Abstract
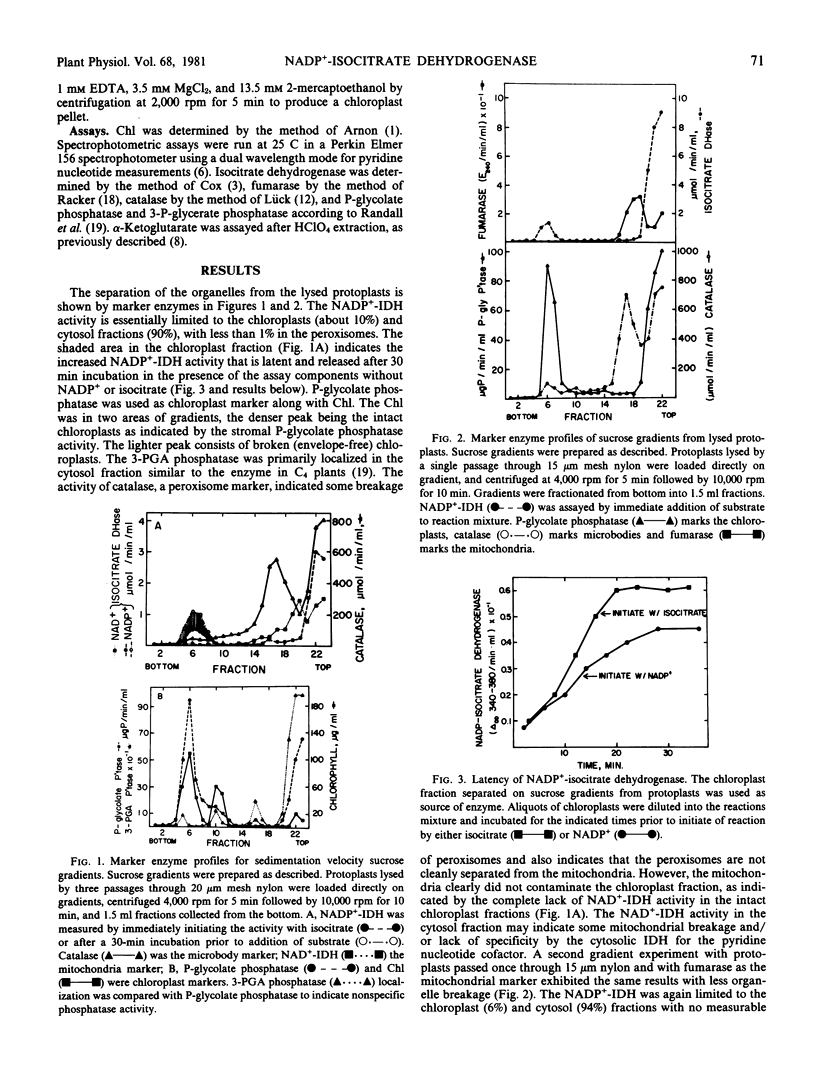
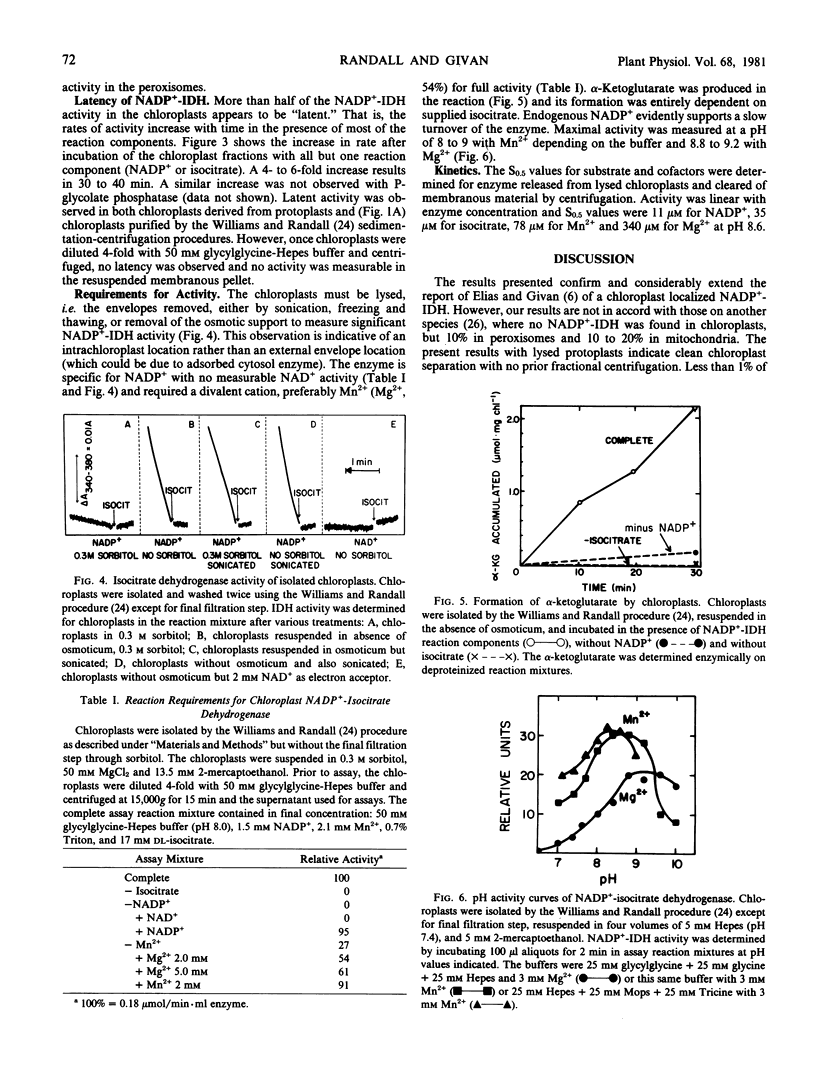
The subcellular location of NADP+-isocitrate dehydrogenase was investigated by preparing protoplasts from leaves of pea seedlings. Washed protoplasts were gently lysed and the whole lysate separated on sucrose gradients by a rate-zonal centrifugation. Organelles were located by marker enzymes and chlorophyll analysis. Most of the NADP+-isocitrate dehydrogenase was in the soluble fraction. About 10% of the NADP+-isocitrate dehydrogenase was present in the chloroplasts as a partially latent enzyme. Less than 1% of the activity was found associated with the peroxisome fraction. NADP+-isocitrate dehydrogenase was partially characterized from highly purified chloroplasts isolated from shoot homogenates. The enzyme exhibited apparent Km values of 11 micromolar (NADP+), 35 micromolar (isocitrate), 78 micromolar (Mn2+), 0.3 millimolar (Mg2+) and showed optimum activity at pH 8 to 8.5 with Mn2+ and 8.8 to 9.2 with Mg2+. The NADP+-isocitrate dehydrogenase activity previously claimed in the peroxisomes by other workers is probably due to isolation procedures and/or nonspecific association. The NADP+-isocitrate dehydrogenase activity in the chloroplasts might help supply α-ketoglutarate for glutamate synthase action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultate T. P., Dennis D. T. Regulatory properties of a plant NAD: isocitrate dehydrogenase. The effect of inorganic ions. Eur J Biochem. 1969 Jan;7(2):153–158. doi: 10.1111/j.1432-1033.1969.tb19586.x. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. Regulation of the nicotinamide adenine dinucleotide-specific isocitrate dehydrogenase from a higher plant. The effect of reduced nicotinamide adenine dinucleotide and mixtures of citrate and isocitrate. J Biol Chem. 1970 Aug 10;245(15):3751–3754. [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias B. A., Givan C. V. Alpha-ketoglutarate supply for amino Acid synthesis in higher plant chloroplasts: intrachloroplastic localization of NADP-specific isocitrate dehydrogenase. Plant Physiol. 1977 Apr;59(4):738–740. doi: 10.1104/pp.59.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan C. V., Givan A. L., Leech R. M. Photoreduction of alpha-Ketoglutarate to Glutamate by Vicia faba Chloroplasts. Plant Physiol. 1970 May;45(5):624–630. doi: 10.1104/pp.45.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackert M. L., Harris B. A., Poulsen L. L. Purification and crystallization of NADP+-specific isocitrate dehydrogenase from Escherichia coli using polyethylene glycol. Biochim Biophys Acta. 1977 Apr 12;481(2):340–347. doi: 10.1016/0005-2744(77)90267-4. [DOI] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Graham D., Akazawa T. Isolation of intact chloroplasts and other cell organelles from spinach leaf protoplasts. Plant Physiol. 1976 Sep;58(3):309–314. doi: 10.1104/pp.58.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran R. G., Dennis D. T. Nicotinamide adenine dinucleotide phosphate-specific isocitrate dehydrogenase from a higher plant. Isolation and charactertization. Plant Physiol. 1971 Jan;47(1):43–47. doi: 10.1104/pp.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E., Gremel D. 3-Phosphoglycerate Phosphatase in Plants: II. Distribution, Physiological Considerations, and Comparison with P-Glycolate Phosphatase. Plant Physiol. 1971 Oct;48(4):480–487. doi: 10.1104/pp.48.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Randall D. D. Pyruvate Dehydrogenase Complex from Chloroplasts of Pisum sativum L. Plant Physiol. 1979 Dec;64(6):1099–1103. doi: 10.1104/pp.64.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Enzymic characterization of leaf peroxisomes. J Biol Chem. 1970 Oct 10;245(19):5137–5144. [PubMed] [Google Scholar]


