Abstract
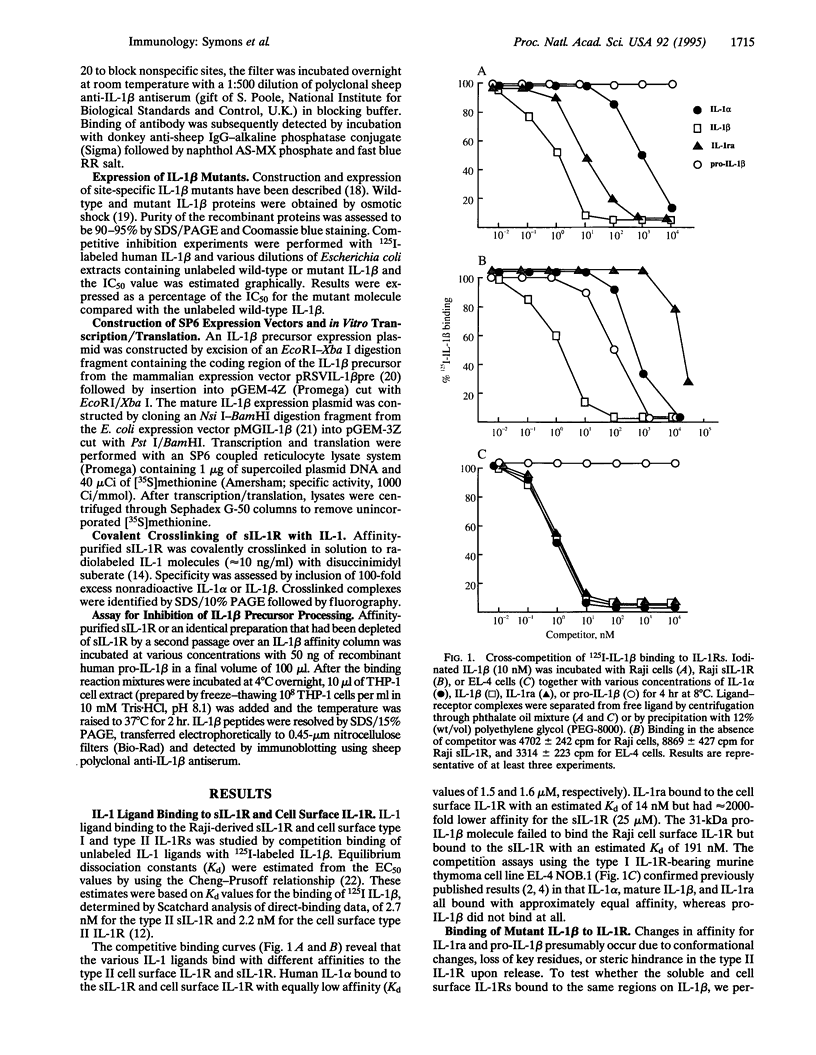
Two IL-1 receptors have been identified, termed type I and type II. The extracellular domain of the type II IL-1 receptor is released from certain cells and can function as a specific inhibitor of IL-1 beta activity. We assessed the ligand-binding properties of the type II membrane-bound and soluble IL-1 receptor (sIL-1R) from the human B cell line Raji by competition. Upon release, the affinity of sIL-1R for IL-1 alpha and IL-1 beta remained constant, and both soluble and cell surface IL-1 receptors bound to the same regions on the IL-1 beta molecule as defined by binding of a series of IL-1 beta mutant molecules. However, the affinity of sIL-1R for the IL-1 receptor antagonist (IL-1ra) decreased by a factor of 2000 when compared with the cell surface receptor. Type II sIL-1R and IL-1ra had an additive effect in inhibiting the binding of IL-1 beta to cell surface IL-1 receptors. In contrast, the combination of recombinant type 1 sIL-1R with IL-1ra abrogated the inhibition seen with each of the individual agents alone. The type II cell surface IL-1 receptor failed to bind the biologically inactive IL-1 beta precursor molecule, but binding to the IL-1 beta precursor was observed on cellular release of the receptor; this was confirmed with 35S-labeled IL-1 beta. Binding of IL-1 beta precursor by sIL-1R inhibited the precursor's ability to be processed to the mature, biologically active 17-kDa species. These observations suggest that the type II sIL-1R inhibits IL-1 beta at two steps, by preventing processing of propeptide and by blocking the interaction of mature IL-1 beta with type I IL-1 receptor. In addition, type II sIL-1R does not interfere with inhibition mediated by IL-1ra.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcamí A., Smith G. L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992 Oct 2;71(1):153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Antin J. H., Weinstein H. J., Guinan E. C., McCarthy P., Bierer B. E., Gilliland D. G., Parsons S. K., Ballen K. K., Rimm I. J., Falzarano G. Recombinant human interleukin-1 receptor antagonist in the treatment of steroid-resistant graft-versus-host disease. Blood. 1994 Aug 15;84(4):1342–1348. [PubMed] [Google Scholar]
- Cerretti D. P., Kozlosky C. J., Mosley B., Nelson N., Van Ness K., Greenstreet T. A., March C. J., Kronheim S. R., Druck T., Cannizzaro L. A. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992 Apr 3;256(5053):97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Colotta F., Re F., Muzio M., Bertini R., Polentarutti N., Sironi M., Giri J. G., Dower S. K., Sims J. E., Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993 Jul 23;261(5120):472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dower S. K., Wignall J. M., Schooley K., McMahan C. J., Jackson J. L., Prickett K. S., Lupton S., Cosman D., Sims J. E. Retention of ligand binding activity by the extracellular domain of the IL-1 receptor. J Immunol. 1989 Jun 15;142(12):4314–4320. [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Duff G. W. Identification of an interleukin-1 beta binding protein in human plasma. FEBS Lett. 1990 Jan 29;260(2):213–216. doi: 10.1016/0014-5793(90)80106-s. [DOI] [PubMed] [Google Scholar]
- Giri J. G., Newton R. C., Horuk R. Identification of soluble interleukin-1 binding protein in cell-free supernatants. Evidence for soluble interleukin-1 receptor. J Biol Chem. 1990 Oct 15;265(29):17416–17419. [PubMed] [Google Scholar]
- Granowitz E. V., Clark B. D., Mancilla J., Dinarello C. A. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J Biol Chem. 1991 Aug 5;266(22):14147–14150. [PubMed] [Google Scholar]
- Hannum C. H., Wilcox C. J., Arend W. P., Joslin F. G., Dripps D. J., Heimdal P. L., Armes L. G., Sommer A., Eisenberg S. P., Thompson R. C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990 Jan 25;343(6256):336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hazuda D. J., Lee J. C., Young P. R. The kinetics of interleukin 1 secretion from activated monocytes. Differences between interleukin 1 alpha and interleukin 1 beta. J Biol Chem. 1988 Jun 15;263(17):8473–8479. [PubMed] [Google Scholar]
- Hazuda D. J., Strickler J., Kueppers F., Simon P. L., Young P. R. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem. 1990 Apr 15;265(11):6318–6322. [PubMed] [Google Scholar]
- Joseph-Liauzun E., Leplatois P., Legoux R., Guerveno V., Marchese E., Ferrara P. Human recombinant interleukin-1 beta isolated from Escherichia coli by simple osmotic shock. Gene. 1990 Feb 14;86(2):291–295. doi: 10.1016/0378-1119(90)90293-z. [DOI] [PubMed] [Google Scholar]
- Krasney P. A., Young P. R. Further aspects of IL-1 beta secretion revealed by transfected monkey kidney cells. Cytokine. 1992 Mar;4(2):134–143. doi: 10.1016/1043-4666(92)90048-v. [DOI] [PubMed] [Google Scholar]
- Labriola-Tompkins E., Chandran C., Kaffka K. L., Biondi D., Graves B. J., Hatada M., Madison V. S., Karas J., Kilian P. L., Ju G. Identification of the discontinuous binding site in human interleukin 1 beta for the type I interleukin 1 receptor. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11182–11186. doi: 10.1073/pnas.88.24.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillquist J. S., Simon P. L., Summers M., Jonak Z., Young P. R. Structure-activity studies of human IL-1 beta with mature and truncated proteins expressed in Escherichia coli. J Immunol. 1988 Sep 15;141(6):1975–1981. [PubMed] [Google Scholar]
- McMahan C. J., Slack J. L., Mosley B., Cosman D., Lupton S. D., Brunton L. L., Grubin C. E., Wignall J. M., Jenkins N. A., Brannan C. I. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991 Oct;10(10):2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Dower S. K., Gillis S., Cosman D. Determination of the minimum polypeptide lengths of the functionally active sites of human interleukins 1 alpha and 1 beta. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4572–4576. doi: 10.1073/pnas.84.13.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Urdal D. L., Prickett K. S., Larsen A., Cosman D., Conlon P. J., Gillis S., Dower S. K. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987 Mar 5;262(7):2941–2944. [PubMed] [Google Scholar]
- Priestle J. P., Schär H. P., Grütter M. G. Crystal structure of the cytokine interleukin-1 beta. EMBO J. 1988 Feb;7(2):339–343. doi: 10.1002/j.1460-2075.1988.tb02818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. A., Black R. A., Kronheim S. R., Greenstreet T. A., Sleath P. R., Salvesen G. S., Pickup D. J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992 May 15;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Simon P. L., Kumar V., Lillquist J. S., Bhatnagar P., Einstein R., Lee J., Porter T., Green D., Sathe G., Young P. R. Mapping of neutralizing epitopes and the receptor binding site of human interleukin 1 beta. J Biol Chem. 1993 May 5;268(13):9771–9779. [PubMed] [Google Scholar]
- Sims J. E., Gayle M. A., Slack J. L., Alderson M. R., Bird T. A., Giri J. G., Colotta F., Re F., Mantovani A., Shanebeck K. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6155–6159. doi: 10.1073/pnas.90.13.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., March C. J., Cosman D., Widmer M. B., MacDonald H. R., McMahan C. J., Grubin C. E., Wignall J. M., Jackson J. L., Call S. M. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988 Jul 29;241(4865):585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- Slack J., McMahan C. J., Waugh S., Schooley K., Spriggs M. K., Sims J. E., Dower S. K. Independent binding of interleukin-1 alpha and interleukin-1 beta to type I and type II interleukin-1 receptors. J Biol Chem. 1993 Feb 5;268(4):2513–2524. [PubMed] [Google Scholar]
- Stylianou E., O'Neill L. A., Rawlinson L., Edbrooke M. R., Woo P., Saklatvala J. Interleukin 1 induces NF-kappa B through its type I but not its type II receptor in lymphocytes. J Biol Chem. 1992 Aug 5;267(22):15836–15841. [PubMed] [Google Scholar]
- Symons J. A., Duff G. W. A soluble form of the interleukin-1 receptor produced by a human B cell line. FEBS Lett. 1990 Oct 15;272(1-2):133–136. doi: 10.1016/0014-5793(90)80466-v. [DOI] [PubMed] [Google Scholar]
- Symons J. A., Eastgate J. A., Duff G. W. A soluble binding protein specific for interleukin 1 beta is produced by activated mononuclear cells. Cytokine. 1990 May;2(3):190–198. doi: 10.1016/1043-4666(90)90015-l. [DOI] [PubMed] [Google Scholar]
- Symons J. A., Eastgate J. A., Duff G. W. Purification and characterization of a novel soluble receptor for interleukin 1. J Exp Med. 1991 Nov 1;174(5):1251–1254. doi: 10.1084/jem.174.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Young P. R., Sylvester D. Cloning of rabbit interleukin-1 beta: differential evolution of IL-1 alpha and IL-1 beta proteins. Protein Eng. 1989 May;2(7):545–551. doi: 10.1093/protein/2.7.545. [DOI] [PubMed] [Google Scholar]