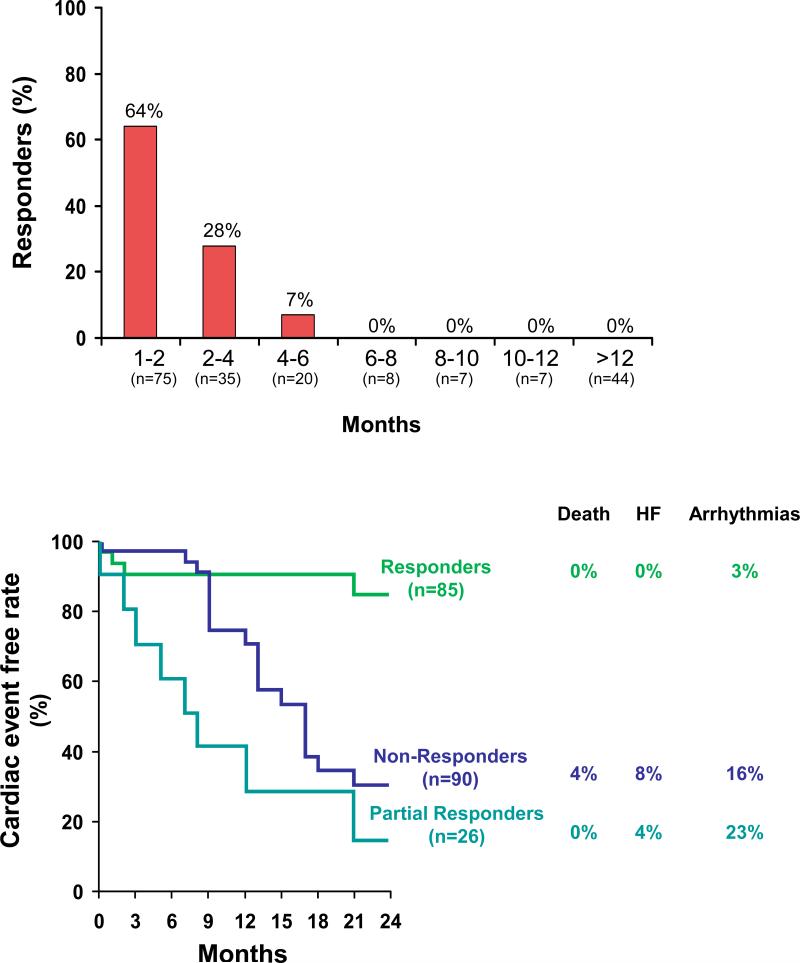
Induction of cardiomyocyte injury is a key distinguishing feature of this type of chemotherapy-induced cardiotoxicity with anthracyclines as the prototype class of drugs in this category. Given the imposition of structural changes, it has become widely accepted that this type of cardiotoxicity is not reversible. Moreover, anthracycline-induced cardiomyopathy has been considered to be associated with a prognosis that is worse than that for ischemic or dilated cardiomyopathies and possibly even for the primary cancer for which it was given.29,30 These views have been refined by the observation of reversibility as a function of timely institution of appropriate therapy.25 While anthracycline-induced cardiomyopathy was found to be rarely and never fully reversible when recognized and treated late, resolution can be noted with close surveillance and prompt institution of therapy early on (Figure 3).25
Figure 3.
Illustration of the percentage of patients with improvement of left ventricular ejection fraction (LVEF) to >50% depending on the timing of initiation of heart failure therapy after diagnosis of anthracycline-induced cardiomyopathy (upper panel) and survival free of cardiac events for patients with improvement of LVEF below (partial responders) or above 50% (responders) (From Journal of the American College of Cardiology,25 with permission).
Traditionally, a distinction has been made between an acute and a chronic form of anthracyline-induced cardiotoxicity. The acute from develops at the time (or within 1 week) of administration of anthracyclines and resembles an acute toxic myocarditis with myocyte damage (pyknotic debris), inflammatory infiltrates, and interstitial edema.31 It manifests primarily with ECG changes (20-30%) and arrhythmias (up to 3%), and occasionally with reversible cardiac dysfunction, even acute heart failure and peri-/myocarditis. The aforementioned clinically much more recognized type is the chronic form of anthracycline-induced cardiotoxicity with early onset within one year or late onset more than one year after completion of therapy.32 This type is marked by cardiac dysfunction rather than electrocardiographic abnormalities. On histology, cytoplasmic vacuolization due swelling of the sarcoplasmic reticulum and mitochondria can be noted, as well as disruption of organelles, myocyte death, myofibrillar loss, and/or myofibrillar disarray.31,33 Studies on the exact mechanisms responsible for the cardiotoxicity of anthracyclines have remained without a unifying explanation. The prevailing theory has been the “iron and free radical hypothesis”.34 Accordingly, reductases in cardiomyocytes catalyze the addition of an electron to the quinone moiety of anthracyclines, which leads to the formation of a semiquinone that then regenerates the quinone state by reducing molecular oxygen to superoxide anion and its dismutation product hydrogen peroxide (so-called “redox cycling”).34 The propensity towards toxicity is greatly enhanced when these products interact with low-molecular iron generating a surge of oxidative stress (Fenton’s reaction). Oxidative modification of proteins, lipids, and genomic and mitochondrial DNA damage are the downstream consequences.34,35 Uncoupling of the electron transport chain with impairment of oxidative phosphorylation and ATP synthesis contributes further to mitochondrial dysfunction and damage.36,37 Finally, inhibition of topoisomerase 1-β in cardiomyocytes has recently been proposed as an additional, if not key, mediator of anthracycline-induced cardiomyopathy.38 Anthracyclines thereby induce DNA damage and impair its repair, which are the very mechanisms responsible for tumor cell death. In crucial distinction, however, it is inhibition of topoisomerase 1-[.alpha] in cancer cells.37
Other drugs that cause structural damage to the heart include alkylating agents such as cyclophosphamide at high doses (Table 1).39,40 The pathology is that of an acute myopericarditis that does not take the course of chronic injury, and hence would not meet all criteria for a type 1 pattern. Similarly, the tyrosine kinase inhibitor sunitinib manifests cardiotoxicity that meets some but not all of the above criteria and has, in fact, been considered to represent a type II pattern. 41,42 Histology is not completely unremarkable and shows cardiomyocyte hypertrophy with mild degenerative changes and myocyte vacuolization.15 However, edema, inflammation, regional infarct or focal cell necrosis, or fibrosis are not seen.15 Transmission electron micrography (EM) shows normal sarcomere structure but does provide evidence of mitochondrial injury.15 Mechanistically, sunitinib inhibits AMP-activated protein kinase, which interferes with the ability of the cardiomyocyte to adapt to energy demands with untoward consequences on cardiac function.43,44 Other studies furthermore suggest that inhibition of the platelet-derived growth factor (PDGF) receptor pathway and the vascular endothelial growth factor (VEGF) receptor pathway leads to hypoxia, hypoxia-inducible genes, and a pattern of myocardial hibernation rather than infarction.37 This may explain reversibility of sunitinib cardiomyopathy in most but not all patients.17
Chemotherapy-related cardiotoxicity type 2
This type of treatment-induced cardiotoxicity is marked by the absence of structural abnormalities and is reversible upon cessation of therapy. Trastuzumab (Herceptin) is the prototype drug, and the key pathophysiological mechanism is interference with the HER-2/ErbB2-regulated signaling pathways in cardiomyocytes.45 These are key stress, adaption, and survival pathways, which explains why the incidence of cardiotoxicity is extremely high when trastuzumab is given in close temporal relationship with anthracyclines.42 Furthermore, mice with a cardiac-specific deletion of the ErbB2 receptor develop a dilated cardiomyopathy and are unable to adapt to and tolerate high afterload (blood pressure) challenges.45,46 As not all HER-2/ErbB2 pathway inhibitors, however, share the same potential for cardiomyopathy, the ultimate consequences must be dictated by the net effect these agents have on multiple downstream targets.14
Another example of a drug that can cause type II treatment-induced cardiotoxicity is bevacizumab, which is used for the treatment of metastatic kidney cancer, metastatic colon cancer, non-squamous non-small cell lung cancer, and glioblastoma multiforme. In distinction from previously mentioned drugs, the cardiomyocyte does not appear to be the primary culprit. Rather, bevacizumab binds to and prevents VEGF from interacting with its receptor(s), which reside primarily on endothelial cells. Still, cardiotoxicity including clinical heart failure has been reported with this medication.47 Reversibility of cardiac dysfunction has been noted suggesting that no structural damage to the cardiomyocytes is induced, though histological confirmation is lacking.48 The underlying mechanisms likely include interference with endothelial function, impairment of endothelial-myocardial coupling, and capillary rarefaction.37 Indeed, mice with cardiomyocyte-specific deletion of VEGF develop hypovascular, non-necrotic cardiac contractile dysfunction.49
Radiation therapy-related cardiotoxicity
Radiation therapy entails the utilization of high-energy particles, X-rays, or gamma rays that fragment cellular DNA and thereby interfere with cell proliferation and viability. This impacts cancer cells in particular given their high metabolic and proliferation index. The impact on normal cells and tissues is related to their particular susceptibility and the extent of ionizing radiation exposure. For instance, radiation therapy to the chest can harm the cardiovascular system, and even more so if doses exceed 30 Gy.50 This had been the case with mantle field radiation and is still the case with involved field radiation therapy (IFRT) in patients with Hodgkin's lymphoma (up to a total dose of 20-35 Gy). Doses for adjuvant radiation therapy in breast cancer patients can be even higher (in the order of 45 to 50 Gy). Modifications of radiation protocols, careful radiation field planning, and techniques such as breath holding have been implemented to reduce the radiation dose to the cardiovascular structures. As outlined in recent studies and not without controversy, however, there may not be a threshold level below which radiation therapy is safe to the heart and the vascular system.51-53 From a current practice standpoint though, radiation-induced heart disease remains of significance as most patients seen today are those who had higher exposures 20 to 30 years ago.2,51,54
Radiation therapy induces a spectrum of cardiotoxicities that differ considerably from chemotherapy-related cardiotoxic effects and affect all layers of the heart. Acute pericarditis used to be the most frequent complication but advances reduced its incidence from 25% to 2%.2 Chronic pericarditis still develops with a clinical incidence of 3% at 20 years and 12% at 30 years in those who underwent chest radiation at a dose of ≥35 Gy.55 Fibrinous exudates, fibrous adhesions, and collagenous thickening (predominantly of the parietal pericardium) are characteristic features.56 Similar fibrotic changes can be noted in the endocardium and the valve apparatus, initially causing retraction and regurgitation and over time (>20 years) also stenosis, especially of the left-sided valves.57 Diffuse interstitial fibrosis as well as thickening and narrowing of arterioles and capillaries are characteristic changes in the myocardium.56 Capillary rarefaction has also been noted, and injury to the endothelium is considered an integral part of radiation-induced heart disease. The theory has been that microvascular insufficiency leads to ischemia and cardiomyocyte death with replacement fibrosis; however, this has not been substantiated by histological observations.58,59 Instead, barrier breakdown of the endothelium with micro-hemorrhage and aggravation of (radiation-induced) oxidative stress and inflammation seems to be an important pathomechanism.60 Furthermore, extravasation of albumin can lead to amyloid formation and predisposition to sudden cardiac death. These very recent observations add another dimension to the restrictive cardiomyopathy phenotype observed after radiation therapy. They may also have important implications for early detection of radiation-induced cardiotoxicity and the identification of patients at risk of malignant cardiac arrhythmias.61 Conceivably, radiation therapy may lead to more tissue fibrosis and substrate for magnetic resonance imaging.52,62 This has not been the case for anthracycline-based chemotherapy though, irrespective of induction of histological changes and impairment in ventricular function.52,62,63 How the combination of anthracycline-based chemotherapy and radiation therapy influences these aspects remains to be defined. Alterations in cardiac function and valve disease are most profound with combined therapies.55,57 On the other hand, the incidence of radiation-induced pericardial disease is not increased by concomitant anthracycline therapy and only limited, inconclusive data are available for coronary artery disease (CAD).57,58
Given the sensitivity and vulnerability of endothelial cells, it is not surprising that radiation therapy induces and accelerates atherosclerosis.64-66 A dose correlation has been noted and hence coronary artery segments with the highest degree of exposure are at greatest risk of disease. For mantle and even involved field radiation, this is the proximal left main and right coronary artery (RCA).67,68 For left- and right-sided breast cancer radiation, this is the mid left anterior descending artery (LAD) and proximal to mid RCA, respectively.69 Clinically, these changes emerge after 15 years with an increase in the incidence of myocardial infarctions.55 An intriguing aspect is that radiation therapy has a long-reaching impact despite a very confined exposure period. This points to the induction of a smoldering process that continues after the initiating stimulus, and similar to the mechanisms discussed for anthracycline-induced cardiomyopathy, this may be mitochondrial DNA damage and perturbed mitochondrial function.70,71 Another unique factor could be vasa vasorum compromise.33,72 DNA damage and activation of key pro-inflammatory pathways such as the NFκB pathway has been pointed out for traditional cardiovascular risk factors (CVRF) as well, which may explain why traditional CVRF can influence the type and extent of atherosclerosis after chest radiation.51,57,62,71,73-75
Part 2: Evaluation for Cancer Therapy-Induced Cardiotoxicity
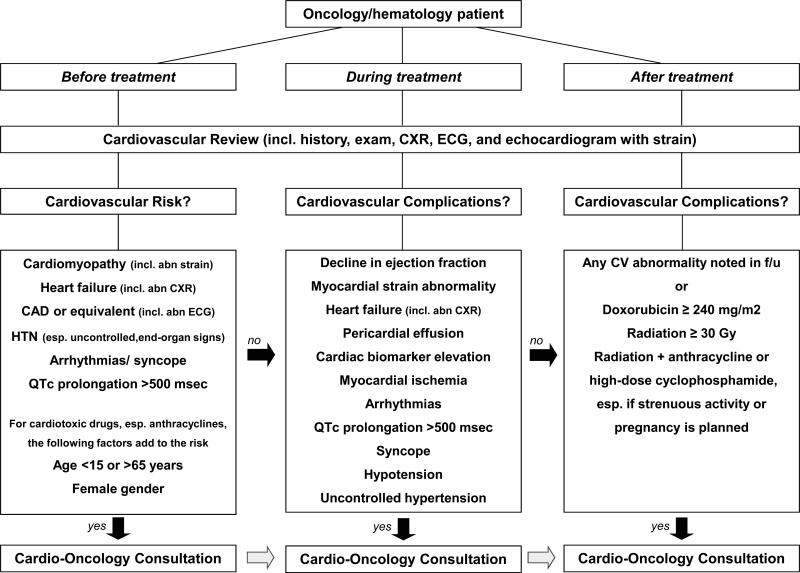
A multidisciplinary approach incorporating cardiology and oncology expertise is needed to evaluate and manage short- and long-term effects of cancer treatments enumerated above.7 The following sections are devoted to discuss the principles of practice of cardiooncology before, during, and after chemo- and/or radiation therapy (Figure 1).
Figure 1.
Outline of a general Cardio-Oncology algorithm. CAD = coronary artery disease; CXR = chest x-ray; ECG = electrocardiogram; HTN = hypertension.
Cardiovascular Evaluation of Patients Before and During Cancer Therapy
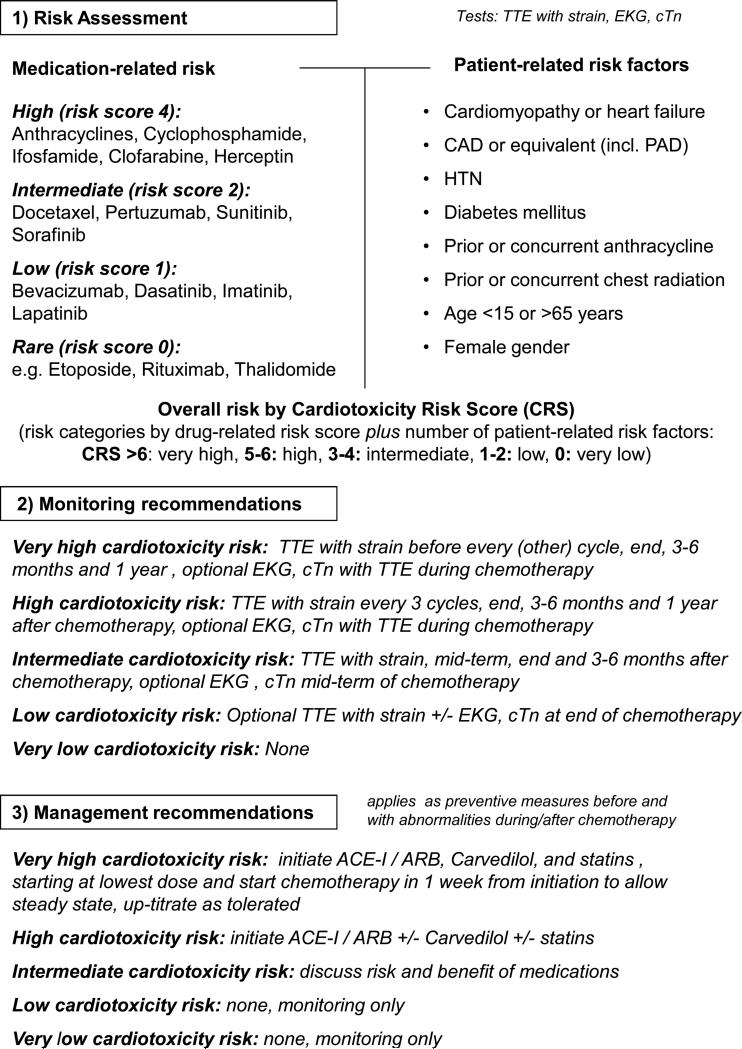
Before initiation of cancer therapy, a thorough patient history and physical examination should be taken to determine the baseline cardiovascular risk. Traditionally there has been considerable variability in the use of adjunctive tests such as ECG or echocardiography, influenced mainly by the cardiotoxicity profile of the planned treatment regimen and individual practice styles. However, it is advisable to standardize the assessment of cancer patients and to stratify them in their cardiotoxicity risk profile routinely. Such an approach allows for a universal standard of care for all patients, facilitates communication across disciplines, and aids in treatment decisions and follow-up planning. Recent meta-analyses support operational models that incorporate underlying patient-related risk factors.76 However, full assessment should also include the cardiac toxicity potential of cancer therapies (as suggested in Figure 2) and the anticipated therapeutic benefit of the anti-cancer regimen. Whether and which additional tests would further refine this risk assessment remains unanswered at present.
Figure 2.
Outline of a putative risk assessment, monitoring, and management model for patients undergoing chemotherapy. The central concept is that patient- and medication-related risk factors can be used to generate an overall risk score that can then be used to determine monitoring intervals and thresholds for preventive strategies. Such models need to be accounted for the fact that not all chemotherapeutics and patient-related risk factors weigh the same, and hence the ultimate prediction models will need to be more stratified and will need to be verified.
ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CAD = coronary artery disease; cTn = cardiac troponin; CXR = chest x-ray; ECG = electrocardiogram; HTN = hypertension; IFN-α = interferon-alpha; TTE = transthoracic echocardiogram.
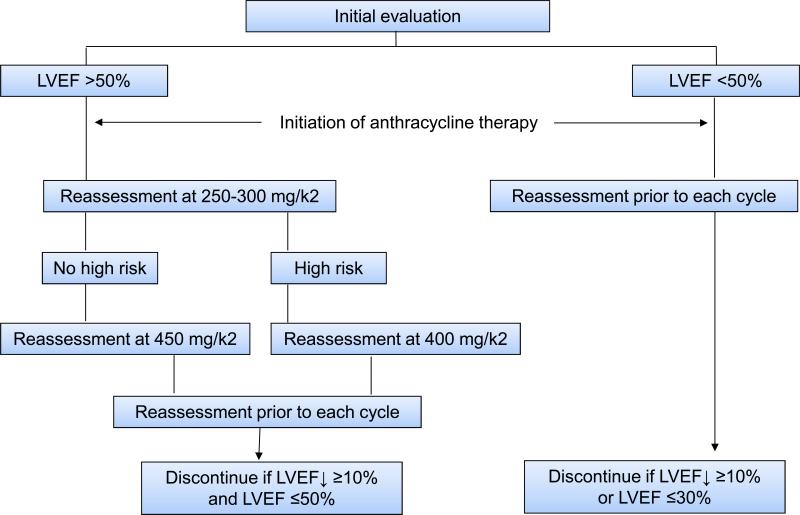
Once a patient has started treatment, it must be further decided which patients require cardiovascular follow-up. This depends on the baseline cardiovascular risk profile, the specific cancer treatment regimen, and the development of cardiac symptoms and/or events. Monitoring protocols were developed and validated for patients undergoing anthracycline-based therapy in the 1970s and 1980s (Figure 4).77 These were based on radionucleotide angio- (ventriculo-) gram (RNA, also known as multiple-gated acquisition or MUGA scan) and the pivotal observation that a decline in LVEF noted by this technique would precede clinically overt heart failure.78,79 Importantly, adhering to this protocol led to significantly better clinical outcomes.80
Figure 4.
Established algorithm for the monitoring of patients undergoing anthracycline-based chemotherapy (Adapted from The American Journal of Medicine,80 with permission. A variation to this algorithm is the reassessment prior to each cycle after 250-300 mg/kg2 in those at high risk. ECG = electrocardiogram; LVEF = left-ventricular ejection fraction; RNA = radionuclide angiography; TTE = transthoracic echocardiogram.
RNA is operator-independent and highly reproducible but has been largely replaced by echocardiography. Historically, variability of LVEF by 2D transthoracic echocardiography (TTE) has been reported as high as 10%. However, LVEF assessment by contrast-enhanced 2D TTE or 3D TTE is superior in this regard and comparable to RNA and cardiac MRI.81-84 As such, the algorithms validated for RNA may apply to TTE imaging using these newer echocardiographic techniques. Widespread availability, feasibility, lack of radiation exposure, and acquisition of additional cardiac imaging information (valvular, pericardial, and hemodynamic data) make echocardiography a very attractive option for serial imaging, even though service fees might be higher.
Of particular interest is strain imaging, which is a measure of regional deformation of the myocardium. It is currently mainly obtained by angle-independent 2-Dimensional speckle tracking echocardiography (2D-STE), which can evaluate all three domains of myocardial mechanics (longitudinal, circumferential and radial) and derive data for deformation and rate of deformation for each myocardial segment.85 2D-STE has been used in multiple independent studies, demonstrating changes in cardiac (mechanical) function before a drop in LVEF and even before changes in diastolic function after chemotherapy.86-89 The degree of change in strain imaging is quite consistent across different laboratories and studies, and a greater than 10% change in global longitudinal strain after completion of anthracycline chemotherapy relative to baseline is predictive of a future drop in LVEF.86,90 Importantly, these (high risk) changes can be noted in up to 70% of patients.86,90 Conceivably, but subject to further studies, abnormal strain values before cancer therapy may signal higher baseline risk for chemotherapy-induced cardiotoxicity.
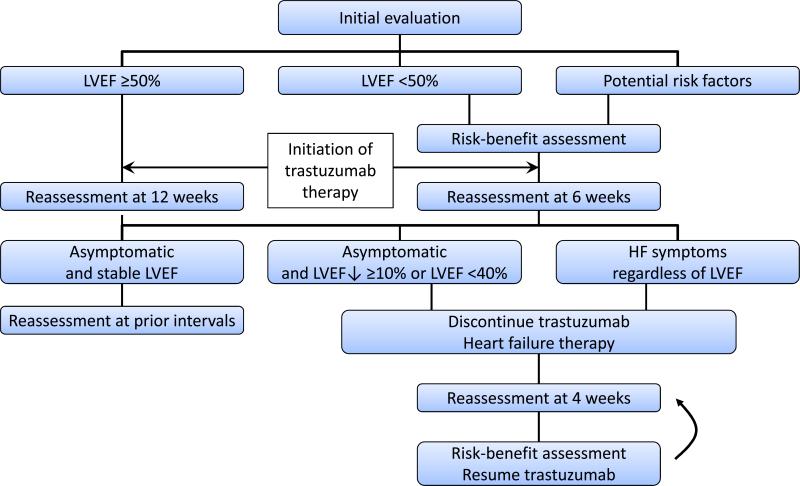
Based on the above, it seems appropriate to include strain imaging in monitoring algorithms for cardiotoxicity. The dynamics after a cumulative dose of 200 mg/m2 of doxorubicin may be particularly instructive and have been suggested to serve as a bench mark with reassessment after each additional 50-100 mg/m2 thereafter.42,91 After completion of therapy, reassessment is recommended at 6 and 12 months and as early as 3 months for those at highest risk, e.g. after doxorubicin equivalent doses >400 mg/m2.42 The aforementioned observation of clinical outcome as a function of time-to-treatment (Figure 3) would support expansion of these efforts to even earlier time points as would the outlined data on strain imaging.42,86 Importantly, how well changes in these imaging parameters predict the development of clinical heart failure has not been established. Also, how these algorithms should be modified for other drugs, for instance, trastuzumab, remains to be defined. Changes in strain values, however, have been found to precede changes in LVEF by a minimum of three months in this setting as well.88
One unique aspect with trastuzumab is the prolonged (one year) treatment duration. Algorithms are in place with LVEF as a central evaluation parameter; however, there is no guidance on how to interpret changes in strain imaging data over time (Figure 5). One may argue that recognition of abnormalities in these echocardiographic parameters should prompt initiation of effective cardiac therapies with continuation rather than suspension of cancer therapy as long as LVEF is preserved and signs and symptoms of heart failure are absent. Specific guidelines addressing the use of strain imaging in cancer patients will be released by the American Society of Echocardiography later in 2014. The current (2012) clinical practice guidelines by the European Society of Medical Oncology (ESMO) are summarized in Supplemental Tables 3 and 4.27
Figure 5.
Algorithm for the monitoring of patients undergoing Herceptin chemotherapy (Adapted from The Oncologist,137 with permission). ECG = electrocardiogram; EF = left-ventricular ejection fraction; RNA = radionuclide angiography (MUGA); TTE = transthoracic echocardiogram.
Regarding the use of circulatory biomarkers, only cardiac troponin (cTn) has stood the test of time while brain natriuretic peptides and C-reactive protein have not and emerging data on myeloperoxidase are yet to be confirmed.86,92,93 The replacement, additive, or synergistic role of cTn in the outlined monitoring algorithms also requires further investigation. With anthracycline-based protocols, cTn serum concentrations peak during and early after completion of chemotherapy but their predictive value for a future drop in LV function is not superior to strain imaging.86,94 With trastuzumab, new cTn elevation is mainly seen with the first or second cycle, and only early and persistent elevations seem to carry the greatest prognostic weight.95 These data are intriguing if trastuzumab is to lead only to myocardial dysfunction and not myocardial injury.
Cardiovascular Evaluation of Patients After Cancer Therapy
After completion of cancer therapy, follow-up recommendations are to be individualized according to the overall survival prognosis of the underlying malignancy, the specific anti-cancer therapy administered, each patient's unique cardiovascular risk and comorbidity profile, and whether they suffered adverse cardiac effects during therapy. Goals of management should be explained and managed together with the patient and other sub-specialists involved in the cancer care of the patient.
Serial, long-term post-exposure cardiac surveillance does not pertain to drugs that are associated with acute but not chronic injury patterns in the absence of any persistence of complications after completion of therapy. One example is cyclophosphamide, and even if acute cardiotoxicity were to occur, ongoing follow-up is not necessary following recovery. Similarly, cardiotoxicity has not been reported to develop late after completion of trastuzumab therapy, and hence there is no need to continue with cardiac monitoring after completion of therapy (which is typically for the duration of 12 months). The need for post-treatment cardiovascular follow-up is hence confined to only those patients who have ongoing cardiovascular disease processes or are at risk of late cardiotoxicity.
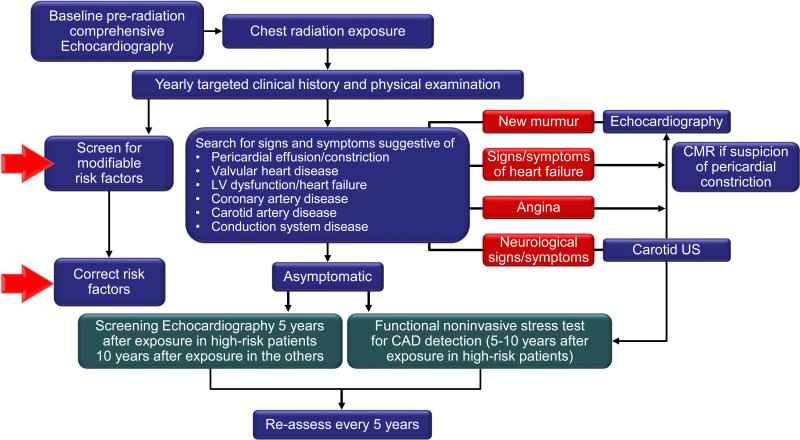
Breast cancer and lymphoma patients who have undergone anthracycline-based therapy and patients who have had mediastinal radiation therapy are prime candidates for long-term cardiac surveillance programs (ideally integrated into cancer survivorship programs). A recently published expert consensus statement from the European Association of Cardiovascular Imaging and the American Society of Echocardiography recommends evaluation based on signs and symptoms and echocardiographic surveillance starting 5 years after treatment in high-risk patients and 10 years in all other patients. High-risk patients should also receive a functional non-invasive stress test within 5 to 10 years of completion of chest radiation therapy (Figure 6).96
Figure 6.
Monitoring algorithm of patients after radiation therapy (From J Am Soc Echocardiogr, 96 with permission). US = ultrasound.
Intriguingly, even in the highest risk group of Hodgkin Lymphoma patients who received ≥35 Gy of radiation to the chest, treadmill exercise ECGs do not reflect the burden of CAD.97 Moreover, stress echocardiography and sestamibi can be quite discordant in their results - the former being more sensitive for abnormalities at rest, the latter being more sensitive for stress-related abnormalities.97 However, it should be noted that these results do not reflect the technological advances, which have been made, specifically as they relate to the use of ultrasound-enhancing contrast agents in stress echocardiography. Still, significant stenoses (>70%) are noted on coronary angiography much more frequently than suggested by stress tests, and left main disease alone is found in >10% of Hodgkin's disease patients more than 10 years after chest radiation therapy.97
As outlined above, the disease process may be more regional in patients undergoing radiation therapy for breast cancer and the yield of nuclear stress testing may thus be higher in these patients.98,99 For CAD evaluation after mediastinal radiation therapy, one must therefore consider the type and extent of therapy as well as baseline cardiac status along with advantages and limitations of various test modalities.50 Coronary computed tomography angiography (CCTA) is an appealing option but detection remains limited to those without significant coronary calcifications.100 Combination with perfusion imaging may help overcome these limitations and increases the yield of CCTA.101 Radiation exposure has been a concern but recently developed CT scanners have led to dose reduction. The role of cardiac MRI remains to be defined but it is an excellent method to reliably assess ventricular structure and function and can be used for perfusion stress imaging. However, it poses significant logistic and financial challenges. For detection of cardiomyopathy, there is evidence that stress tests that assess exercise capacity and reserve are of superior yield, unmasking otherwise unrecognized (subclinical) impairment.102,103
One subgroup of patients in need of particular attention are women with a history of chemo- or radiation therapy who are pregnant or are planning to become pregnant, as the pregnancy can unmask “smoldering” (subclinical) cardiomyopathy. The recommendation of the Children's Oncology Group (COG) is that these patients should be evaluated by a cardiologist if they received a cumulative anthracycline dose of ≥300 mg/m2, a radiation dose to the heart or surrounding tissues of ≥30 Gy, or any chest radiation plus an anthracycline or high-dose cyclophosphamide. These recommendations have been incorporated into the Mayo Clinic Lymphoma Survivorship program (Supplemental Table 5).
Part 3: Management of Cancer Therapy-Induced Cardiotoxicity
Management of patients who sustain cardiotoxicity during or after cancer therapy should be in keeping with the AHA/ACC heart failure guidelines.104 The efficacy of angiotensin converting enzyme (ACE) inhibitors as first line therapy was elegantly demonstrated in breast cancer patients who developed a significant drop in LVEF and heart failure following epirubicin-based chemotherapy. Neither diuretics nor digoxin but prompt institution of ACE inhibitor therapy restored LV systolic function.105 Maintenance therapy was necessary although duration remains undefined.105 While such data are not available for angiotensin receptor blocker (ARBs), these should still be considered in those with contraindications to ACE inhibitors. The precise role of aldosterone receptor antagonists (e.g. spironolactone) in the treatment of chemotherapy-induced cardiomyopathy is currently unknown but may be considered in those with NYHA class symptoms >1 and an LVEF ≤35%.104
Beta-blockers are the second main class of drugs for patients with chemotherapy-induced cardiomyopathy. This is supported by reports on the initiation of carvedilol after initial successful commencement of low-dose enalapril with up-titration of both drugs as tolerated in patients who were found to have an LVEF ≤45% after completion or during chemotherapy without any other identifiable cause.25 Over the course of 3 years, this combined intervention restored LVEF to >50% in 42% and partially improved it by 10% to <50% in another 13% of patients. Intriguingly, clinical outcome was equally poor in those with a partial response and the 45% of patients with no response (Figure 3).25 As outlined before, timing from completion of anthracycline-based chemotherapy to initiation of heart failure therapy was identified as the crucial determinant of the response rate (Figure 3).25 These observations substantiate the paradigm shift to early detection and early treatment of chemotherapy-induced cardiotoxicity.
Hemodynamic device support may become necessary if medical therapy fails. Acute support can be temporarily lifesaving as in those with acute myopericarditis due to cyclophosphamide.39,106 Alternatively, chronic left ventricular assist device (LVAD) support may become a bridge to transplantation or destination therapy as recently reported. 107,108 One unique aspect is the relatively high rate of biventricular failure in these patients compared with those receiving LVAD therapy for other indications.107 The most severe forms of heart failure are usually observed in patients receiving both chemotherapy and radiation therapy because of the profound injury and in those of very young age.109,110 Two key processes have been noted in children developing cardiotoxicity: reduction in contractility and/or increase in afterload. These have not been described in adults but may be of significance for the choice of therapy.110-112
At present it is unknown if the above outlined medical therapies are necessary for agents that cause type II cardiotoxicity. Initial studies on trastuzumab cardiomyopathy reported improved cardiac function after withholding therapy alone.113 This has remained the primary approach common to all published management algorithms for trastuzumab cardiotoxicity. However, HER-2 inhibition is a vital element in the treatment of breast cancer patients over-expressing this receptor, and thus “drug holiday” is of concern.114-116 It is currently undefined whether institution of cardioactive medication is necessary and would allow continuation of therapy without concerns for cardiac side effects. As the Akt/PKB signaling pathway is one of the key pathways affected by HER-2/ErbB2 signaling and is involved in trastuzumab cardiotoxicity, up-regulation of this pathway in the heart may be of merit. Intriguingly, statins and nebivolol induce potent upregulation of this pathway.117,118 Whether such strategies would be counterproductive to the anti-cancer effects of therapy, however, remains an unanswered question.
The treatment of patients with radiation-induced heart disease is challenged by the multiplicity of processes and the high propensity for surgical intervention over time. One aspect often not considered is the fact that radiation therapy to the chest increases the level of complexity for open heart surgery and risk of complications.54 The internal mammary artery (IMA) is often not a prime bypass conduit in these patients due to radiation damage. Furthermore, radiation-induced left subclavian artery disease may cause both a traditional steal phenomenon and flow limitation to the left IMA. Aortic calcification may impose significant challenges for aortocoronary (saphenous and arterial) bypasses. Assessment of the ascending aorta, aortic arch, and IMAs is recommended if these patients are considered for coronary artery bypass surgery.50 Given the surgical challenges in these patients, percutaneous coronary intervention might be preferred over C whenever possible.2 A “heart team” approach should be pursued and a comprehensive risk and benefit assessment should be made. As with patients with CAD in general, medical therapy remains the cornerstone of treatment. However, experimental data indicate that while radiation induces an inflammatory and thrombotic phenotype, conventionally used drugs such as statins and clopidogrel are not effective, though clopidogrel seems more favorable.119 Similarly, neither aspirin nor the newer nitric oxide-donating aspirin reduce the amount of plaque development in radiation-induced atherosclerosis despite efficacy for age-related atherosclerosis.119
Another important consideration is that these patients may require more than one open heart surgery (e.g. bypass surgery, valve replacement). Post-radiation mediastinal fibrosis poses a challenge at baseline and limits the number of redo surgeries. It is therefore advisable, if surgery is pursued, to address as many cardiac disease processes as comprehensively as possible in one operation and to use alternative interventions when possible until surgery becomes the only remaining option.
Furthermore, it is important to consider the propensity for constrictive pericarditis in these patients. If a patient is considered for pericardectomy, evaluation for the coexistence of restrictive cardiomyopathy is necessary since radiation therapy can also cause myocardial and endocardial fibrosis. Removal of the pericardium will unmask the presence of underlying restrictive cardiomyopathy without providing much symptom relief. Thus, hemodynamic catheterization is advised to differentiate between a primarily constrictive or restrictive process. Cardiac MRI might help to define the burden of cardiac fibrosis, especially in those with equivocal hemodynamic findings. It can also outline the pericardium, but in most cases there is no inflammation and these patients do not benefit from anti-inflammatory therapy.
A high level of clinical suspicion for arrhythmia needs to be maintained in patients after radiation therapy to the chest since interstitial fibrosis may lead to ventricular tachycardias and degeneration of the conduction system may cause bradyarrhythmias.2,57 Calcification of the aortomitral ridge is a characteristic clue for those at risk of heart block.120 Treatment of these ultimate complications should be in keeping with current guidelines.
Part 4: Prevention of Cancer Therapy-Induced Cardiotoxicity
The poor prognosis of cancer therapy-induced heart disease and the lack of a universal response to the institution of therapy argue for a preventive approach. This has to be based on the premise that the perceived benefit is greater than the perceived risk in terms of both, side effects and reduction of the anti-cancer effects. Ideally, these approaches should be supported by prospective randomized trials that also define target subsets of patients at varying levels of risk.
This has been the case for dexrazoxane, the drug with the best level of evidence for the prevention of chemotherapy-induced cardiotoxicity. Based on a meta-analysis of eight trials with more than 1,500 patients, dexrazoxane reduced the incidence of clinical heart failure by more than 80% (RR 0.18, 95% CI 0.10-0.32, p<0.0001).121 However, even though progression-free and overall survival rates were similar, there was a trend towards lower response rate.121 These and other observations have raised enough concerns that dexrazoxane may reduce the anti-tumor efficacy of the primary cancer therapy. For this reason, its FDA- and EMA-approved use is only for patients with metastatic breast cancer who may benefit from further anthracycline therapy after having already received 300 mg/m2.38
With regards to cardiovascular medications, the currently available evidence is summarized in Table 2.122-134 Two studies provide retrospective evidence for beta-blockers and statins but remain limited in therapy details.122,123 This is important as experimental studies have demonstrated that not all beta-blockers provide cardioprotection from chemotherapy-induced cardiotoxicity. Non-selective beta-blockers such as propranolol may, in fact, potentiate cardiotoxicity, likely related to inhibition of beta-2 activity.135,136 On the other hand, beta-blockers with proven evidence for cardioprotection in this setting include carvedilol and nebivolol whereas the effect of metoprolol appears neutral.124-128
Table 2.
Adjunctive pharmacological strategies for the prevention of chemotherapy-induced cardiotoxicity
| Study | Year | Cohort (n) | F/u time | Cardiotoxic chemotherapy | Radiation therapy | Preventive therapy | Cardiotoxicity definition | Outcome with vs. without prev. therapy |
|---|---|---|---|---|---|---|---|---|
| Observational studies | ||||||||
| Seicecan et al.122 | 2012 | Breast cancer (n=628) | 2.6±1.7 yrs | Anthracyclines | 66% | Any statin therapy during CT | Rate of new HF admission [%] | 6.0 vs. 17.2,a HR 0.3 (0.1-0.9) |
| Seicecan et al.123 | 2013 | Breast cancer (n=318) | 3±2 yrs | Anthracyclines +/− Herceptin | 59% | Any BB therapy during CT | Rate of new HF admission [%] | 4.7 vs. 12.7,a HR 0.2 (0.1-0.7) |
| Randomized controlled trials | ||||||||
| Kalay et al.124 | 2006 | Breast cancer (68%), Lymphoma (18%) | 6 mo. | Anthracyclines: Doxorubicin 520 mg/m2 or epirubicin 780 mg/m2 | 0% | Carvedilol 12.5 mg per day, started before CT and continued for 6 months | LVEF [%] | Carvedilol: no change, Control: significant decrease (68.9 to 52.3) |
| El-Shitany et al.125 | 2012 | Children with ALL (n=50) | 1 week after CT | Doxorubicin 120 mg/m2 | 0% | Carvedilol 0.1-1 mg/day, started 5 days before CT | FS [%] GPSS [%] cTnI [ng/mL] |
39.5±6.3 vs. 33.5±6.2a −19.3±2.0 vs. −15.1±1.8a 0.02±0.02 vs. 0.06±0.05a |
| Elitok et al.126 | 2013 | Breast cancer (n=80) | 6 mo. | Anthracyclines 520 mg/m2 | 0% | Carvedilol 12.5 mg/d started before CT and continued for 6 mo. | Peak systolic strain,septal [%] Peak systolic strain,lateral [%] LVEF [%] |
20±5.3 vs. 16±4.3a 18±5.6 vs. 14±6.1a 64±5.1 vs. 63±4.8 |
| Kaya et al.127 | 2013 | Breast cancer (n=45) | 6 mo. | Anthracyclines: Doxorubicin 246 mg/m2 or epirubicin 354 mg/m2 | 27% | Nebivolol 5 mg/day, started 7 days before CT and continued for 6 mo. | LVEF [%] NT-proBNP [pmol/L] |
63.8±3.9 vs. 57.5±5.6a 152±69 vs. 204±73a |
| Georgakopoulos et al. 128 | 2010 | HL and NHL (n=125) | 12 mo. 30 mo. |
ABVD R-CHOP |
21% | Metoprolol 25-50 mg/BID or Enalapril 2.5-10 mg/BID started with CT | HF LVEF decline >10% to <50% |
NS NS |
| Bosch et al. (OVERCOME trial) 129 | 2013 | Acute leukemia (n=36) or HSCT (n=54) | 6 mo. | Anthracyclines (40% before, 40% during, cumulative 265 mg/m2) | 18% | Carvedilol (6.25-25 mg BID) and Enalapril (2.5-10 mg BID), started 24h before CT and continued in f/u | LVEF [%], absolute change by TTE LVEF [%], absolute change by CMR | −0.17 vs. −3.28a 0.36 vs. −3.04 |
| Silber et al. (AAA study) 130 | 2004 | Ped. cancer survivors with ≥1 cardiac abnorm. in f/u (n=135) | 35 mo. | Anthracyclines 300 mg/m2 | 36% | Enalapril 0.05-0.15 mg/kg/d | FS [%] LVESWS [g/cm2] MCI [l/min/m2] |
NS NS NS |
| Cardinale et al. 131 | 2006 | HDC (n=114, 60% NHL and breast cancer) + cTnI >ULN within 3 days of any cycle | 12 mo. | Various, cumulative doxorubicin equivalent dose 335 mg/m2 | 11% | Enalapril 2-20 mg per day, started after cTnI elevation and continued in f/u | LVEF decline >10% to <50%, rate [%] HF rate [%] Arrhythmia rate [%] |
0 vs. 43a 0 vs. 24a 2 vs. 17a |
| Nakamae et al. 132 | 2005 | NHL (n=40) | Day 3 after initiation | CHOP | 0% | Valsartan 80 mg/d started and continued with CT | LVEDD [mm] BNP [pmol/L] QTc [msec] |
45 vs. 49a 30 vs. 80a 420 vs. 435a |
| Dessi et al. 133 | 2011 | Various (n=49, breast cancer 37%) | 12 mo. | Epirubicin 400 mg/m2 | 0% | Telmisartan 40 mg/d, 1 wk before −6 mo. after CT | Strain rate | 1.75 vs. 1.5a |
| Acar et al. 134 | 2011 | Various (n=40) | 6 mo. | Anthracyclines : doxorubicin 256 mg/m2, idarubicin 297 mg/m2 | NA | Atorvastatin 40 mg per day, started before and continued for 6 mo after CT | LVEF [%], absolute change LVEDD [mm], absolute change LVESD [mm], absolute change |
1.3 vs. −7.9a −0.15 vs. 2.0a −1.35 vs. 2.1a |
p<0.05; ABVD = Adriamycin, Bleomycin, Vinblastine, and Dacarbazine; ALL = acute lymphocytic leukemia; BB = beta-blocker; BID = twice a day; BNP = brain natriuretic peptide; CHOP = Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone; CMR = cardiac magnetic resonance imaging; CT = chemotherapy; cTnI = cardiac troponin I; F/u = follow-up; FS = fractional shortening; HF
Contrary to experimental data, randomized clinical trial data supporting the use of statins remain scarce with only one reported so far.134 In keeping with the reported benefit in the treatment of patients with chemotherapy-induced heart failure, ACE inhibitors have been the first ones tested and contended. The ACE inhibitor After Anthracycline (AAA) study in survivors of pediatric cancer did not find any significant long-term benefit.130 However, the qualifying enrollment criteria included a broad spectrum of cardiac abnormalities and treatment was not commenced until at least 2 years out from anthracycline therapy.130 Still, a prospective, randomized, controlled study comparing monotherapy with enalapril or metoprolol to placebo in patients undergoing ABVD or R-CHOP therapy for Hodgkin or Non-Hodgkin lymphoma, respectively, did not find any benefit in the prevention of clinical or subclinical cardiotoxicity.128 At the other end of the spectrum, confined to only those with cardiac troponin I elevation within 3 days of initiation of high-dose chemotherapy, initiation of enalapril therapy was of remarkable benefit.131 The surveillance intensity of such a protocol, however, may pose a barrier for general use even though it underscores the merit of defining a high risk-high yield patient population.
With regards to combination therapies, the preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies (OVERCOME) trial was conducted with universal consideration of enalapril and carvedilol for all patients referred for intensive chemotherapy or stem cell transplantation.129 The study remained positive in its primary endpoint of prevention of LVEF reduction at 6 months and even outlined a benefit in terms of the combined secondary endpoint of death or heart failure.129 No interaction was observed in terms of the primary endpoint and cTnI or BNP elevation; however, the LVEF benefits remained largely confined to patients with acute leukemia.129 This observation thus supports the limitation of preventive strategies to those patients at highest presumed risk of cardiotoxicity based on treatment-related and patient-related factors as outlined in the beginning (Figure 2). This is in keeping with the ESMO guidelines (Supplemental Table 3), which recommend ACE inhibitors as first-line agents, but as outlined above and summarized in Table 2, carvedilol, nebivolol, and statins should be considered as well.27 Obviously, patients already on these therapies should continue with them. The exception, however, as implied, is that efforts should be undertaken to switch patients from beta-blockers other than carvedilol and nebivolol to these specific agents given their evidence-based level of benefit for the prevention of chemotherapy-induced cardiomyopathy.
Summary
Advances in cancer therapy and the outcome limiting impact of cardiovascular side effects have generated a growing need for Cardio-Oncology. With this, the paradigm has shifted towards early recognition and treatment of cardiotoxicity and even pre-cancer therapy cardiovascular risk assessment and prevention. The key practical steps in the Cardio-Oncology approach outlined in this review comprise the following:
Step 1: Ongoing interaction between Cardiologists, Oncologists or Hematologists and General Practitioners in a “Cardio-Oncology Team” approach, ideally with the development of “Cardio-Oncology Clinics” staffed by dedicated specialists. These efforts should be linked to and even start with evaluation efforts of potential cardiotoxic side effects of chemotherapeutics.
Step 2: Clinical screening of cancer patients for underlying or developing cardiovascular disease, followed by stratified evaluation and management based on patient presentation relative to the timing of cancer therapy, i.e. before, during or after (Figure 1).
Step 3: Prior to cancer therapy, cardiotoxicity risk stratification should be pursued which then guides further follow-up (Figure 2 and Supplemental Table 3).
Step 4: During cancer therapy, specific surveillance algorithms have been formulated for the two most notoriously known drugs to cause cardiotoxicity, i.e. anthracycline and trastuzumab (Figures 4 and 5 and Supplemental Table 4), but may include others.
Step 5: Following cancer therapy, clinical and echocardiographic screening for cardiotoxicity depends on estimated cardiovascular risk and any cardiovascular toxicity observed during treatment (Figure 6 and Supplemental Table 5).
Step 6: Consideration of cardiovascular medications with reported benefit to be instituted as a preventive or therapeutic measure for chemotherapy-induced cardiotoxicity as patients qualify. These include carvedilol, nebivolol, ACE inhibitors and ARBs, and/or statins; additional cardiovascular treatments as required based on standard of care.
Step 7: Treatment of radiation-induced heart disease is to follow standard practice guidelines with the recognition that exposure was to the entire heart with the potential for multi-level involvement; treatment approaches thus need to be integrative and individualized with a very cautious approach to surgical interventions.
These steps aim to minimize the burden of cardiovascular morbidity and mortality in cancer patients treated with cardiotoxic agents and thus to improve their clinical outcome and survivorship. They will need to be applied on an individual basis and require ongoing re-evaluation as the field of cardio-oncology continues to evolve.
Supplementary Material
Article highlights.
Advances in cancer therapy have allowed for increasing numbers of long-term cancer survivors but have also generated increasing potential and significance of cardiovascular complications
Involvement of cardiovascular disease specialists has therefore become advisable from the initial assessment through survivorship, and this integrative approach has been coined “Cardio-Oncology”
Cardiotoxicity related to cancer therapy is currently defined by a decline in cardiac function and conceptualized into two types: irreversible injury type (type 1) or reversible dysfunction type (type 2)
Monitoring and management algorithms for either type of chemotherapy-induced cardiomyopathy are evolving around the central paradigm of early recognition and early treatment
Radiation-induced cardiotoxicity encompasses a broad spectrum of cardiac diseases that potentiates any chemotherapy-induced cardiotoxicity
Treatment of cardiovascular conditions of cancer patients generally follows AHA/ACC guidelines with some particular nuances
Preventive efforts should be considered for patients at estimated high risk for cancer therapy-related cardiotoxicity with the preferred drugs being ACE inhibitors and the specific beta-blockers carvedilol or nebivolol
Acknowledgements
We are deeply indebted to Dr. Thomas Gerber for the critical review of this manuscript.
Abbreviations
- ACE
angiotensin converting enzyme
- CAD
coronary artery disease
- DNA
deoxyribonucleic acid
- ECG
electrocardiogram
- LVEF
left ventricular ejection fraction
- MRI
magnetic resonance imaging
- RNA
radionucleotide angio- (ventriculo-) gram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial support and disclosure: This work was supported by a prospective grant of the Division of Cardiovascular Diseases, Mayo Clinic Rochester.
References
- 1.Lee CK, Aeppli D, Nierengarten ME. The need for long-term surveillance for patients treated with curative radiotherapy for Hodgkin's disease: University of Minnesota experience. International journal of radiation oncology, biology, physics. 2000;48:169–179. doi: 10.1016/s0360-3016(00)00647-7. [DOI] [PubMed] [Google Scholar]
- 2.Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 3.Aapro M, Bernard-Marty C, Brain EG, et al. Anthracycline cardiotoxicity in the elderly cancer patient: a SIOG expert position paper. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:257–267. doi: 10.1093/annonc/mdq609. [DOI] [PubMed] [Google Scholar]
- 4.Serrano C, Cortes J, De Mattos-Arruda L, et al. Trastuzumab-related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:897–902. doi: 10.1093/annonc/mdr348. [DOI] [PubMed] [Google Scholar]
- 5.Tarantini L, Gori S, Faggiano P, et al. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: a multicenter cohort analysis. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:3058–3063. doi: 10.1093/annonc/mds127. [DOI] [PubMed] [Google Scholar]
- 6.Cardinale D, Colombo A, Lamantia G, et al. Cardio-oncology: a new medical issue. Ecancermedicalscience. 2008;2:126. doi: 10.3332/ecancer.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio oncological prevention. Journal of the National Cancer Institute. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. Journal of the American College of Cardiology. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 9.Coleman RE, Maisey MN, Knight RK, Rubens RD. Mitoxantrone in advanced breast cancer--a phase II study with special attention to cardiotoxicity. European journal of cancer & clinical oncology. 1984;20:771–776. doi: 10.1016/0277-5379(84)90215-3. [DOI] [PubMed] [Google Scholar]
- 10.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug safety : an international journal of medical toxicology and drug experience. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 11.Cerny J, Hassan A, Smith C, Piperdi B. Coronary vasospasm with myocardial stunning in a patient with colon cancer receiving adjuvant chemotherapy with FOLFOX regimen. Clinical colorectal cancer. 2009;8:55–58. doi: 10.3816/CCC.2009.n.009. [DOI] [PubMed] [Google Scholar]
- 12.Lenihan D, Suter T, Brammer M, Neate C, Ross G, Baselga J. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;Pooled analysis of cardiac safety in patients with cancer treated with pertuzumab.23:791–800. doi: 10.1093/annonc/mdr294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 14.Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clinic proceedings. Mayo Clinic. 2008;83:679–686. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 15.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Lorenzo G, Autorino R, Bruni G, et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20:1535–1542. doi: 10.1093/annonc/mdp025. [DOI] [PubMed] [Google Scholar]
- 17.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19:1613–1618. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 18.Hall PS, Harshman LC, Srinivas S, Witteles RM. The Frequency and Severity of Cardiovascular Toxicity From Targeted Therapy in Advanced Renal Cell Carcinoma Patients. JACC Heart Fail. 2013;1:72–78. doi: 10.1016/j.jchf.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. The New England journal of medicine. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 20.Kruit WH, Punt KJ, Goey SH, et al. Cardiotoxicity as a dose-limiting factor in a schedule of high dose bolus therapy with interleukin-2 and alpha-interferon. An unexpectedly frequent complication. Cancer. 1994;74:2850–2856. doi: 10.1002/1097-0142(19941115)74:10<2850::aid-cncr2820741018>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Leighl NB, Raez LE, Besse B, et al. A multicenter, phase 2 study of vascular endothelial growth factor trap (Aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J Thorac Oncol. 2010;5:1054–1059. doi: 10.1097/jto.0b013e3181e2f7fb. [DOI] [PubMed] [Google Scholar]
- 22.Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119:1658–1665. doi: 10.1016/j.ophtha.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 24.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. Journal of the American College of Cardiology. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 26.Sawaya H, Plana JC, Scherrer-Crosbie M. Newest echocardiographic techniques for the detection of cardiotoxicity and heart failure during chemotherapy. Heart failure clinics. 2011;7:313–321. doi: 10.1016/j.hfc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Curigliano G, Cardinale D, Suter T, et al. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. Vol. 23. Suppl 7: 2012. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. pp. vii155–166. [DOI] [PubMed] [Google Scholar]
- 28.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 29.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. The New England journal of medicine. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 30.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. European journal of heart failure. 2001;3:315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 31.Ferrans VJ. Overview of cardiac pathology in relation to anthracycline cardiotoxicity. Cancer treatment reports. 1978;62:955–961. [PubMed] [Google Scholar]
- 32.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Annals of internal medicine. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Berry GJ, Jorden M. Pathology of radiation and anthracycline cardiotoxicity. Pediatric blood & cancer. 2005;44:630–637. doi: 10.1002/pbc.20346. [DOI] [PubMed] [Google Scholar]
- 34.Minotti G, Cairo G, Monti E. Role of iron in anthracycline cardiotoxicity: new tunes for an old song? FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:199–212. [PubMed] [Google Scholar]
- 35.Lebrecht D, Walker UA. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovascular toxicology. 2007;7:108–113. doi: 10.1007/s12012-007-0009-1. [DOI] [PubMed] [Google Scholar]
- 36.Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell biology and toxicology. 2007;23:15–25. doi: 10.1007/s10565-006-0140-y. [DOI] [PubMed] [Google Scholar]
- 37.Ky B, Vejpongsa P, Yeh ET, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circulation research. 2013;113:754–764. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nature medicine. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 39.Katayama M, Imai Y, Hashimoto H, et al. Fulminant fatal cardiotoxicity following cyclophosphamide therapy. J Cardiol. 2009;54:330–334. doi: 10.1016/j.jjcc.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Gottdiener JS, Appelbaum FR, Ferrans VJ, Deisseroth A, Ziegler J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Archives of internal medicine. 1981;141:758–763. [PubMed] [Google Scholar]
- 41.Hariharan S, Lowry S. Cardiotoxicity associated with sunitinib. Lancet. 2008;371:1244–1245. doi: 10.1016/S0140-6736(08)60552-8. author reply 1245. [DOI] [PubMed] [Google Scholar]
- 42.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nature reviews. Cardiology. 2010;7:564–575. doi: 10.1038/nrcardio.2010.121. [DOI] [PubMed] [Google Scholar]
- 43.Kerkela R, Woulfe KC, Durand JB, et al. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clinical and translational science. 2009;2:15–25. doi: 10.1111/j.1752-8062.2008.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greineder CF, Kohnstamm S, Ky B. Heart failure associated with sunitinib: lessons learned from animal models. Current hypertension reports. 2011;13:436–441. doi: 10.1007/s11906-011-0225-8. [DOI] [PubMed] [Google Scholar]
- 45.Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954–960. doi: 10.1161/CIRCULATIONAHA.107.690487. [DOI] [PubMed] [Google Scholar]
- 46.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nature medicine. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 47.Choueiri TK, Mayer EL, Je Y, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 48.Hawkes EA, Okines AF, Plummer C, Cunningham D. Cardiotoxicity in patients treated with bevacizumab is potentially reversible. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:e560–562. doi: 10.1200/JCO.2011.35.5008. [DOI] [PubMed] [Google Scholar]
- 49.Giordano FJ, Gerber HP, Williams SP, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groarke JD, Nguyen PL, Nohria A, Ferrari R, Cheng S, Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non invasive imaging for detection of cardiovascular disease. European heart journal. 2013 doi: 10.1093/eurheartj/eht114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. The New England journal of medicine. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 52.van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 53.Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 54.Wu W, Masri A, Popovic ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127:1476–1484. doi: 10.1161/CIRCULATIONAHA.113.001435. [DOI] [PubMed] [Google Scholar]
- 55.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart JR, Fajardo LF, Gillette SM, Constine LS. Radiation injury to the heart. International journal of radiation oncology, biology, physics. 1995;31:1205–1211. doi: 10.1016/0360-3016(94)00656-6. [DOI] [PubMed] [Google Scholar]
- 57.van Leeuwen-Segarceanu EM, Bos WJ, Dorresteijn LD, et al. Screening Hodgkin lymphoma survivors for radiotherapy induced cardiovascular disease. Cancer treatment reviews. 2011;37:391–403. doi: 10.1016/j.ctrv.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Lee PJ, Mallik R. Cardiovascular effects of radiation therapy: practical approach to radiation therapy-induced heart disease. Cardiology in review. 2005;13:80–86. doi: 10.1097/01.crd.0000131188.41589.c5. [DOI] [PubMed] [Google Scholar]
- 59.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Human pathology. 1996;27:766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 60.Seemann I, Gabriels K, Visser NL, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;103:143–150. doi: 10.1016/j.radonc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 61.Ordovas KG, Higgins CB. Delayed contrast enhancement on MR images of myocardium: past, present, future. Radiology. 2011;261:358–374. doi: 10.1148/radiol.11091882. [DOI] [PubMed] [Google Scholar]
- 62.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. Journal of the National Cancer Institute. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 63.Ylanen K, Poutanen T, Savikurki-Heikkila P, Rinta-Kiikka I, Eerola A, Vettenranta K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. Journal of the American College of Cardiology. 2013;61:1539–1547. doi: 10.1016/j.jacc.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 64.Gabriels K, Hoving S, Seemann I, et al. Local heart irradiation of ApoE(−/−) mice induces microvascular and endocardial damage and accelerates coronary atherosclerosis. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;105:358–364. doi: 10.1016/j.radonc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Hoving S, Heeneman S, Gijbels MJ, et al. Irradiation induces different inflammatory and thrombotic responses in carotid arteries of wildtype C57BL/6J and atherosclerosis-prone ApoE(−/−) mice. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;105:365–370. doi: 10.1016/j.radonc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Stewart FA, Heeneman S, Te Poele J, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. The American journal of pathology. 2006;168:649–658. doi: 10.2353/ajpath.2006.050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D OHI, Garot J. Radiation-induced heart disease. Circulation. Heart failure. 2011;4:e1–2. doi: 10.1161/CIRCHEARTFAILURE.110.958454. [DOI] [PubMed] [Google Scholar]
- 68.Koh ES, Tran TH, Heydarian M, et al. A comparison of mantle versus involved-field radiotherapy for Hodgkin's lymphoma: reduction in normal tissue dose and second cancer risk. Radiation oncology. 2007;2:13. doi: 10.1186/1748-717X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. International journal of radiation oncology, biology, physics. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 70.Barjaktarovic Z, Shyla A, Azimzadeh O, et al. Ionising radiation induces persistent alterations in the cardiac mitochondrial function of C57BL/6 mice 40 weeks after local heart exposure. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;106:404–410. doi: 10.1016/j.radonc.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Yu E, Calvert PA, Mercer JR, et al. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. 2013;128:702–712. doi: 10.1161/CIRCULATIONAHA.113.002271. [DOI] [PubMed] [Google Scholar]
- 72.Herrmann J, Lerman LO, Mukhopadhyay D, Napoli C, Lerman A. Angiogenesis in atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1948–1957. doi: 10.1161/01.ATV.0000233387.90257.9b. [DOI] [PubMed] [Google Scholar]
- 73.Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. Journal of the American College of Cardiology. 2010;55:1237–1239. doi: 10.1016/j.jacc.2009.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halle M, Hall P, Tornvall P. Cardiovascular disease associated with radiotherapy: activation of nuclear factor kappa-B. Journal of internal medicine. 2011;269:469–477. doi: 10.1111/j.1365-2796.2011.02353.x. [DOI] [PubMed] [Google Scholar]
- 75.Mahmoudi M, Mercer J, Bennett M. DNA damage and repair in atherosclerosis. Cardiovascular research. 2006;71:259–268. doi: 10.1016/j.cardiores.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 76.Lotrionte M, Biondi-Zoccai G, Abbate A, et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. The American journal of cardiology. 2013;112:1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz RG, Jain D, Storozynsky E. Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2013;20:443–464. doi: 10.1007/s12350-013-9707-1. [DOI] [PubMed] [Google Scholar]
- 78.Choi BW, Berger HJ, Schwartz PE, et al. Serial radionuclide assessment of doxorubicin cardiotoxicity in cancer patients with abnormal baseline resting left ventricular performance. American heart journal. 1983;106:638–643. doi: 10.1016/0002-8703(83)90080-7. [DOI] [PubMed] [Google Scholar]
- 79.Alexander J, Dainiak N, Berger HJ, et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. The New England journal of medicine. 1979;300:278–283. doi: 10.1056/NEJM197902083000603. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz RG, McKenzie WB, Alexander J, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. The American journal of medicine. 1987;82:1109–1118. doi: 10.1016/0002-9343(87)90212-9. [DOI] [PubMed] [Google Scholar]
- 81.Hoffmann R, Barletta G, von Bardeleben S, et al. Analysis of Left Ventricular Volumes and Function: A Multicenter Comparison of Cardiac Magnetic Resonance Imaging, Cine Ventriculography, and Unenhanced and Contrast- Enhanced Two-Dimensional and Three-Dimensional Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2014 doi: 10.1016/j.echo.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 82.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. Journal of the American College of Cardiology. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 83.Eder V, Herault S, Hudelo C, et al. Evaluation of left ventricular systolic function by 3D echocardiography: a comparative study with X-ray angiography and radionuclide angiography. European journal of ultrasound : official journal of the European Federation of Societies for Ultrasound in Medicine and Biology. 2000;11:105–115. doi: 10.1016/s0929-8266(00)00077-x. [DOI] [PubMed] [Google Scholar]
- 84.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? European heart journal. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 85.Fine NM, Crowson CS, Lin G, Oh JK, Villarraga HR, Gabriel SE. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Annals of the rheumatic diseases. 2013 doi: 10.1136/annrheumdis-2013-203314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circulation. Cardiovascular imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2012;25:733–740. doi: 10.1016/j.echo.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. Journal of the American College of Cardiology. 2011;57:2263–2270. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 89.Xu YH J, Pellikka P, Ansell S, Cha S, HR V. Early changes in 2D-speckle tracking echocardiography may predict a decrease in left ventricular ejection fraction in lymphoma patients undergoing anthracycline chemotherapy. Journal of the American Society of Echocardiography. 2013;26:B52. [Google Scholar]
- 90.Stoodley PW, Richards DA, Hui R, et al. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2011;12:945–952. doi: 10.1093/ejechocard/jer187. [DOI] [PubMed] [Google Scholar]
- 91.Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. British journal of cancer. 2002;86:1697–1700. doi: 10.1038/sj.bjc.6600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. Journal of the American College of Cardiology. 2000;36:517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 93.Ky B, Putt M, Sawaya H, et al. Early Increases in Multiple Biomarkers Predict Subsequent Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang Y, Xu X, Cheng L, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. European journal of heart failure. 2013 doi: 10.1002/ejhf.8. [DOI] [PubMed] [Google Scholar]
- 95.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 96.Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013–1032. doi: 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:43–49. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 98.Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:3031–3037. doi: 10.1200/JCO.2006.08.6595. [DOI] [PubMed] [Google Scholar]
- 99.Zagar TM, Marks LB. Breast cancer radiotherapy and coronary artery stenosis: location, location, location. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:350–352. doi: 10.1200/JCO.2011.38.9304. [DOI] [PubMed] [Google Scholar]
- 100.Kruk M, Noll D, Achenbach S, et al. Impact of Coronary Artery Calcium Characteristics on Accuracy of CT Angiography. JACC. Cardiovascular imaging. 2013 doi: 10.1016/j.jcmg.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 101.Rochitte CE, George RT, Chen MY, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. European heart journal. 2013 doi: 10.1093/eurheartj/eht488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 103.Jarfelt M, Kujacic V, Holmgren D, Bjarnason R, Lannering B. Exercise echocardiography reveals subclinical cardiac dysfunction in young adult survivors of childhood acute lymphoblastic leukemia. Pediatric blood & cancer. 2007;49:835–840. doi: 10.1002/pbc.21289. [DOI] [PubMed] [Google Scholar]
- 104.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 105.Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracycline cardiotoxicity: a prospective, blinded, long-term observational study of outcome in 120 patients. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2002;13:699–709. doi: 10.1093/annonc/mdf132. [DOI] [PubMed] [Google Scholar]
- 106.Schweiger M, Dave H, Lemme F, et al. Acute chemotherapy-induced cardiomyopathy treated with intracorporeal left ventricular assist device in an 8-year-old child. ASAIO journal. 2013;59:520–522. doi: 10.1097/MAT.0b013e3182a0d242. [DOI] [PubMed] [Google Scholar]
- 107.Oliveira GH, Dupont M, Naftel D, et al. Increased Need for Right Ventricular Support in Patients with Chemotherapy-Induced Cardiomyopathy Undergoing Mechanical Circulatory Support: Outcomes from the INTERMACS Registry. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 108.Kurihara C, Nishimura T, Nawata K, et al. Successful bridge to recovery with VAD implantation for anthracycline-induced cardiomyopathy. Journal of artificial organs : the official journal of the Japanese Society for Artificial Organs. 2011;14:249–252. doi: 10.1007/s10047-011-0567-7. [DOI] [PubMed] [Google Scholar]
- 109.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. The New England journal of medicine. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 110.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. The New England journal of medicine. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 111.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 112.Silber JH. Role of afterload reduction in the prevention of late anthracycline cardiomyopathy. Pediatric blood & cancer. 2005;44:607–613. doi: 10.1002/pbc.20351. [DOI] [PubMed] [Google Scholar]
- 113.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 114.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nature reviews. Clinical oncology. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 115.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382:1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 116.Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. The lancet oncology. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nature medicine. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma L, Gul R, Habibi J, et al. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the transgenic (mRen2) rat. American journal of physiology. Heart and circulatory physiology. 2012;302:H2341–2351. doi: 10.1152/ajpheart.01126.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoving S, Heeneman S, Gijbels MJ, et al. Anti-inflammatory and anti-thrombotic intervention strategies using atorvastatin, clopidogrel and knock-down of CD40L do not modify radiation-induced atherosclerosis in ApoE null mice. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2011;101:100–108. doi: 10.1016/j.radonc.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 120.Santoro F, Ieva R, Lupo P, et al. Late calcification of the mitral-aortic junction causing transient complete atrio-ventricular block after mediastinal radiation of Hodgkin lymphoma: multimodal visualization. International journal of cardiology. 2012;155:e49–50. doi: 10.1016/j.ijcard.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 121.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane database of systematic reviews. 2011:CD003917. [Google Scholar]
- 122.Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. Journal of the American College of Cardiology. 2012;60:2384–2390. doi: 10.1016/j.jacc.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 123.Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH. Cardioprotective effect of beta-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow-up study of heart failure. Circulation. Heart failure. 2013;6:420–426. doi: 10.1161/CIRCHEARTFAILURE.112.000055. [DOI] [PubMed] [Google Scholar]
- 124.Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. Journal of the American College of Cardiology. 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 125.El-Shitany NA, Tolba OA, El-Shanshory MR, El-Hawary EE. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. Journal of cardiac failure. 2012;18:607–613. doi: 10.1016/j.cardfail.2012.06.416. [DOI] [PubMed] [Google Scholar]
- 126.Elitok A, Oz F, Cizgici AY, et al. Effect of carvedilol on silent anthracycline- induced cardiotoxicity assessed by strain imaging: A prospective randomized controlled study with 6-month follow-up. Cardiology journal. 2013 doi: 10.5603/CJ.a2013.0150. [DOI] [PubMed] [Google Scholar]
- 127.Kaya MG, Ozkan M, Gunebakmaz O, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: A randomized control study. International journal of cardiology. 2012 doi: 10.1016/j.ijcard.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 128.Georgakopoulos P, Roussou P, Matsakas E, et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. American journal of hematology. 2010;85:894–896. doi: 10.1002/ajh.21840. [DOI] [PubMed] [Google Scholar]
- 129.Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). Journal of the American College of Cardiology. 2013;61:2355–2362. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 130.Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:820–828. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 131.Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 132.Nakamae H, Tsumura K, Terada Y, et al. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer. 2005;104:2492–2498. doi: 10.1002/cncr.21478. [DOI] [PubMed] [Google Scholar]
- 133.Dessi M, Piras A, Madeddu C, et al. Long-term protective effects of the angiotensin receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress and myocardial dysfunction. Experimental and therapeutic medicine. 2011;2:1003–1009. doi: 10.3892/etm.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Acar Z, Kale A, Turgut M, et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. Journal of the American College of Cardiology. 2011;58:988–989. doi: 10.1016/j.jacc.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 135.Choe JY, Combs AB, Folkers K. Potentiation of the toxicity of adriamycin by propranolol. Research communications in chemical pathology and pharmacology. 1978;21:577–580. [PubMed] [Google Scholar]
- 136.Bernstein D, Fajardo G, Zhao M, et al. Differential cardioprotective/cardiotoxic effects mediated by beta-adrenergic receptor subtypes. American journal of physiology. Heart and circulatory physiology. 2005;289:H2441–2449. doi: 10.1152/ajpheart.00005.2005. [DOI] [PubMed] [Google Scholar]
- 137.Martin M, Esteva FJ, Alba E, et al. Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: review and expert recommendations. The oncologist. 2009;14:1–11. doi: 10.1634/theoncologist.2008-0137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.