Abstract
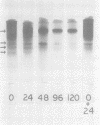
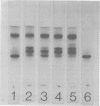
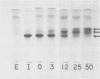
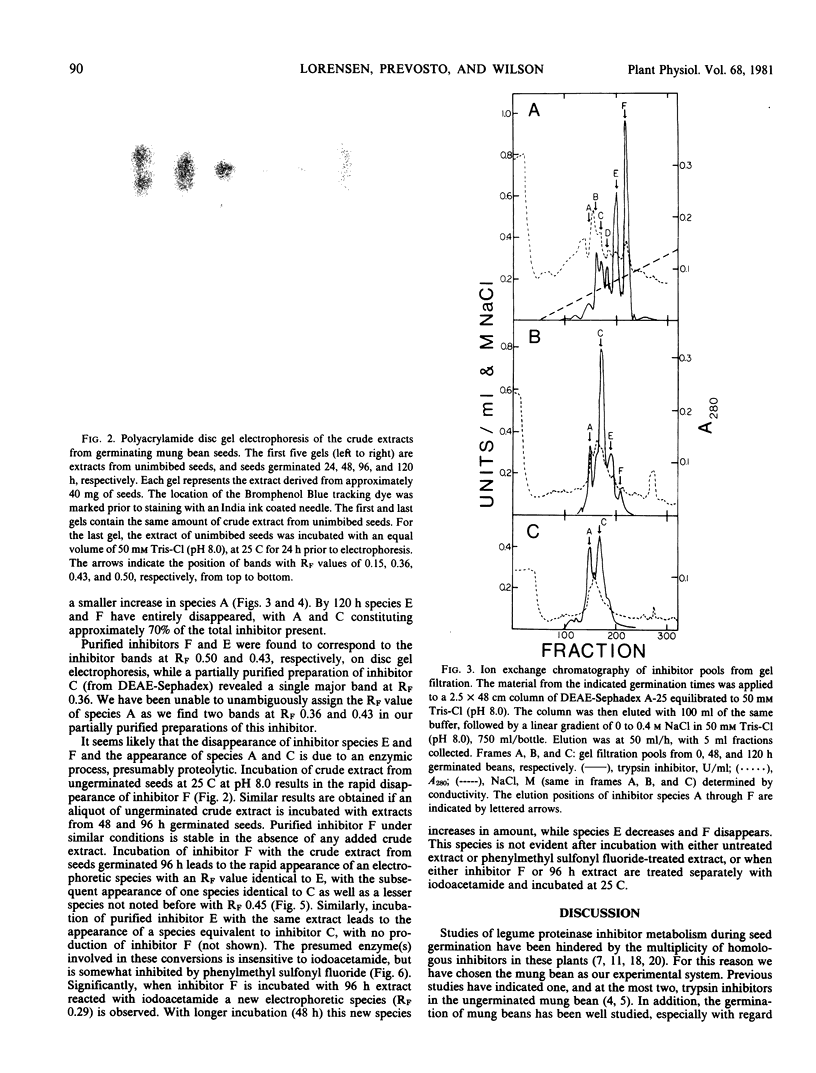
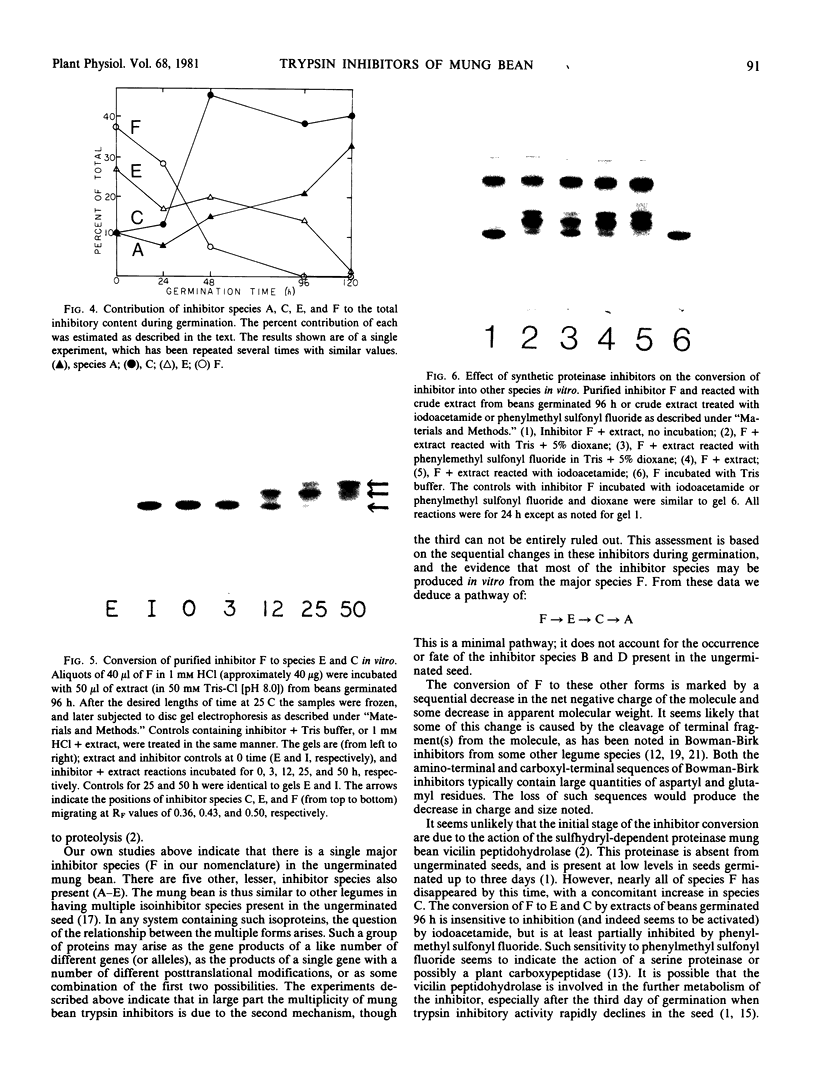
Ungerminated seeds of mung bean contain a single major species (F) of trypsin inhibitor with five minor species (A-E) separable on diethylaminoethyl-cellulose. During germination the level of trypsin inhibitory activity decreases from 1.8 units/grams dry weight in ungerminated cotyledons to 1.2 units/grams in cotyledons from seeds germinated 5 days. This decrease is accompanied by major changes in the distribution of inhibitory activity among the inhibitor species. By 48 hours of germination, inhibitor F has largely disappeared with an accompanying rapid increase in inhibitor C. Similarly, though less rapidly, inhibitor E decreases while inhibitor A increases. A similar sequence of changes is found in vitro when purified inhibitor F is incubated with extracts from seeds germinated 96 hours. The combined in vivo and in vitro data suggest a conversion sequence of: F → E → C → A. The in vitro conversion is inhibited by phenylmethyl sulfonyl fluoride but not by iodoacetamide, indicating that at least the initial phases of inhibitor conversion are not catalyzed by the mung bean vicilin peptidohydrolase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner B., Chrispeels M. J. Partial characterization of a protease inhibitor which inhibits the major endopeptidase present in the cotyledons of mung beans. Plant Physiol. 1976 Jul;58(1):1–6. doi: 10.1104/pp.58.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner B., Chrispeels M. J. Purification and characterization of vicilin peptidohydrolase, the major endopeptidase in the cotyledons of mung-bean seedlings. Eur J Biochem. 1977 Jul 15;77(2):223–233. doi: 10.1111/j.1432-1033.1977.tb11661.x. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Baumgartner B. Trypsin inhibitor in mung bean cotyledons: purification, characteristics, subcellular localization, and metabolism. Plant Physiol. 1978 Apr;61(4):617–623. doi: 10.1104/pp.61.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chü H. M., Chi C. W. The isolation and crystallization of two trypsin inhibitors of low molecular weight from mung bean (Phaseolus aureus Roxb.). Sci Sin. 1965 Oct;14(10):1441–1453. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Haynes R., Feeney R. E. Fractionation and properties of trypsin and chymotrypsin inhibitors from lima beans. J Biol Chem. 1967 Nov 25;242(22):5378–5385. [PubMed] [Google Scholar]
- KASSELL B., RADICEVIC M., BERLOW S., PEANASKY R. J., LASKOWSKI M., Sr THE BASIC TRYPSIN INHIBITOR OF BOVINE PANCREAS. I. AN IMPROVED METHOD OF PREPARATION AND AMINO ACID COMPOSITION. J Biol Chem. 1963 Oct;238:3274–3279. [PubMed] [Google Scholar]
- Odani S., Ikenaka T. Studies on soybean trypsin inhibitors, XII. Linear sequences of two soybean double-headed trypsin inhibitors, D-II and E-I. J Biochem. 1978 Mar;83(3):737–745. doi: 10.1093/oxfordjournals.jbchem.a131967. [DOI] [PubMed] [Google Scholar]
- Odani S., Ikenaka T. Studies on soybean trypsin inhibitors. X. Isolation and partial characterization of four soybean double-headed proteinase inhibitors. J Biochem. 1977 Dec;82(6):1513–1522. doi: 10.1093/oxfordjournals.jbchem.a131845. [DOI] [PubMed] [Google Scholar]
- Uriel J., Berges J. Characterization of natural inhibitors of trypsin and chymotrypsin by electrophoresis in acrylamide-agarose gels. Nature. 1968 May 11;218(5141):578–580. doi: 10.1038/218578b0. [DOI] [PubMed] [Google Scholar]
- Wilson K. A., Laskowski M., Sr Isolation of three isoinhibitors of trypsin from garden bean, Phaseolus vulgaris, having either lysine or arginine at the reactive site. J Biol Chem. 1973 Feb 10;248(3):756–762. [PubMed] [Google Scholar]
- Wilson K. A., Laskowski M., Sr The partial amino acid sequence of trypsin inhibitor II from garden bean, Phaseolus vulgaris, with location of the trypsin and elastase-reactive sites. J Biol Chem. 1975 Jun 10;250(11):4261–4267. [PubMed] [Google Scholar]
- Yoshida C., Yoshikawa M. Purification and characterization of proteinase inhibitors from adzuki beans (Phaseolus angularis). J Biochem. 1975 Nov;78(5):935–945. doi: 10.1093/oxfordjournals.jbchem.a131000. [DOI] [PubMed] [Google Scholar]