Abstract
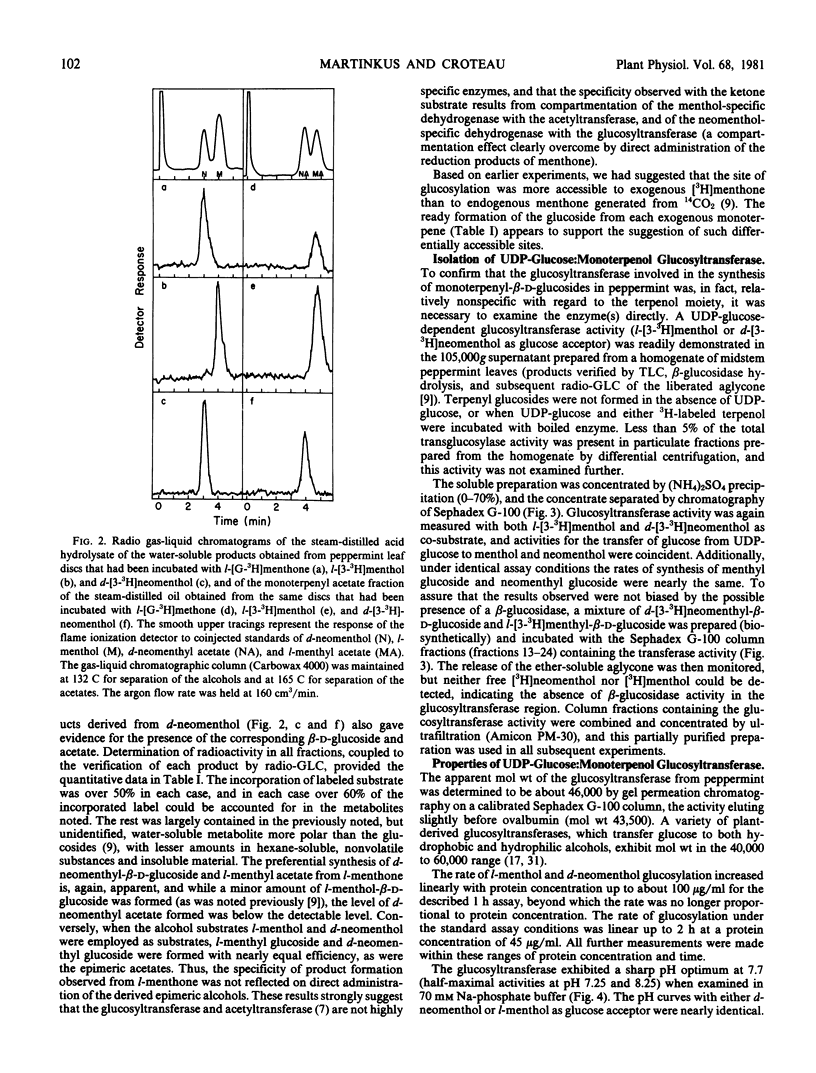
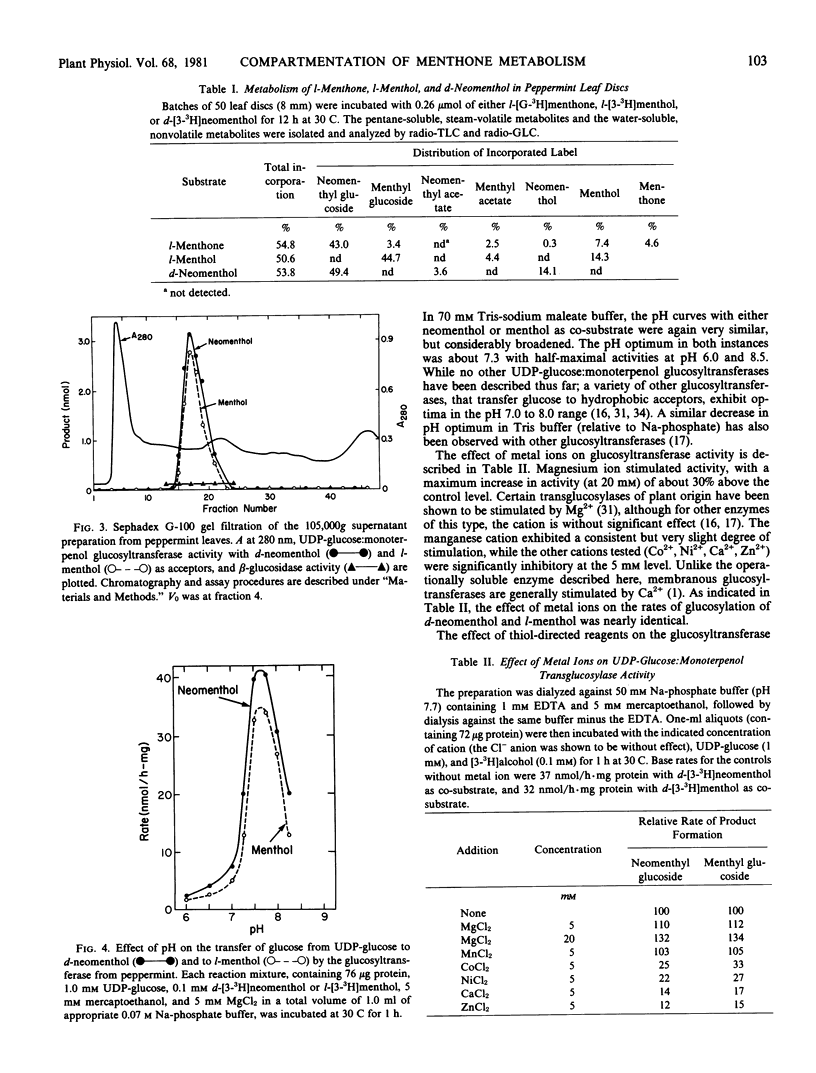
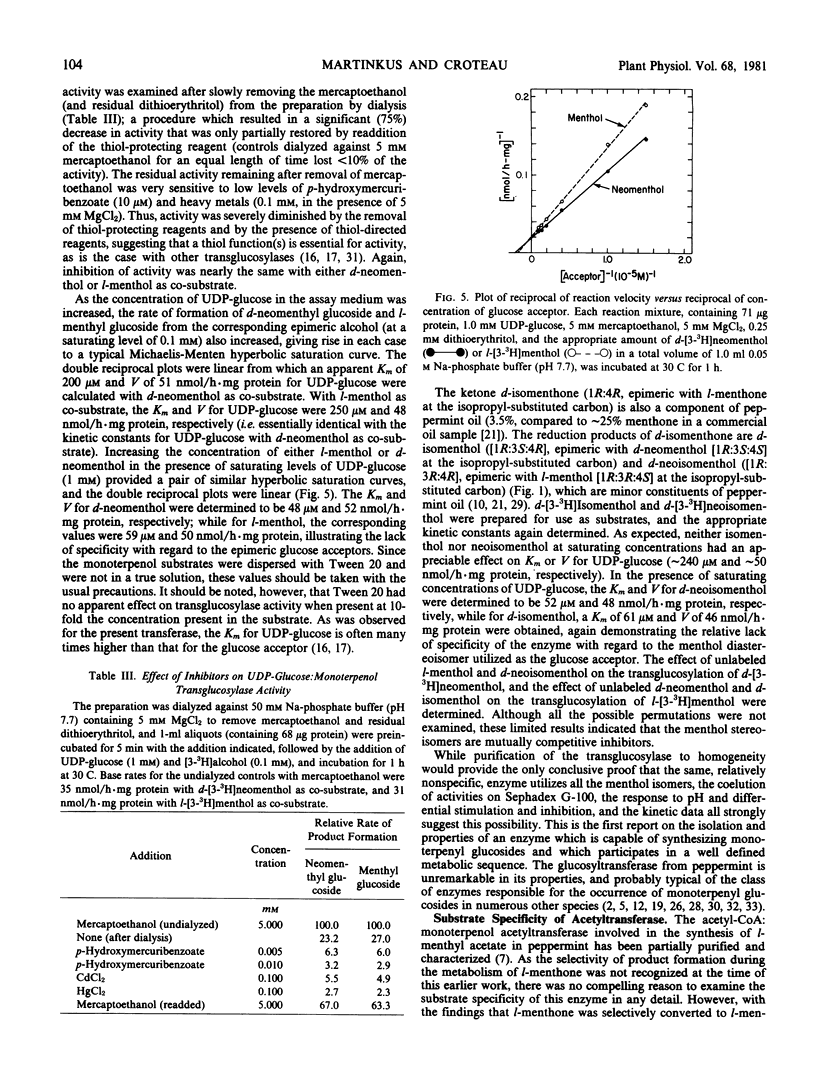
Previous studies have shown that the monoterpene ketone l-[G-3H]-menthone is reduced to the epimeric alcohols l-menthol and d-neomenthol in leaf discs of flowering peppermint (Mentha piperita L.), and that a portion of the menthol is converted to menthyl acetate while the bulk of the neomenthol is transformed to neomenthyl-β-d-glucoside (Croteau, Martinkus 1979 Plant Physiol 64: 169-175). The metabolic disposition of the epimeric reduction products of the ketone, which is a major constituent of peppermint oil, is highly specific, in that little neomenthyl acetate and little menthyl glucoside are formed. However, when l-[3-3H]menthol and d-[3-3H]neomenthol are separately administered to leaf discs, both menthyl and neomenthyl acetates and menthyl and neomenthyl glucosides are formed with nearly equal facility, suggesting that the metabolic specificity observed with the ketone precursor was not a function of the specificity of the transglucosylase or transacetylase but rather a result of compartmentation of each stereospecific dehydrogenase with the appropriate transferase. A UDP-glucose:monoterpenol glucosyltransferse, which utilized d-neomenthol or l-menthol as glucose acceptor, was demonstrated in the 105,000g supernatant of a peppermint leaf homogenate, and the enzyme was partially purified and characterized. Co-purification of the acceptor-mediated activities, and differential activation and inhibition studies, provided strong evidence that the same UDP-glucose-dependent enzyme could transfer glucose to either l-menthol or d-neomenthol. Determination of Km and V for the epimeric monoterpenols provided nearly identical values. The acetylcoenzyme A:monoterpenol acetyltransferase previously isolated from peppermint extracts (Croteau, Hooper 1978 Plant Physiol 61: 737-742) was re-examined using l-[3-3H]menthol and d-[3-3H]neomenthol as acetyl acceptors, and the Km and V for both epimers were, again, very similar. These results demonstrate that the specific in vivo conversion of l-menthone to l-menthyl acetate and d-neomenthyl-β-d-glucoside cannot be attributed to the selectivity of the transferases, and they clearly indicate that the metabolic specificity observed is a result of compartmentation effects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burbott A. J., Loomis W. D. Effects of light and temperature on the monoterpenes of peppermint. Plant Physiol. 1967 Jan;42(1):20–28. doi: 10.1104/pp.42.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbott A. J., Loomis W. D. Evidence for metabolic turnover of monoterpenes in peppermint. Plant Physiol. 1969 Feb;44(2):173–179. doi: 10.1104/pp.44.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Hooper C. L. Metabolism of Monoterpenes: Acetylation of (-)-Menthol by a Soluble Enzyme Preparation from Peppermint (Mentha piperita) Leaves. Plant Physiol. 1978 May;61(5):737–742. doi: 10.1104/pp.61.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Karp F. Biosynthesis of monoterpenes: enzymatic concersion of neryl pyrophosphate to 1,8-cineole, alpha-terpineol, and cyclic monoterpene hydrocarbons by a cell-free preparation from sage (Salvia officinalis). Arch Biochem Biophys. 1976 Oct;176(2):734–746. doi: 10.1016/0003-9861(76)90217-4. [DOI] [PubMed] [Google Scholar]
- Croteau R., Martinkus C. Metabolism of Monoterpenes: Demonstration of (+)-Neomenthyl-beta-d-Glucoside as a Major Metabolite of (-)-Menthone in Peppermint (Mentha Piperita). Plant Physiol. 1979 Aug;64(2):169–175. doi: 10.1104/pp.64.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R. Site of Monoterpene Biosynthesis in Majorana hortensis Leaves. Plant Physiol. 1977 Mar;59(3):519–520. doi: 10.1104/pp.59.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim R. K., Grisebach H. Purification and properties of UDP-glucose: coniferyl alcohol glucosyltransferase from suspension culturesof Paul's scarlet rose. Arch Biochem Biophys. 1976 Oct;176(2):700–708. doi: 10.1016/0003-9861(76)90214-9. [DOI] [PubMed] [Google Scholar]
- Lang E., Hörster H. An Zucker gebundene reguläre Monoterpene Teil II Untersuchungen zur Olbidung und Akkumulation ätherischer Ole in Ocimum basilicum Zellkulturen. Planta Med. 1977 Feb;31(2):112–118. doi: 10.1055/s-0028-1097501. [DOI] [PubMed] [Google Scholar]
- Loomis W. D. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- Skopp K., Hörster H. An Zucker gebundene reguläre Monoterpene, teil I Thymol-und carvacrolglykoside in thymus vulgaris. Planta Med. 1976 May;29(3):208–215. doi: 10.1055/s-0028-1097653. [DOI] [PubMed] [Google Scholar]
- Storm D. L., Hassid W. Z. Partial Purification and Properties of a beta-d-Glucosyltransferase Occurring in Germinating Phaseolus aureus Seeds. Plant Physiol. 1974 Dec;54(6):840–845. doi: 10.1104/pp.54.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAHA T., CARDINI C. E. The biosynthesis of plant glycosides. I. Monoglucosides. Arch Biochem Biophys. 1960 Jan;86:127–132. doi: 10.1016/0003-9861(60)90379-9. [DOI] [PubMed] [Google Scholar]


