Abstract
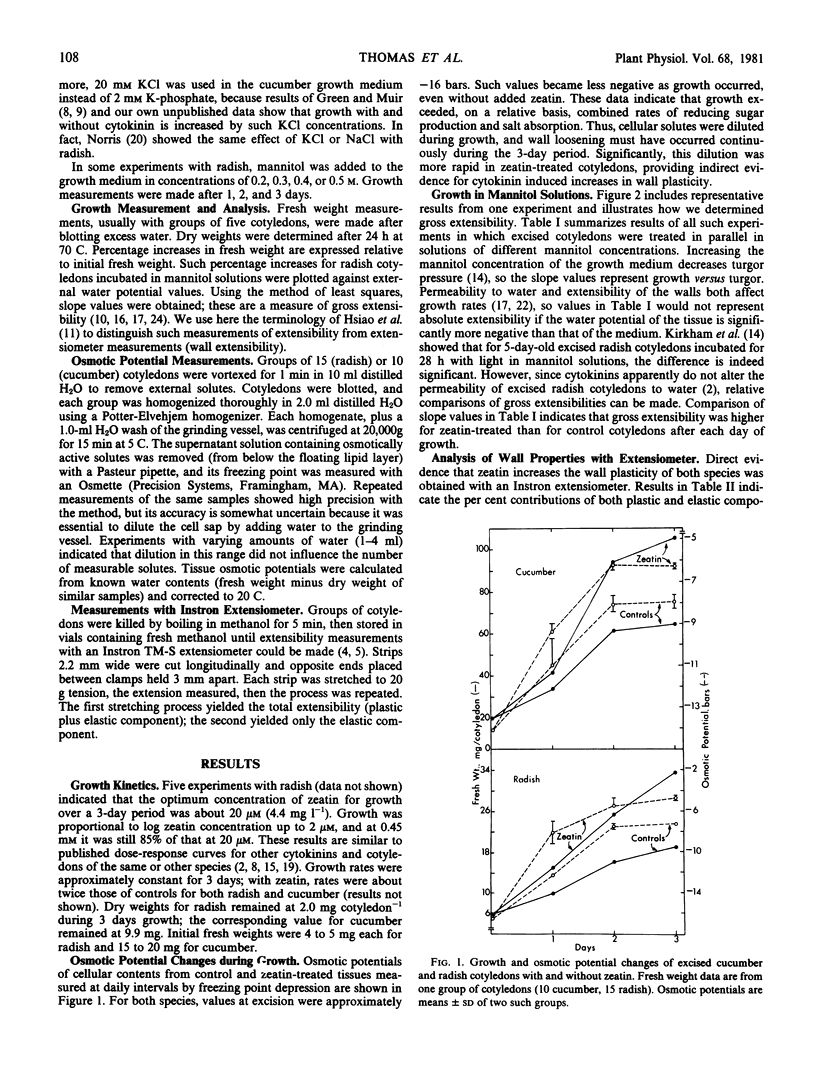
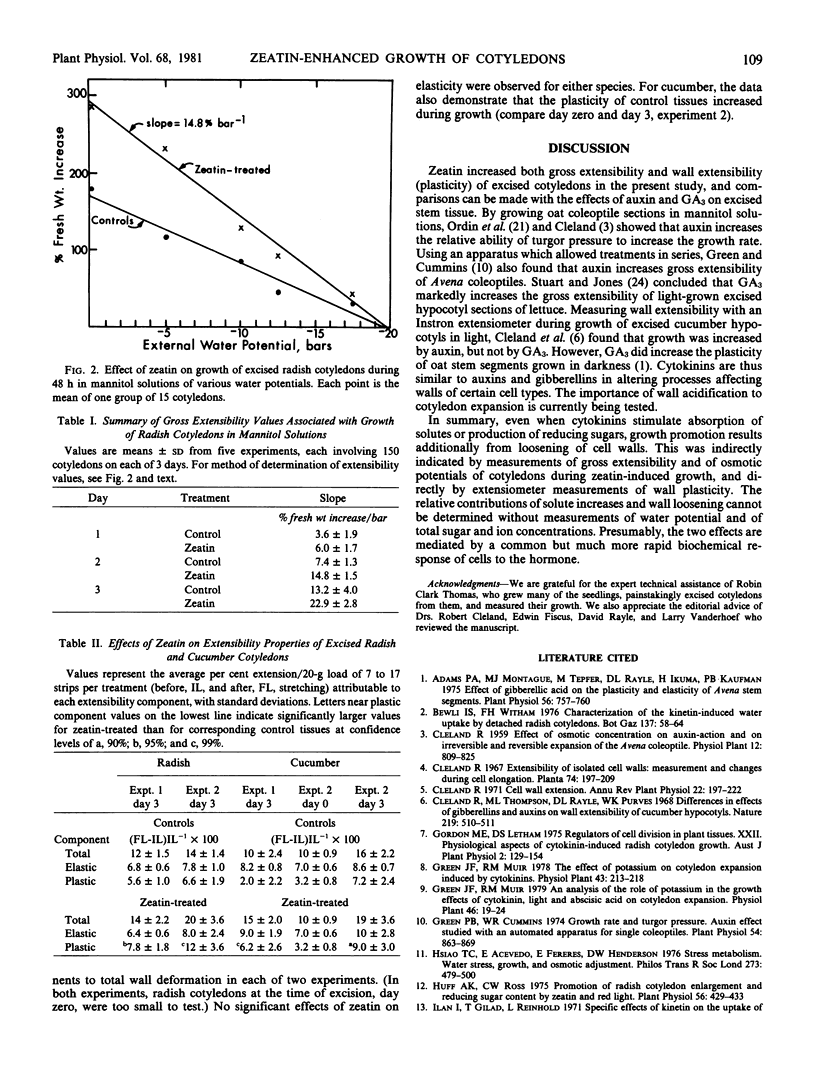
The mechanism of cytokinin-induced cell expansion in cotyledons excised from dark-grown seedlings of radish (Raphanus sativus L.) and cucumber (Cucumus sativus L.) was studied. Cotyledons were incubated in dim light with or without 17 micromolar zeatin for periods up to 3 days. Fresh weights and osmotic potentials were measured daily. Cell wall extensibility properties were measured before and after the growth period. Also, experiments in which radish cotyledons were grown in mannitol solutions of various concentrations were performed. Comparisons of growth rates and increases of tissue osmotic potentials (toward zero) during growth without mannitol indicate that wall extensibility increased during the growth period and that this extensibility was enhanced by zeatin.
Extensibility values derived from growth rates in mannitol provided indirect evidence of zeatin-increased wall extensibility. These conclusions were verified by direct measurements of plasticity with an Instron extensiometer. Thus, growth stimulation of excised cotyledons by cytokinins apparently involves wall loosening, in addition to previously demonstrated increases of K+ absorption and formation of reducing sugars.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. A., Montague M. J., Tepfer M., Rayle D. L., Ikuma H., Kaufman P. B. Effect of gibberellic Acid on the plasticity and elasticity of Avena stem segments. Plant Physiol. 1975 Dec;56(6):757–760. doi: 10.1104/pp.56.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beth Kirkham M., Gardner W. R., Gerloff G. C. Regulation of cell division and cell enlargement by turgor pressure. Plant Physiol. 1972 Jun;49(6):961–962. doi: 10.1104/pp.49.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R., Thompson M. L., Rayle D. L., Purves W. K. Difference in effects of gibberellins and auxins on wall extensibility of cucumber hypocotyls. Nature. 1968 Aug 3;219(5153):510–511. doi: 10.1038/219510a0. [DOI] [PubMed] [Google Scholar]
- Green P. B., Cummins W. R. Growth rate and turgor pressure: auxin effect studies with an automated apparatus for single coleoptiles. Plant Physiol. 1974 Dec;54(6):863–869. doi: 10.1104/pp.54.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff A. K., Ross C. W. Promotion of radish cotyledon enlargement and reducing sugar content by zeatin and red light. Plant Physiol. 1975 Sep;56(3):429–433. doi: 10.1104/pp.56.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. An analysis of irreversible plant cell elongation. J Theor Biol. 1965 Mar;8(2):264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Ordin L., Applewhite T. H., Bonner J. Auxin-Induced Water Uptake by Avena Coleoptile Sections. Plant Physiol. 1956 Jan;31(1):44–53. doi: 10.1104/pp.31.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D. A., Jones R. L. Roles of Extensibility and Turgor in Gibberellin- and Dark-stimulated Growth. Plant Physiol. 1977 Jan;59(1):61–68. doi: 10.1104/pp.59.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]



