Abstract
Context:
Laryngoscopy and endotracheal intubation activates the sympathetic nervous system, causing tachycardia and hypertension. Dexmedetomidine has an affinity for alpha2 receptors 8 times greater than that of clonidine. It diminishes norepinephrine release and inhibits sympathetic activity leading to decreased heart rate (HR) and blood pressure.
Aims:
The aim was to compare the effect of intravenous (IV) infusion of dexmedetomidine and clonidine on the pressor response among patients undergoing tracheal intubation in elective surgeries under general anesthesia.
Settings and Design:
A prospective, randomized control study.
Subjects and Methods:
Seventy-five adult patients of age 18-55 years in American Society of Anesthesiologists physical status I and II were included in this study. Patients were allocated randomly into Group P, Group D and Group C of 25 patients each. In the operation theatre, clonidine (3 μg/kg) or dexmedetomidine (0.5 μg/kg) or placebo (0.9% normal saline) diluted in 100 ml NaCl 0.9% were infused over a period of 10 min.
Statistical Analysis Used:
Statistical analysis was done using Statistical Package for Social Sciences version 15.0. Chi-square test, ANOVA, Student's t-test, and Paired t-test were used.
Results:
As compared to Group P, the mean systolic blood pressure in Group D and Group C were significantly lower (<0.01) after intubation and all the subsequent intervals. After infusion and after the induction interval, the maximum value was observed in Group D while minimum mean value was observed in Group C. As compared to Group P, the mean arterial pressure in Group D was significantly higher at after infusion and after induction intervals but significantly lower after intubation and subsequent intervals. However, in Group C, the mean value was significantly lower as compared to Group P at all-time intervals except after infusion and after induction intervals. As compared to Group P, the mean HR in Group D was significantly higher at after infusion and after induction intervals. However at all the subsequent intervals, Group D was significantly lower as compared to Group P.
Conclusion:
It was found that attenuating response to hemodynamic changes were observed with dexmedetomidine and clonidine IV infusion. The early onset of dexmedetomidine makes it a promising choice. Hence premedication with IV infusion of dexmedetomidine can safely be recommended for attenuation of hemodynamic response to endotracheal intubation.
Keywords: American Society of Anesthesiologists physical status I and II, clonidine, dexmedetomidine, randomized control study
INTRODUCTION
Intense noxious stimuli such as laryngoscopy and endotracheal intubation activate the sympathetic nervous system, inducing tachycardia and hypertension.[1] Controlling this postintubation pressor response is an important goal for modern anesthesia.
Dexmedetomidine and clonidine are two pharmacologically related to alpha agonists.[2] Dexmedetomidine has an affinity for alpha2 receptors 8 times greater than that of clonidine.[3] It diminishes norepinephrine release and inhibits sympathetic activity.[4] The inhibition of sympathetic activity causes decreased heart rate (HR) and blood pressure.
In the present study, we compared the effect of dexmedetomidine and clonidine administered by intravenous (IV) infusion on the pressor response among patients undergoing tracheal intubation in elective surgeries under general anesthesia (GA).
SUBJECTS AND METHODS
Seventy-five adult patients aged between 18 and 55 years in American Society of Anesthesiologists (ASA) physical status I and II were included in this prospective, randomized, controlled study after obtaining the hospital Ethical Committee approval. After obtaining written informed consent, the patients were allocated randomly into Group P, Group D and Group C of 25 patients each. In the operation theatre, placebo (P,0.9% normal saline [NS]) or dexmedetomidine (D, 0.5 μg/kg) or clonidine (C, 3 μg/kg) diluted in 100 ml NaCl 0.9% were infused over a 10 min period. Thereafter the patients received injection glycopyrrolate 0.2 mg IV. After preoxygenation with 100% oxygen for 3 min patients were induced with injection propofol (2 mg/kg). Endotracheal intubation was facilitated following a paralyzing dose of injection succinylcholine (2 mg/kg) IV maintenance of anesthesia was carried out with inhalation anesthetics (sevofluran) and nitrous oxide: Oxygen (60:40). Intraoperative relaxation was maintained with injection atracurium 0.5 mg/kg (bolus dose) followed by 0.1 mg/kg incremental doses on return of respiration. On the conclusion of surgery, patients were reversed with an injection neostigmine (50 μg/kg) and injection glycopyrrolate (10 μg/kg) and extubated. Patients were monitored for SpO2, electrocardiography, HR and invasive blood pressure intraoperatively.
Heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) were noted at:
T0-Before administration of the drug
T1-After completion of the administration of the drug
T2-After induction
T3-After intubation
T4 to T6-2, 4, 6, 8 and 10 min after intubation.
Inclusion criteria
Adult patients aged between 18 and 55 years
ASA-I and II physical status
Scheduled for elective surgery under GA.
Exclusion criteria
Patients with significant coronary artery disease or ischemic heart disease, chronic obstructive pulmonary disease, renal failure, hepatic dysfunction, morbid obesity, moderate or severe anemia, hypertension
Patients are not willing to enroll for the study.
Statistical tools employed
The statistical analysis was performed using Statistical Package for Social Sciences version 15.0 statistical analysis software. The values were represented in number (%) and mean ± standard deviation. Test used Chi-square test, ANOVA, Student's t-test, Paired t-test.
RESULTS
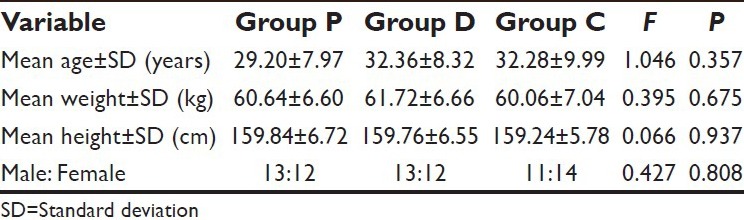
Comparison of the three study groups for age, weight, height, and gender ratio revealed no statistically significant intergroup difference (P > 0.05) [Table 1].
Table 1.
Demographic characteristics of the patients
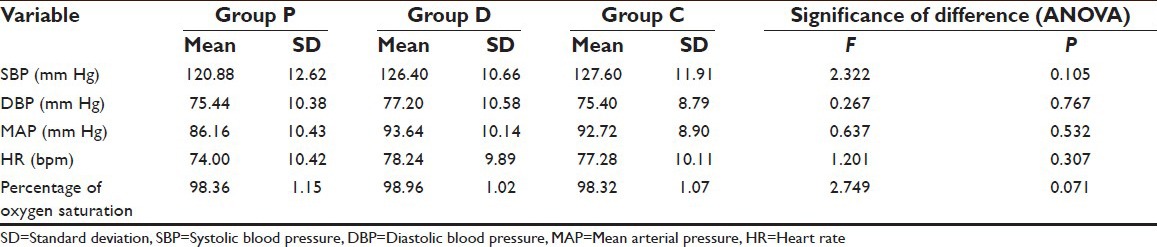
The three groups were matched for all the hemodynamic parameters at baseline showing no significant intergroup difference (P > 0.05) [Table 2].
Table 2.
Comparison of three groups for hemodynamic variables at baseline
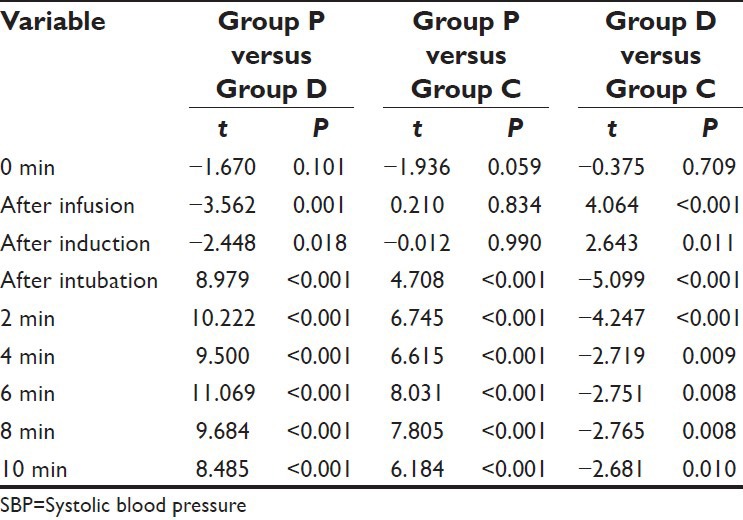
As compared to Group P, the mean SBP in Group D and Group C was significantly lower after intubation and all the subsequent intervals. On comparing the difference between Group D and Group C, the mean value of Group D was observed to be significantly higher at after infusion and after induction intervals, however at all the subsequent follow-up intervals, the mean value of Group D was significantly lower as compared to Group C [Table 3].
Table 3.
Comparison (between group) of mean SBP at different time intervals (student's t-test)
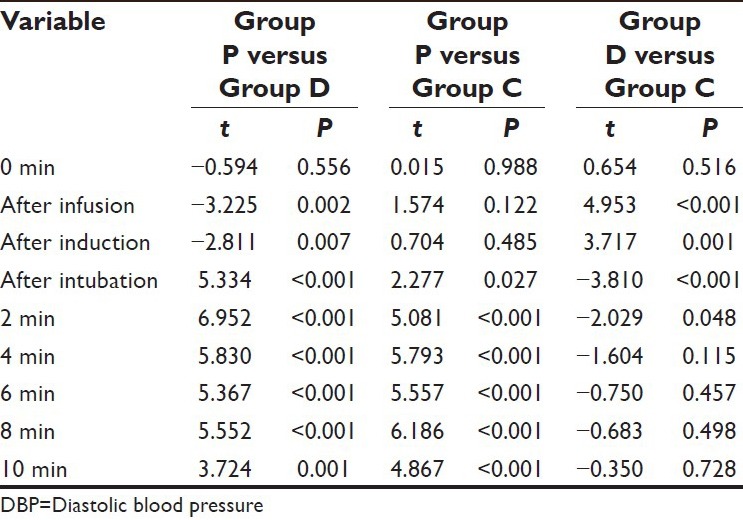
On comparing the difference between Group D and Group C, the mean value of Group D was observed to be significantly higher at after infusion and after induction intervals, however at all the subsequent follow-up intervals till 2 min after induction, the mean value of Group D was significantly lower as compared to Group C. Although the mean value in Group D was lower as compared to Group C at 4 min and subsequent follow-ups after intubation yet the difference between the two was not significant statistically (P > 0.05) [Table 4].
Table 4.
Comparison of mean DBP between groups at different time intervals (student's t-test)
At baseline, there was no significant difference among the groups (P = 0.532).
However, at all the time intervals during the procedure a statistically significant difference among groups were observed (P < 0.001). After infusion and after the induction interval, the maximum value was observed in Group D, while minimum mean value was observed in Group C. However, from after intubation interval till the end Group D showed minimum value and Group P had the maximum value [Table 5].
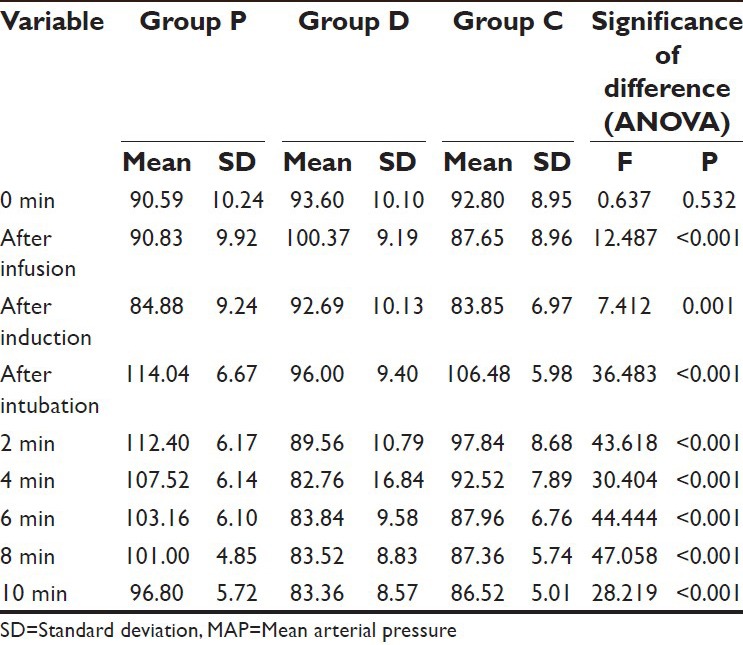
Table 5.
Comparison of MAP in different groups at different time intervals
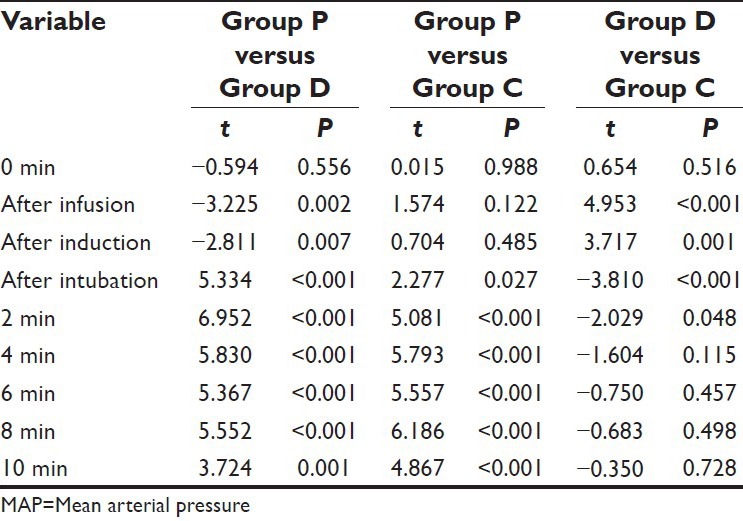
All the intra-procedure comparisons between the groups were significant statistically except between Group P and Group C at after infusion and after induction intervals and between Group D and Group C from 4 min postintubation till the end of follow-up.
As compared to Group P, the mean MAP in Group D was significantly higher at after infusion and after induction intervals but significantly lower after intubation and subsequent intervals. However, in Group C, the mean value was significantly lower as compared to Group P at all-time intervals except after infusion and after induction intervals.
On comparing the difference between Group D and Group C, the mean value of Group D was observed to be significantly higher at after infusion and after induction intervals, however at all the subsequent follow-up intervals till 2 min after intubation, the mean value of Group D was significantly lower as compared to Group C. Although the mean value in Group D was lower as compared to Group C at 4 min and subsequent follow-ups after intubation yet the difference between the two was not significant statistically (P > 0.05) [Table 6].
Table 6.
Comparison of mean MAP between groups at different time intervals (student's t-test)
At baseline, there was no significant difference among the groups (P = 0.307).
However, at all the time intervals during the procedure a statistically significant difference among groups was observed (P < 0.001). After infusion and after the induction interval, the maximum value was observed in Group D while minimum mean value was observed in Group C. However, from after intubation interval till the end of follow-up Group D showed minimum value and Group P had the maximum value [Table 7].
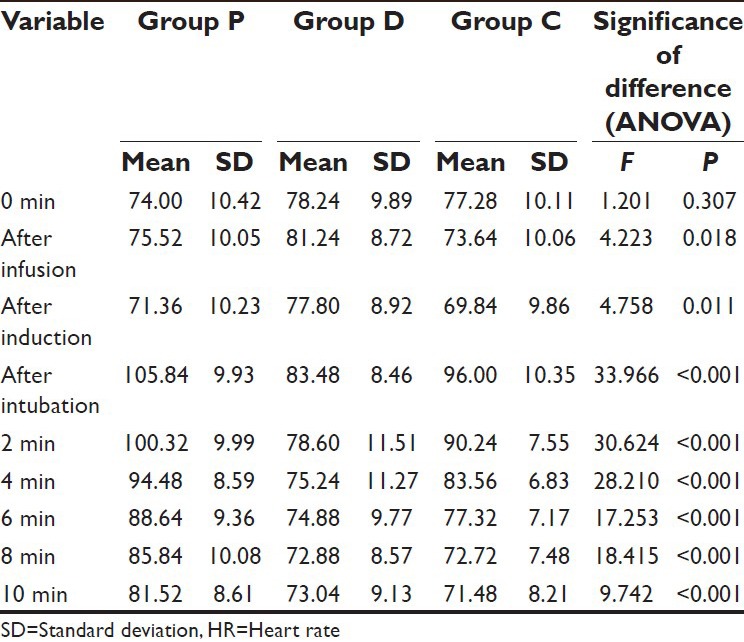
Table 7.
Comparison of HR in different groups at different time intervals
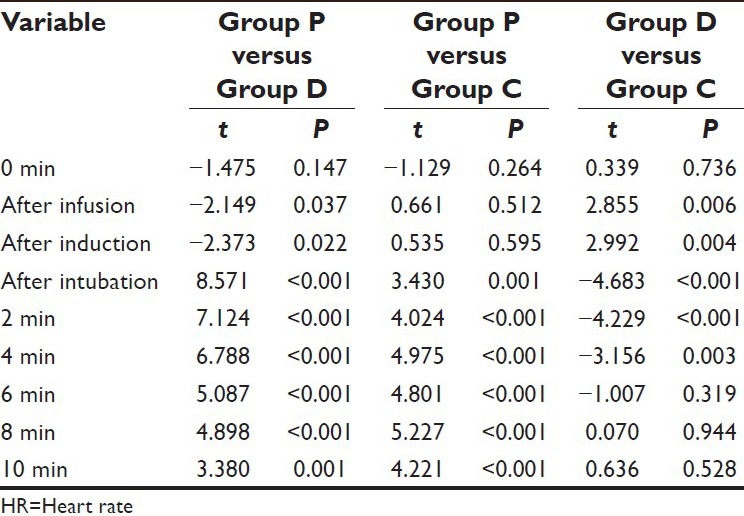
All the intra-procedure comparisons between the groups were significant statistically except between Group P and Group C at after infusion and after induction intervals and between Group D and Group C from 6 min postintubation till the end of follow-up.
As compared to Group P, the mean HR in Group D was significantly higher at after infusion and after induction intervals. However at all the subsequent intervals, Group D was significantly lower as compared to Group P.
On comparing the difference between Group D and Group C, the mean value of Group D was observed to be significantly higher at after infusion and after induction intervals, however at all the subsequent follow-up intervals till 2 min after induction, the mean value of Group D was significantly lower as compared to Group C [Table 8].
Table 8.
Comparison of mean HR between the groups at different time intervals (student's t-test)
DISCUSSION
Manipulation of the respiratory tract such as in laryngoscopy and tracheal intubation is associated with hemodynamic and cardiovascular responses (Matot et al., 2000).[5] In the recent decade, several studies have focused on clonidine and recently on dexmedetomidine premedication to attenuate the hemodynamic responses following laryngoscopy and intubation. However, there was no comparative study. The present study is an attempt to fill that gap.
A total of 75 patients were recruited in three groups of 25 each. Group C – 25 patients received placebo (0.9% NS) infused over a period of 10 min; Group D – 25 patients received dexmetodimine (0.5 μg/kg) infused over a period of 10 min and Group P – 25 patients received clonidine (3 μg/kg) infused over a period of 10 min. The baseline parameters for demography and hemodynamic variables were matched in the three group.
It was observed that mean SBP, DBP and MAP in the dexmedetomidine group remained close to the baseline throughout the study period showing a significant difference from both the placebo and clonidine groups throughout the study period following the induction interval. Clonidine group too showed significantly lower mean values for SBP and DBP as compared to placebo the group at all-time intervals, however, the extent of the difference between placebo and dexmedetomidine group was higher as compared to that of the clonidine group. Similar trends were obtained for HR too. Oxygen saturation was maintained between 98% and 100% in all the cases throughout.
One of the interesting findings was the relatively higher efficacy of dexmedetomidine group to show minimal changes from baseline. In the placebo and clonidine groups, a steep increase in all the hemodynamic parameters (SBP, DBP, MAP and HR) was observed between induction to intubation intervals, however, no such change was observed in the dexmedetomidine group. It was observed that the changes in all the parameters in dexmedetomidine groups were consistent and did not show a steep rise or fall at any time interval. Although as compared to the placebo and controlled groups, the mean MAP values in dexmedetomidine groups were significantly lower for all the measurements following infusion yet at none of the times the mean values were below the threshold level of 60 mm of Hg.
In the placebo group, maximum mean SBP was observed to be 153.76 ± 12.82 mm of Hg at after intubation interval which was significantly higher to both clonidine as well as dexmedetomidine group at the corresponding time interval. In the placebo group, the minimum mean SBP at any postintubation interval was 136.32 ± 9.29 mm of Hg at 10 min whereas the corresponding values for clonidine and dexmedetomidine groups were 119.56 ± 9.87 and 111.52 ± 11.28 mm of Hg respectively. In dexmedetomidine group, the minimum mean SBP was observed at 8 min postintubation interval (110.96 ± 12.02 mm Hg), whereas the corresponding values in placebo and clonidine groups were 140.40 ± 9.31 and 119.48 ± 9.64 mm Hg respectively. As regards the hike in mean SBP between induction to intubation intervals, the change was distinctly sharp in the placebo group (from 115.36 ± 12.82 to 153.76 ± 12.82 mm of Hg) as compared to clonidine group (115.40 ± 10.70 to 138.88 ± 9.23 mm of Hg) and dexmedetomidine group (123.20 ± 10.16 to 125.96 ± 8.67 mm of Hg). The minimum to a maximum range of SBP was between 115.36 ± 12.38 and 153.75 ± 12.82 mm of Hg in the placebo group, from 115.40 ± 10.70 to 138.88 mm Hg in clonidine group and from 110.96 ± 12.02 to 131.28 mm of Hg in the dexmedetomidine group. It was observed that in the placebo group at none of the time intervals the mean SBP was below the baseline value whereas in the clonidine group too, the mean SBP was higher as compared to baseline at after intubation and 2 min after intubation intervals. However, in the dexmedetomidine group, at none of the postinduction intervals the mean value of SBP was higher as compared to the baseline value.
Study of pattern of these relative changes gives the idea of the hemodynamic stability brought in by dexmedetomidine as compared to placebo and clonidine group. Similar trends were obtained for DBP, MAP and HR too.
Taittonen et al. (1997)[6] have also shown similar response patterns while comparing clonidine, dexmedetomidine and placebo and attributed this to be the distinguishing feature of α2-agonists, including clonidine and dexmedetomidine. They reported that use of α2-agonists improves the oxygen saturation and reduces tachycardia, hypertension and sympathetic activity.
In all groups, blood pressure and HR increased after tracheal intubation; both were significantly lower in the dexmedetomidine and clonidine groups than in the placebo group (P < 0.05). A similar observation was made by Yildiz et al. (2006).[7]
In some studies, it is observed that MAP was decreased by low dosage of dexmedetomidine (0.25 μg/kg) and MAP was increased transiently, and HR was decreased significantly by high dosage of (1–4 μg/kg) dexmedetomidine.[8,9] Scheinin et al.[10] reported that the use of α2-agonist leads to bradycardia. Belleville et al.[11] found that the dexmedetomidine given in 2 min in the doses of 1–2 μg/kg causes irregular ventilation and apnea episodes. Ebert et al.[9] did not observe any episode of apnea, airway obstruction and hypoxemia with bolus doses of dexmedetomidine in their study, and they reported that the depression of respiration may be seen due to deep sedation, for the reason that the α2 adrenergic agonists don’t have an active role on the respiration center. However, in the present study a transient rise of MAP and HR was observed immediately following dexmedetomidine infusion. In another study in which the infusion of opioid and α2 adrenergic agonists were compared, it was concluded that dexmedetomidine doesn’t cause significant respiratory depression, and it decreases the risk of apnea.[12]
Carabine et al.[13] demonstrated that 0.625 and 1.25 μg/kg clonidine IV 15 min prior to induction of anesthesia attenuates the pressor response to laryngoscopy and intubation. In contrast, Wright et al.[14] observed in noncardiac ASA physical status I patients that under almost identical conditions 1.25 μg/kg clonidine IV was not effective. In the present study, we achieved a reasonable attenuation effect of clonidine using a dose of 3 μg/kg infused over a period of 10 min.
A number of studies have indicated the oral premedication of clonidine to achieve the attenuating effect of clonidine before laryngoscopy intubation.[15,16] However, as the bioavailability after oral intake varies between 70% and 90%, we chose the IV route of administration to relate pharmacodynamic effects more precisely to a certain dose. The infusion time was also spanned over 10 min in order to ascertain the maximum bioavailability. The IV infusion of clonidine was demonstrated to be safe in our patients. In early investigations the fast injection of clonidine increases blood pressure and as a consequence, decreases cardiac index.[17] These effects can be attributed to peripheral α2 stimulation by high clonidine plasma levels. Despite the lack of adverse effects, even this slow IV infusion obviously affected peripheral α2 -adrenoceptors because blood pressure increased slightly, but significantly during the 10 min after the start of infusion.
There are only limited studies in the literature that have followed a design similar to that reported in the present study, where a single dose schedule for both clonidine and dexmedetomidine groups led to promising attenuating effects during intubation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Fernandez-Galinski S, Bermejo S, Mansilla R, Pol O, Puig MM. Comparative assessment of the effects of alfentanil, esmolol or clonidine when used as adjuvants during induction of general anaesthesia. Eur J Anaesthesiol. 2004;21:476–82. doi: 10.1017/s0265021504006106. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman BB, Lefkowitz RJ, Taylor P. Neurotransmission: The autonomic and somatic motor nervous systems. In: Hardman JG, Limbird LE, Goodman GA, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw Hill Professional; 2001. pp. 137–82. [Google Scholar]
- 3.Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001;7:221–6. doi: 10.1097/00075198-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bustillo MA, Lazar RM, Finck AD, Fitzsimmons B, Berman MF, Pile-Spellman J, et al. Dexmedetomidine may impair cognitive testing during endovascular embolization of cerebral arteriovenous malformations: A retrospective case report series. J Neurosurg Anesthesiol. 2002;14:209–12. doi: 10.1097/01.ANA.0000017492.93942.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matot Idit, Sichel J.Y, Yofe Valeri, Gozal Yaacov. The Effect of Clonidine Premedication on Hemodynamic Responses to Microlaryngoscopy and Rigid Bronchoscopy and Rigid Bronchoscopy. Anesth Analg. 2000;91:828–33. doi: 10.1097/00000539-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Taittonen MT, Kirvelä OA, Aantaa R, Kanto JH. Effect of clonidine and dexmedetomidine premedication on perioperative oxygen consumption and haemodynamic state. Br J Anaesth. 1997;78:400–6. doi: 10.1093/bja/78.4.400. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: Perioperative haemodynamics and anaesthetic requirements Drugs R D. 2006;7:43–52. doi: 10.2165/00126839-200607010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenel responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. Br J Anaesth. 1992;68:126–31. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 11.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–33. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Cortinez LI, Hsu YW, Sum-Ping ST, et al. Dexmedetomidine pharmacodynamics: Part I: Crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–76. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Carabine UA, Allen RW, Moore J. Partial attenuation of the pressor response to endotracheal intubation. A comparison of the effects of intravenous clonidine and fentanyl. Eur J Anaesthesiol. 1992;9:325–9. [PubMed] [Google Scholar]
- 14.Wright PM, Carabine ZA, Kearney E, Howe JP. Intravenous clonidine: Effect on the cardiovascular response to intubation. Anesth Analg. 1991;72:5327. [Google Scholar]
- 15.Batra YK, Indu B, Puri GD. Attenuation of pulse rate and blood pressure response to laryngoscopy and tracheal intubation by clonidine. Int J Clin Pharmacol Ther Toxicol. 1988;26:360–3. [PubMed] [Google Scholar]
- 16.Talebi H, Nourozi A, Fateh S, Mohammadzadeh A, Eghtesadi-Araghi P, Jabbari S, et al. Effects of oral clonidine premedication on haemodynamic response to laryngoscopy and tracheal intubation: A clinical trial. Pak J Biol Sci. 2010;13:1146–50. doi: 10.3923/pjbs.2010.1146.1150. [DOI] [PubMed] [Google Scholar]
- 17.Mroczek WJ, Davidov M, Finnerty FA., Jr Intravenous clonidine in hypertensive patients. Clin Pharmacol Ther. 1973;14:847–51. doi: 10.1002/cpt1973145847. [DOI] [PubMed] [Google Scholar]


