Abstract
Mycobacterium protein tyrosine phosphatase B (mPTPB) is a potential drug target of Tuberculosis (TB). Small molecule inhibitors of mPTPB could be a treatment to overcome emerging TB drug resistance. Using a Diversity-Oriented Synthesis (DOS) strategy, we successfully developed a salicylic acid based and drug-like mPTPB inhibitor with an IC50 of 2 μM and >20-fold specificity over many human PTPs, making it an excellent lead molecule for anti-TB drug discovery. In addition, DOS generated bicyclic salicylic acids are also promising starting points for acquiring inhibitors targeting other PTPs.
Protein tyrosine phosphatases (PTPs) have emerged as the next generation drug targets due to their complementary roles to protein tyrosine kinases in controlling protein tyrosine phosphorylation levels.[1] Small molecule PTP inhibitors thus possess enormous therapeutic potentials. However, there are two major challenges in PTP inhibitor development: specificity and cell permeability, due to conserved and highly positively charged active sites.[2] Consequently, most existing PTP inhibitors mimic the negatively charged pTyr substrate.[1d,3] We discovered that salicylic acid could serve as a novel pTyr mimetic, affording PTP inhibitors with excellent potency, specificity, and cell permeability.[4] To expand the salicylic acid based chemistry, we sought to employ Diversity-Oriented Synthesis (DOS)[5] strategy to develop salicylic acid based PTP inhibitors with novel and diverse structures, and more importantly, drug-like properties.
Tuberculosis (TB) is a worldwide threat to public health with 9 million new infections and 1.8 million deaths yearly.[6] Existing TB treatments require administration of antibiotics targeting mycobacterial RNA transcription, protein translation, and cell wall biogenesis for 6–9 months.[7] The lengthy treatment often leads to patient noncompliance, which contributes to the emergence of multidrug-resistant (MDR) and extensively resistant (XDR) TB. Thus there is urgent need to develop new and more effective therapies against TB.[8] Mycobacterium protein tyrosine phosphatase B (mPTPB) was identified as a promising target for novel anti-TB agents. It is secreted into host macrophages by Mtb and is a virulence factor to attenuate host immune responses.[9] Deletion of mPTPB impaired the ability of the mutant strain to survive in interferon-γ (IFN-γ) activated macrophages and severely reduced the bacterial load in a clinically-relevant guinea pig model.[10] Hence small molecules that inhibit mPTPB posses great potentials as anti-TB agents with nonoverlapping mechanism of action with existing drugs.[4c] Although a handful of mPTPB inhibitors have been reported, they often have high molecular weight, high lipophilicity, and flexible structures, and are thus not ideal lead molecules.[11] Herein we report our DOS strategy in generating novel salicylic acid based mPTPB inhibitors with good potency, specificity, cell activity, and more importantly, lean and compact structures with drug-likeness.
Given that bicyclic salicylic acid are more active than salicylic acid itself in inhibiting PTP activity as a result of enhanced interactions with the PTP active site,[4] our target molecules in DOS strategy encompass a range of novel bicyclic heterocycles (Fig. 1). To this end, we aim to install heteroatoms in adjacent positions on the parent benzene ring of salicylic acid, which furnishes molecular handles for the generation of the second heterocycles. The substituents can be amino, hydroxyl, halogen, and other groups that are easily functionalized. As a proof of concept, we synthesized protected salicylic acids with substituents of 4-hydroxy-5-amino (1), 4,5-diamino (2), 4-amino-5-iodo (3), and 4-amino-3-iodo (4). The syntheses of these intermediates are straightforward from commercially available starting materials (Scheme 1). 1 was obtained from 4-hydroxy salicylic acid in a sequence of nitration, protection, and reduction with 46% overall yield. Similarly, 2 was prepared in 5 steps including acetylation, nitration, hydrolysis, esterification, and reduction, with overall yield of 23%. Although iodine gave amino and iodo substituted 3 and 4 in low yields, using 1.1 equivalents of iodine monochloride successfully delivered products 3 and 4 in excellent yield, which were easily separated by flash chromatography.
Fig. 1.
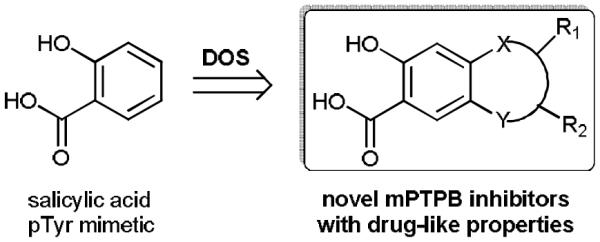
Structure of salicylic acid as pTyr mimetic and the design of DOS for discovery of novel and drug-like mPTPB inhibitors.
Scheme 1.
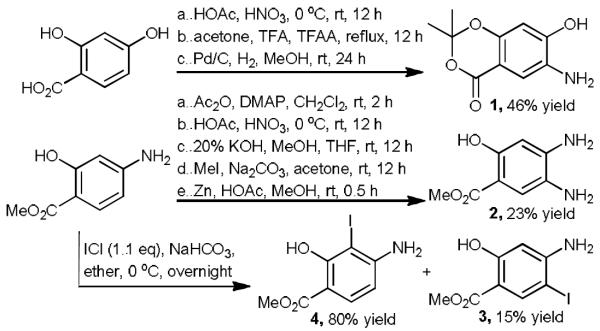
The synthesis of 3,4 or 4,5-disubstituted intermediates as precursors for DOS.
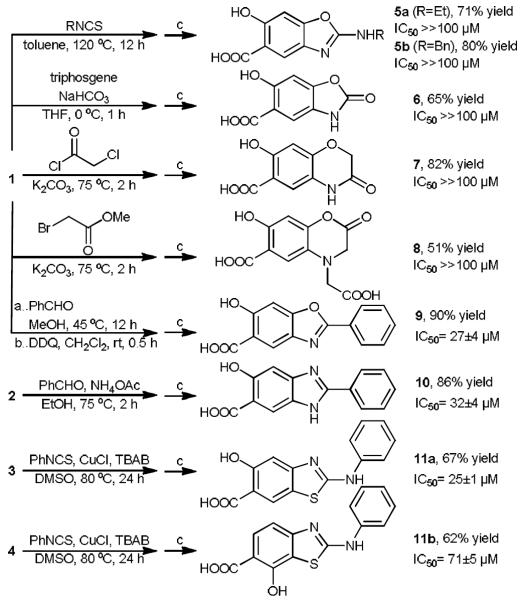
Intermediates 1–4 were treated with various cyclization reagents to make the second heterocyclic rings, which were then hydrolyzed in 10% LiOH/MeOH (v/v=1:1) to afford bicyclic salicylic acids (Scheme 2). Examples from intermediate 1 include isothiocynates for aminooxazole 5, triphosgene for cyclic carbamate 6, chloro acetic chloride and bromo acetic acid methyl ester for six membered lactam 7 and lactone 8, and a sequence of benzaldehyde and DDQ for oxazole 9.[12] From intermediate 2, the method for synthesizing oxazole 9 failed to give cyclised product 10, however, treatment of 2 with benzaldehyde in the presence of ammonium acetate successfully afforded benzimidazole 10 in excellent yield, and oxygen in the air was the oxidant for cycliation.[13] Intermediates 3 and 4 were reacted with phenyl isothiocynate to give aminobenzthiazole compounds 11a and 11b, respectively.[14,15] The reaction occurs in a sequence of thiourea formation and Cu(I) catalyzed S-arylation, and TBAB was proposed to play a role as ligand and phase-transfer catalyst. All products generated by the DOS strategy were obtained in moderate to good yields, and were purified by reversed phase HPLC to give structurally diverse bicyclic heterocycles with salicylic acid moiety. The ability of the compounds to inhibit the mPTPB catalyzed para-nitrophenyl phosphate hydrolysis was evaluated. Several structural motifs, including benzoxazole 9, benzimidazole 10, and aminobenzthiazole 11a, stand out as the most active among the DOS generated bicyclic salicylic acids 5–11, with IC50 values from 20 to 30 μM, while bicycles such as 6, 7, 8, did not exhibit activity at 100 μM, likely due to lack of aromaticity. Interestingly, 11a is 3-fold more active than its close analogues 11b, indicating that the hydroxyl group on the parent phenyl ring contributes to the affinity, and its location on the benzene ring influences compound's activity against mPTPB. Compound 11a's easy access, good activity, low molecule weight (MW = 286), coupled with the prevalence of the aminothiazole motif in FDA approved drugs such as Dasatinib and Norvir, make it ideal for further elaboration to develop drug-like mPTPB inhibitors with improved potency.
Scheme 2.
DOS of various bicyclic salicylic acids as mPTPB inhibitors. Reaction condition c: 10% LiOH/MeOH (v/v=1:1), 80 °C, 1 h.
Thus, a focused library of aminothiazole-salicylic acids was prepared by reacting precursor 3 with a set of aryl isothiocynates. Generally, o, m, or p-substituted phenyl isothiocyanates with either electron donating or withdrawing properties, as well as 1-naphthyl isothiocyanate, were tolerated to afford products in moderate to good yields, except that alkyl isothiocynates did not react with 3 under the same reaction conditions (Fig. 2). All hydrolyzed products were purified by HPLC to ensure high purity. SAR analysis indicated that a substituent at m position of isothiocynate is most beneficial, and compound 11h with m-bromo was found to be the most potent mPTPB inhibitor with an IC50 of 2.0 μM, representing a 12-fold increase in comparison to 11a. It should be noted that salicylic acid exhibits an IC50 value of 55±8 mM for mPTPB. Thus, compound 11h improved salicylic acid mPTPB inhibitory activity by 27,500 fold. Subsequent kinetic studies revealed that it is a noncompetitive inhibitor against pNPP with a Ki of 2.2±0.1 μM (Fig. S1, ESI†). To determine the specificity of 11h towards mPTPB, its inhibitory activity against representative human PTPs including cytosolic PTPs, PTP1B, SHP2, SHP1, PTP-Meg2, the receptor-like PTPs, CD45 and PTPε, and the dual specificity phosphatases VHR and Laforin, were measured. As shown in Table 1, 11h is highly selective for mPTPB, exhibiting over 20-fold preference against these PTPs. The basis of the high specificity of 11h was also studied by molecular modeling, which shows that salicylic acid head group in 11h strongly interacts with mPTPB active site P-loop residues CFAGKDR (Fig. 3), however, 11h has weaker interactions with cognate residues in the P-loop of PTP1B (highlighted in green color). This has been validated by sequence alignment showing that CFAGKDR is unique to mPTPB in comparison to many human PTPs (Fig. S2, S3, ESI†). In addition, benzthiazole and 3-bromo benzene of 11h interact with helices α3A, α7 and α8 of mPTPB, which are not present in human PTPs such as PTP1B and VHR (Fig. S3, ESI†). mPTPB secreted by Mtb down-regulates Erk1/2 activation in macrophage cells to block IL-6 production, while up-regulates Akt to promote survival, and mPTPB inhibitors can rescue these processes.[4c] Gratifyingly, 11h increased Erk1/2 and decreased Akt phosphorylations in mPTPB transfected Raw264.7 cells at 5 μM, 10 μM, in a dose dependent manner. By contrast, 11a (IC50 = 25 μM) has no such effects at 10 μM, validating 11h's excellent cellular activity in targeting mPTPB (Fig. S4, ESI†).
Fig. 2.
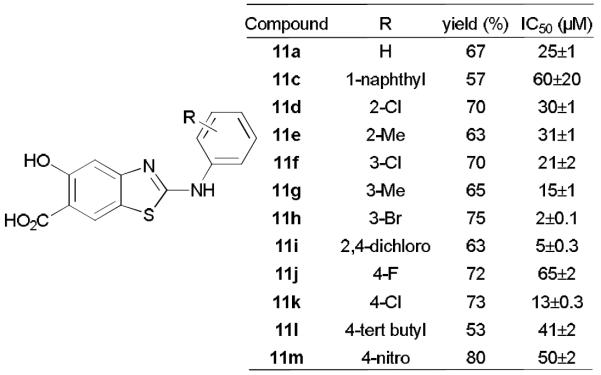
Structures of aminothiazole salicylic acids and their activities against mPTPB.
Table 1.
Specificity studies of compound 11h against a panel of PTPs.
| Enzyme | IC50 (μM) | Enzyme | IC50 (μM) |
| mPTPB | 2±0.1 | CD45 | >>100 |
| PTP1B | 65±1 | PTPε | >> 400 |
| SHP2 | 50±l | VHR | 43±3 |
| SHP1 | 78±8 | Laforin | 56±3 |
| PTP-Meg2 | 69±2 |
Fig. 3.
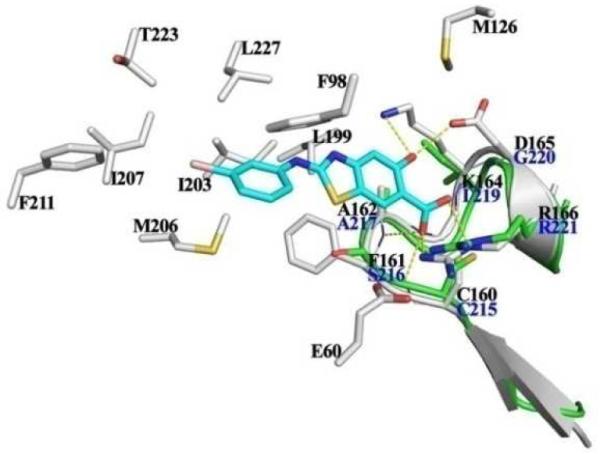
Proposed interactions between 11h and mPTPB. 11h (cyan) and mPTPB residues (gray) within 5Å distance are shown in stick, polar interactions are highlighted in dotted yellow line. The P-loop of PTP1B (green, side chains in blue) was superimposed into 11h•mPTPB complex, showing that 11h has weaker interactions with the P-loop of PTP1B.
In summary, we have successfully applied a DOS strategy for the discovery of a range of novel bicyclic salicylic acids as mPTPB inhibitors. A subsequent focused library provided compound 11h with improved potency and excellent specificity for mPTPB. With advantages of efficient synthesis, low molecular weight and excellent cell activity, it serves as a promising lead molecule for anti-TB drug discovery targeting mPTPB. Given the favourable pharmacological properties exhibited by the salicylic acid pharmacophore, the bicyclic scaffolds generated by the DOS strategy should also be good starting points for the development of drug-like inhibitors targeting other PTPs.
Supplementary Material
Acknowledgments
This work was supported in part by NIH GrantCA152194.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/b000000x/
Notes and references
- 1.a) Tonks NK, Neel BG. Curr. Opin. Cell Biol. 2001;13:182–195. doi: 10.1016/s0955-0674(00)00196-4. [DOI] [PubMed] [Google Scholar]; b) van Huijisduijnen RH, Bombrun A, Swinnen D. Drug Dicovery Today. 2002;7:1013–1019. doi: 10.1016/s1359-6446(02)02438-8. [DOI] [PubMed] [Google Scholar]; c) Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]; d) Bialy L, Waldmann H. Angew. Chem. Int. Ed. 2005;44:3814–3839. doi: 10.1002/anie.200461517. [DOI] [PubMed] [Google Scholar]; e) Ostman A, Hellberg C, Bohmer FD. Nat. Rev. Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]; f) Tonks NK. Nat. Rev. Mol. Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z-Y. Curr. Opin. Chem. Biol. 2001;5:416–423. 3. doi: 10.1016/s1367-5931(00)00223-4. [DOI] [PubMed] [Google Scholar]
- 3.a) Combs AP. J. Med. Chem. 2010;53:2333–2344. doi: 10.1021/jm901090b. [DOI] [PubMed] [Google Scholar]; b) Thareja S, Aggarwal S, Bhardwaj TR, Kumar M. Med. Res. Rev. 2012;32:459–517. doi: 10.1002/med.20219. [DOI] [PubMed] [Google Scholar]
- 4.a) Yu X, Sun J-P, He Y, Guo X-L, Liu S, Zhou B, Hudmon A, Zhang Z-Y. Proc. Natl. Acad. Sci. USA. 2007;104:19767–19772. doi: 10.1073/pnas.0706233104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang X, He Y, Liu S, Yu Z, Jiang Z-X, Yang Z, Dong Y, Nabinger SC, Wu L, Gunawan AM, Wang L, Chan RJ, Zhang Z-Y. J. Med. Chem. 2010;53:2482–2493. doi: 10.1021/jm901645u. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhou B, He Y, Zhang X, Xu J, Luo Y, Wang Y, Franzblau SG, Yang Z, Chan R, Liu Y, Zheng J, Zhang Z-Y. Proc. Natl. Acad. Sci. USA. 2010;107:4573–4578. doi: 10.1073/pnas.0909133107. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) He Y, Zeng L-F, Yu Z-H, He R, Liu S, Zhang Z-Y. Bioorg. Med. Chem. 2012;20:1940–1946. doi: 10.1016/j.bmc.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Arya P, Chou DTH, Baek M-G. Angew. Chem. Int. Ed. 2001;40:339–346. doi: 10.1002/1521-3773(20010119)40:2<339::AID-ANIE339>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]; b) Schreiber SL. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]; c) Burke MD, Schreiber SL. Angew. Chem. Int. Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]; d) Tan DS. Nat. Chem. Biol. 2005;1:74–84. doi: 10.1038/nchembio0705-74. [DOI] [PubMed] [Google Scholar]; d) Spandl RJ, Bender A, Spring DR. Org. Biomol. Chem. 2008;6:1149–1158. doi: 10.1039/b719372f. [DOI] [PubMed] [Google Scholar]
- 6.WHO Report 2010 on Global TB Control. 2010.
- 7.a) Fox W, Mitchison DA. Lancet. 1976;2:1349–1350. doi: 10.1016/s0140-6736(76)91989-9. [DOI] [PubMed] [Google Scholar]; b) Neff M. Am. Fam. Phys. 2003;68:1854, 1857–1858, 1861–1852. [Google Scholar]
- 8.a) Zhang Y. Annu. Rev. Pharmacol. Toxicol. 2005;45:529–564. doi: 10.1146/annurev.pharmtox.45.120403.100120. [DOI] [PubMed] [Google Scholar]; b) Clatworthy AE, Pierson E, Hung DT. Nat. Chem. Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]; c) Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Lancet. 2010;375:2100–2109. doi: 10.1016/S0140-6736(10)60359-9. [DOI] [PubMed] [Google Scholar]; d) Harries AD, Zachariah R, Corbett EL, Lawn SD, Santos-Filho ET, Chimzizi R, Harrington M, Maher D, Williams BG, De Cock KM. Lancet. 2010;375:1906–1919. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]; e) Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, Jensen P, Bayona J. Lancet. 2010;375:1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 9.Koul A, Herget T, Klebl B, Ullrich A. Nat. Rev. Microbiol. 2004;2:189–202. doi: 10.1038/nrmicro840. [DOI] [PubMed] [Google Scholar]
- 10.a) Koul A, Choidas A, Treder M, Tyagi AK, Drlica K, Singh Y, Ullrich A. J. Bacteriol. 2000;182:5425–5432. doi: 10.1128/jb.182.19.5425-5432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Singh R, Rao V, Shakila H, Gupta R, Khera A, Dhar N, Singh A, Koul A, Singh Y, Naseema M, Narayanan PR, Paramasivan CN, Ramanathan VD, Tyagi AK. Mol. Microbiol. 2003;50:751–762. doi: 10.1046/j.1365-2958.2003.03712.x. [DOI] [PubMed] [Google Scholar]
- 11.a) Noren-Muller A, Reis-Correa I, Jr., Prinz H, Rosenbaum C, Saxena K, Schwalbe H, Vestweber D, Cagna G, Schunk S, Schwarz O, Schiewe H, Waldmann H. Proc. Natl. Acad. Sci. USA. 2006;103:10606–10611. doi: 10.1073/pnas.0601490103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Correa IR, Jr., Noren-Muller A, Ambrosi H-D, Jakupovic S, Saxena K, Schwalbe H, Kaiser M, Waldmann H. Chem. Asian J. 2007;2:1109–1126. doi: 10.1002/asia.200700125. [DOI] [PubMed] [Google Scholar]; c) Noren-Muller A, Wilk W, Saxena K, Schwalbe H, Kaiser M, Waldmann H. Angew. Chem. Int. Ed. 2008;47:5973–5977. doi: 10.1002/anie.200801566. [DOI] [PubMed] [Google Scholar]; d) Weide T, Arve L, Prinz H, Waldmann H, Kessler H. Bioorg. Med. Chem. Lett. 2006;16:59–63. doi: 10.1016/j.bmcl.2005.09.051. [DOI] [PubMed] [Google Scholar]; e) Soellner MB, Rawls KA, Grundner C, Alber T, Ellman JA. J. Am. Chem. Soc. 2007;129:9613–9615. doi: 10.1021/ja0727520. [DOI] [PubMed] [Google Scholar]; f) Grundner C, Perrin D, Huijsduijnen RHV, Swinnen D, Gonzalez J, Gee CL, Wells TN, Alber T. Structure. 2007;15:499–509. doi: 10.1016/j.str.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Tan LP, Wu H, Yang P-Y, Kalesh KA, Zhang X, Hu M, Srinivasan R, Yao SQ. Org. Lett. 2009;11:5102–5105. doi: 10.1021/ol9023419. [DOI] [PubMed] [Google Scholar]; h) He Y, Xu J, Yu Z-H, Gunawan AM, Wu L, Wang L, Zhang Z-Y. J. Med. Chem. 2013;56:832–842. doi: 10.1021/jm301781p. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Zeng L-F, Xu J, He Y, He R, Wu L, Gunawan AM, Zhang Z-Y. ChemMedChem. 2013;8:904–908. doi: 10.1002/cmdc.201300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang J, Zhao K, Pan S. Tetra. Lett. 2002;43:951–954. [Google Scholar]
- 13.Sharghi H, Asemani O, Khalifeh R. Synth. Commun. 2008;38:1128–1136. [Google Scholar]
- 14.Zou B, Yuan Q, Ma D. Angew. Chem. Int. Ed. 2007;46:2598–2601. doi: 10.1002/anie.200700071. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y-J, Tang R-Y, Zhong P, Li J-H. Tetra. Lett. 2010;51:649–652. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.