Abstract
Background and purpose —
Metal artifact reduction sequence (MARS) MRI is widely advocated for surveillance of metal-on-metal hip arthroplasties (MOM-HAs). However, its use is limited by susceptibility artifact at the prosthesis-bone interface, local availability, patient compliance, and cost (Hayter et al. 2011a). We wanted to determine whether CT is a suitable substitute for MARS MRI in evaluation of the painful MOM-HA.
Patients and methods —
50 MOM-HA patients (30 female) with unexplained painful prostheses underwent MARS MRI and CT imaging. 2 observers who were blind regarding the clinical data objectively reported the following outcomes: soft tissue lesions (pseudotumors), muscle atrophy, and acetabular and femoral osteolysis. Diagnostic test characteristics were calculated.
Results —
Pseudotumor was diagnosed in 25 of 50 hips by MARS MRI and in 11 of 50 by CT. Pseudotumors were classified as type 1 (n = 2), type 2A (n = 17), type 2B (n = 4), and type 3 (n = 2) by MARS MRI. CT did not permit pseudotumor classification. The sensitivity of CT for diagnosis of pseudotumor was 44% (95% CI: 25–65). CT had “slight” agreement with MARS MRI for quantification of muscle atrophy (κ = 0.23, CI: 0.16–0.29; p < 0.01). Osteolysis was identified in 15 of 50 patients by CT. 4 of these lesions were identified by MARS MRI.
Interpretation —
CT was found to be superior to MRI for detection of osteolysis adjacent to MOM-HA, and should be incorporated into diagnostic algorithms. CT was unable to classify and failed to detect many pseudotumors, and it was unreliable for assessment of muscle atrophy. Where MARS MRI is contraindicated or unavailable, CT would be an unsuitable substitute and other modalities such as ultrasound should be considered
It is estimated that over 500,000 metal-on-metal (MOM) hip arthroplasties, including both hip resurfacing and total hip replacements (THRs), have been carried out worldwide in the last 15 years (Skinner et al. 2010). There are increasing reports of progressive soft tissue changes in response to metal debris including: solid or cystic, non-malignant masses around the prostheses (termed pseudotumors) (Pandit et al. 2008), perivascular lymphocytic infiltration (Davies et al. 2005), musculotendinous pathology (in particular, wasting of the hip abductors) (Sabah et al. 2011), and periprosthetic osteolysis (Park et al. 2005, Milosev et al. 2006, Korovessis et al. 2006).
There is international agreement that the high failure rate of MOM hip arthroplasties (MOM-HAs) has created the need for surveillance of these devices with cross-sectional imaging (MHRA. 2012). Both pseudotumors and muscle atrophy have been associated with high rates of major complications and poorer outcomes after revision surgery (Grammatopolous et al. 2009). To this end, sensitive detection of periprosthetic changes is vital in order to provide the best outcome for MOM-HA patients with early detection and revision.
Cross-sectional imaging has been shown to be useful for providing a diagnosis in cases of unexplained pain and in planning of revision surgery (Hayter et al. 2011b). A recent European multidisciplinary consensus statement recommended the use of cross-sectional imaging using any of US, MARS MRI, or CT (Hannemann et al. 2013). The gold standard modality is not clear, which has resulted in a variety of diagnostic algorithms being used in different referral centers.
Both CT and MARS MRI similarly offer multi-planar and complete cross-sectional images from which the extent of disease and relationship of the abnormality to normal anatomy can readily be appreciated. MARS MRI has been reliably and extensively used to investigate MOM hip complications (Sabah et al. 2011, Hayter et al. 2012a, Thomas et al. 2013, Nawabi et al. 2013) and has been shown to permit early diagnosis of pseudotumor and other soft tissue pathologies (Toms et al. 2008) associated with pain, loss of function, and higher revision rates. However, the use of MARS MRI is limited by susceptibility artifact at the prosthesis-bone interface, local availability, patient compliance, and cost.
CT is more widely available than MARS MRI (Anderson et al. 2011) and has been used routinely at some centers for the screening of periarticular masses (Bosker et al. 2012). It has been proposed as an alternative to it, for example in cases of claustrophobia, pacemaker, and where there are loose metal implants. CT has been shown to be useful in cases of suspected impingement, acetabular osteolysis (Cahir et al. 2007, Roth et al. 2012), and in identification of prostheses at risk of elevated wear (Hart et al. 2009). The notable success in detecting common complications of hip arthroplasty coupled with widespread accessibility has meant that some centers rely entirely on CT (McMinn. 2012) to follow up patients with suspected MOM-associated bony and soft tissue changes, but to date there have been no published studies comparing CT with MRI.
We investigated whether CT is a suitable substitute for MARS MRI in the evaluation of the painful MOM-HA. We wanted to provide measures of diagnostic accuracy of CT compared to the current gold standard (MARS MRI) for the detection of common periprosthetic complications. The primary outcome measure focused on the detection of pseudotumors, owing to their high prevalence and strong association with adverse outcomes (Hart et al. 2009), with secondary outcome measures for the detection of muscle atrophy and osteolysis.
Patients and methods
Patients
50 patients with unexplained painful prostheses (Oxford hip score ≤ 41 out of 48) were recruited consecutively. Patients who had undergone cross-sectional imaging less than 9 months postoperatively were not included, to avoid detection of normal postoperative inflammatory changes.
Image acquisition
MARS MRI images were acquired using a 1.5-Tesla (T) scanner (MAGNETOM 1.5T; Siemens Medical, Erlangen, Germany) with previously published sequence parameters (Hart et al. 2009, Sabah et al. 2011, Hart et al. 2012). Metal artifact reduction sequences obtained included axial T1-weighted turbo spin-echo (TSE) (echo time (TE) 8 milliseconds; repetition time (TR) 509 ms), axial T2-weighted TSE (TE 67 ms; TR 4,840 ms), coronal T1- weighted TSE (TE 7.1 ms; TR 627 ms), sagittal T2-weighted TSE (TE 68 ms; TR 2,820 ms), and a coronal short tau inversion recovery (STIR) sequence (TE 36 ms; TR 3,770 ms). For all images, section thickness was 5 mm, field of view was 340 × 340 mm, and pixel bandwidth was up to 781 MHz.
Metal artifact reduction CT images were acquired in accordance with the Siemens sensation 64-slice CT scanner (Siemens Medical Solutions, Erlangen, Germany) used in this study.
Image evaluation
All images were retrospectively evaluated by consensus agreement of 2 observers: a consultant musculoskeletal radiologist (KS) and a consultant orthopedic surgeon (SS) with experience in reviewing this type of imaging, both of whom were blind regarding the clinical details. Scans were reviewed on a dedicated PACS workstation with 3 megapixel resolution. CT image evaluation was optimized for assessment of both soft tissue and structures using separate software algorithms. The presence or absence of common MOM-associated pathology was noted including soft tissue lesions, muscle atrophy, and acetabular and femoral osteolysis according to predefined criteria (Table 1).
Table 1.
Objective criteria used to evaluate and compare MARS MRI and CT for the assessment of MOM hips
| Soft tissue lesion | ||
| Soft tissue lesion present or absent? | Yes/No | |
| Imperial classification? | 1/2a/2b/3 | |
| Location(s) in relation to joint? | Anterior/Posterior/Medial/Lateral | |
| Size (mm)? | Anterioposterior x Mediolateral x Craniocaudal | |
| Musculotendinous pathology | ||
| Hip muscle atrophy? | Grade 0/1/2/3 | |
| • Glutei (gluteus maximus, medius, and minimus) | ||
| • Short external rotators (piriformis, obturator internus, obturator externus) | ||
| • Iliopsoas | ||
| • Quadratus femoris | ||
| Bony pathology | ||
| Osteolysis? | Yes/No | |
| If yes, femoral/acetabular/both? |
Soft tissue lesions were uniformly characterized across the 2 modalities according the specified parameters: a previously published pseudotumor classification (1, 2a, 2b, 3; see Table 2), size in 3 dimensions, and location of the lesion in relation to the joint. Lesions seen on MRI were characterized using both T1W and T2W images.
Table 2.
Comparable pseudotumor classifications in MARS MRI and low-dose 3-D CT
| MARS MRI |
CT |
||
|---|---|---|---|
| Type | Description | Type | Description |
| Type 1 | Flat, thin-walled (≤ 2 mm), fluid-like content (hypointense on T1 and hyperintense on T2) | Type 1 | Flat, thin-walled (≤ 2 mm), fluid- like content (less attenuation than skeletal muscle) |
| Type 2a | Not flat, thick-walled (> 2 mm), fluid-like content | Type 2a | Not flat, thick-walled (> 2 mm), fluid-like content |
| Type 2b | Any shape, thick-walled (> 2 mm), atypical fluid (hyperintense on T1 and variable on T2) | Type 2b | Any shape, thick-walled (> 2 mm), atypical fluid (greater attenuation than skeletal muscle) |
| Type 3 | Any shape, mixed signal, solid throughout | Type 3 | Any shape, mixed attenuation, solid throughout |
(Hart et al. 2012) Adapted from (Hart et al. 2012)
Extent of muscle atrophy was comparably defined for both modalities as a decrease in muscle volume using the contralateral, asymptomatic hip as a control. Atrophy was graded using the Bal and Lowe system (Bal and Lowe 2008), which was adapted for CT evaluation to give a standardized grading ranging from 0 (no change) to 3 (greater than 70% decrease in size with the additional evidence of fatty infiltration seen on MRI) (Table 3). Assessment and grading for the hip abductors (gluteus minimus, medius, and maximus), iliopsoas, quadratus femoris, and the short external rotators (piriformis, obturator internus, and obturator externus) was completed. Muscle atrophy was assessed on MRI using T1W images.
Table 3.
Comparable muscle atrophy classification in MARS MRI and low-dose CT
| MARS MRI |
CT |
||
|---|---|---|---|
| Grade | Description | Grade | Description |
| Grade 0 | No change | Grade 0 | No change |
| Grade 1 | < 30% reduction in muscle size | Grade 1 | < 30% reduction in muscle size |
| Grade 2 | 30–70% fatty change and reduction in muscle size | Grade 2 | 30–70% reduction in muscle size |
| Grade 3 | > 70% fatty change and reduction in size | Grade 3 | > 70% reduction in muscle size |
(Bal and Lowe 2008)Adapted from (Bal and Lowe 2008)
Osteolysis was defined as a well demarcated, intraosseous lesion with intermediate to slightly increased signal intensity contrasting with the high-intensity signal of intrameduallary fat on MRI (Hayter et al. 2012b), and as a well demarcated area of lucency without osseous trabeculae on CT (Puri et al. 2002, Park et al. 2004). The anatomical location of any lesion was noted as being acetabular or femoral.
Statistics
The diagnostic accuracy of CT for the detection of periprosthetic soft tissue lesions and muscle atrophy, and of MARS MRI for the detection of periprosthetic osteolysis was quantified by measuring sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Values were calculated using MARS MRI findings as the reference standard for soft tissue lesions and muscle atrophy, and CT was used as the “true” status for the presence of osteolysis.
Cohen’s κ (kappa) coefficient was used to measure the level of agreement between the 2 modalities regarding grading of muscle atrophy. Consistent nomenclature in the measure of relative strength of agreement was achieved using the descriptive thresholds stated by Landis and Koch (1977).
Throughout the study, uncertainty in estimates was expressed using 95% confidence intervals (CIs), with statistical significance taken at the 5% level (p < 0.05). All analyses were conducted using SPSS software version 17.0.
Ethics
Ethical approval for this study was granted in 2009 by the Riverside Ethics Committee, London (COREC 07/Q0401/25).
Results
Demographics
We assessed 50 patients (30 female) with a median age of 55 (IQR: 42–64) years who had undergone MARS MRI and CT with a median difference in timing of 2.5 (IQR: 0–5.5) months between examinations. 37 of the cohort had hip resurfacing-type prostheses (4 ASR, 15 Birmingham Hip Resurfacing, 17 Cormet, and 1 Conserve), while the remaining 13 participants had MOM THRs (2 Corail Pinnacle, 2 Durom, 2 Stanmore, 1 Recap Magnum Cadcam, 1 Furlong, 1 Taperloc Magnum, 1 Wright Profemur, 1 Metasul, 1 Conserve, and 1 Biomet Modular).
Soft tissue lesions
Pseudotumors were diagnosed in 25 of 50 hips by MARS MRI, 17 of which were classified as fluid type-2a lesions. 11 were seen when CT was used for the assessment of unexplained hip pain. None of the lesions were classified by CT, as diagnostic characteristics were not discernible with all lesions iso-attenuated to skeletal muscle. We noted that the inferior soft tissue contrast and residual scatter of the metal artifact frequently hindered the assessment of pseudotumor on CT (Figure 1). The sensitivity of CT for diagnosis of pseudotumor was 44% (CI: 25–65) against the gold standard (MR).
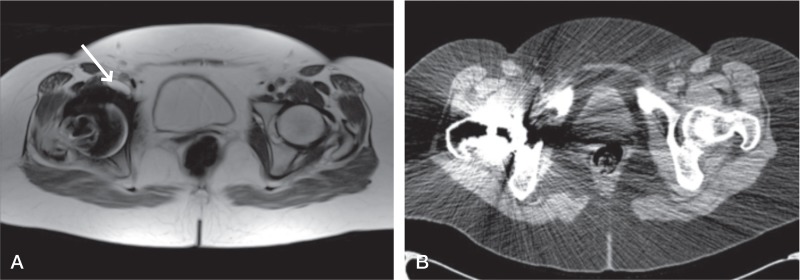
Figure 1.
Patient 6. Type-2a lesion (indicated by arrow) classified on MARS MRI scan (A) but lesion cannot be seen on the equivalent CT scan (B). The high attenuation coefficient of the metal implant on CT has led to significant scatter obscuring much of the periprosthetic anatomy, further compounded by a less clear distinction of soft tissues with this modality.
The volume of lesions detected ranged from 0.48 to 823 cm3. Most lesions detected on CT (7 of 11) corresponded to the largest lesions found on MRI assessment (Figure 2).
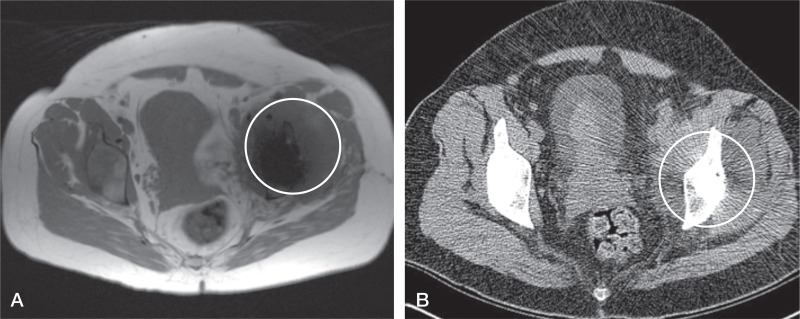
Figure 2.
Large pseudotumor (circled) clearly visible on both MARS MRI scan (A) and CT scan (B), as the anatomy of the affected side is grossly distorted when compared to the contralateral, asymptomatic hip.
Hip musculature
Evidence of moderate or severe muscle atrophy (> 30% reduction in muscle bulk) was present to varying extents in 49 of 50 patients with unexplained hip pain. The overall sensitivity and specificity of CT for the detection of hip muscle wasting was 81% (CI: 77–85) and 37% (CI: 24–52) respectively. The weighted kappa coefficient for muscle atrophy grade found the agreement between the 2 modalities to be “fair” (κ = 0.23, CI: 0.16–0.29; p = < 0.01).
The evaluation of muscles and comparison to contralateral anatomy were hindered on CT by the residual scatter and poor soft tissue resolution, as exemplified by the failure to properly visualize muscles in 12 instances (Figure 3). Although artifact is not completely resolved on MARS MRI, the evaluation of any muscle was not prevented by it in any case.
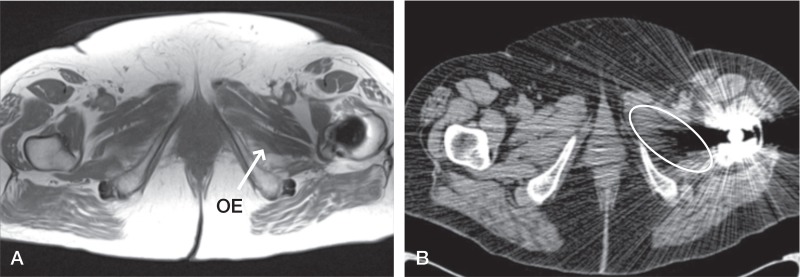
Figure 3.
Patient 13. All muscles visualized on MARS MRI scan (A), but obturator externus (OE) (labelled on left and circled on right) could not be seen on the equivalent CT scan (B) despite being viewed in a soft tissue window.
Bony pathology
Osteolysis was identified in 15 of 50 patients. By MARS MRI evaluation, osteolytic lesions were noted in 4 patients. The sensitivity of MRI for the detection of osetolysis was 27% (CI: 8.9–55) and the specificity was 1% (CI: 1–88). The residual metal artifact of spurious signal voids, high signal areas, and image distortion caused on MRI by the presence of the metallic implant led to a particular distortion of the periactabular anatomy, hindering a comparable evaluation of bony integrity. We noted that the bony interface was much more difficult to discern on MRI images, due to a lower bone contrast with this modality, compared to a bone-windowed CT image (Figure 4).
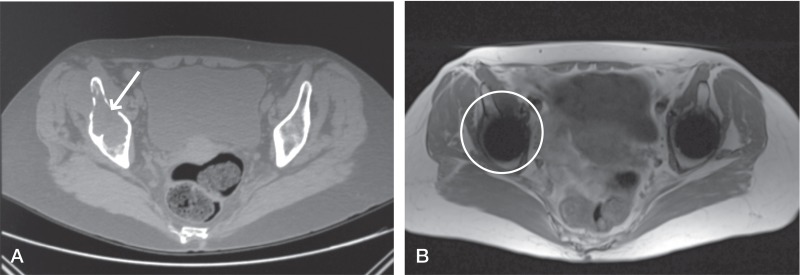
Figure 4.
Comparison of the CT image (A) and the MARS MRI image (B) used to evaluate osteolysis in the same patient illustrates the comparative difficulties in identifying acetabular anatomy on MRI images, which are clear on the corresponding CT image. There is an absence of signal on MRI in this region. Coupled with the inferior bony distinction on MRI, this has prevented the identification of osteolytic changes—which are clear on CT (see arrow).
Discussion
Evaluation of soft tissue pathology
This is the first study to compare CT and MRI in patients with MOM-HA. While it has been shown previously that both MARS MRI and CT are capable of imaging soft tissues in proximity to MOM-HAs (Thomas et al. 2013, Bosker et al. 2012), our study has identified that CT has poor diagnostic performance for soft tissue assessment, which is the main clinical indication for cross-sectional imaging in these patients.
Our findings indicate that little more than two-fifths of all pseudotumor cases apparent on MARS MRI are seen when CT is used to detect the same lesions, with a sensitivity of 44%. We consistently found that substantial scatter generated by the high attenuation coefficient of the metal implant obscured much of the periprosthetic anatomy. This was further compounded by a less clear distinction between soft tissues with this modality, and together they hindered evaluation of soft tissues to the extent possible with MARS MRI, resulting in fewer diagnoses.
We used a well-published reporting form (Sabah et al. 2011), which allows the radiologist to describe with consistency the size, location, and characteristics of pseudotumors seen. This provides orthopedic surgeons with detailed information necessary for patient management and operative planning. The inability of CT to reliably identify lesions, and to classify those it does detect, should preclude its use as a decision-making tool. While pseudotumors are not malignant, they may be associated with catastrophic soft tissue necrosis (Pandit et al. 2008) and a high rate of major complications after revision surgery (Grammatopolous et al. 2009). So early detection and timely revision are vital in the management of such patients, and they can be achieved more reliably through MARS MRI imaging.
This is further supported by the finding that most lesions detected by CT corresponded to the largest lesions visualized on MRI. We found that diagnosis by CT is comparably more difficult until gross changes and distortions to the anatomy have occurred. This further demonstrates that CT is an inferior screening tool—particularly for identifying early changes in MOM patients—and suggests that it should not be used as the first part of an algorithm to screen for changes, as it currently is at some centers (Bosker et al. 2012).
As in previous studies (Sabah et al. 2011), a high prevalence of hip muscle atrophy (87%) was identified in our symptomatic cohort. Hip muscle wasting has been suggested to be a non-specific marker of underlying hip pathology in MOM patients with pain (Sabah et al. 2011) and has been associated with poor outcomes following revision of MOM-HA (Munro et al. 2014). When evaluating hip muscle atrophy, we found that CT was diagnostically inferior and generated a high rate of false negatives, so that almost one-third of all wasting was not identified in a comparable way, with a specificity of 37%. The only “fair” agreement found in the grading of atrophy between CT and MARS MRI reflects the markedly poor soft tissue definition and resolution using CT, making an assessment of the extent of disease less precise.
While MRI allows assessment of both muscle bulk and quality, CT gives an idea of bulk alone. This is particularly important in the evaluation of symptomatic MOM hip patients, as there is a correlation between muscle atrophy and poor function (Hart et al. 2009). A wide range of muscle changes that can occur postoperatively, the most extreme being muscle necrosis (Figure 2). Several studies have found such changes in association with formation of rare solid pseudotumors (Toms et al. 2008, Sabah et al. 2011, Hauptfleisch et al. 2012). Key features that identify an underlying pseudotumor process are: erosion of tissue planes, loss of striated muscle appearance, and integrity of the muscle tendons (Toms et al. 2008, Hart et al. 2012). The inability of CT to differentiate such changes in the quality of the soft tissue hinders both identification and quantification of the underlying disease process.
Consistent with the evaluation of pseudotumors, the residual scatter of the metal artifact and inferior soft tissue contrast also contributed to the inferior performance of CT in detecting and quantifying muscle atrophy—emphasized by the failure to properly visualize 12 muscles in this cohort. These findings illustrate where MARS MRI has the ability to identify muscle atrophy in patients before clinical deterio¬ration adversely affects revision outcomes. CT cannot provide the same level of screening or detection.
This evidence calls into question the value of CT when used as a diagnostic and screening tool for pseudotumors and hip muscle atrophy in symptomatic MOM patients. Detection of soft tissue pathology related to MOM-HA can be achieved more reliably by investigation using MARS MRI, which can provide more detailed information to allow further treatment decisions.
Evaluation of osteolysis
Osteolysis is a commonly recognised complication of MOM prostheses (Park et al. 2005). It affects a significant proportion of symptomatic patients (Korovessis et al. 2006, Carr and DeSteiger 2008, Chang et al. 2012, Hayter et al. 2012a), as found in this study (prevalence 30%). CT has proven efficacy in detecting osteolysis associated with hip arthroplasty, meriting its application in the diagnosis of MOM patients and its preferential use over MRI when osteolysis is suspected (Cahir et al. 2007). Contrary to this premise, a previous cadaveric model comparing MRI and CT for the detection of periprosthetic osteolysis suggested that MRI may in fact be the more sensitive modality (Walde et al. 2005). Our direct, in vivo comparison has shown that MARS MRI is inferior to CT for the detection of osteolytic changes associated with MOM arthroplasty, with a sensitivity of only 27%.
We found that the comparatively poor bone contrast, due to the low water content of cortical bone, and the residual metal artifact, especially distorting the periacetabular anatomy, means that using MARS MRI as a mode of detection and surveillance of periprosthetic bony changes remains a challenge, and is largely unreliable. The promise of new MRI sequences, such as multi-acquisition variable-resonance image combination (MAVRIC)—which has been shown to improve periprosthetic bone visualization (Hayter et al. 2011a)—offer the potential of a single investigation to evaluate symptomatic patients with no radiation exposure. The clinical expectation would be that pulse sequences such as these would become more available routinely in the future. However, very few scanners capable of implementing these sequences are currently available. As such, our study highlights the value of clinically available modalities that are routinely used in the follow-up of symptomatic MOM patients. Our findings lead us to advocate the continued incorporation of CT into diagnostic algorithms where osteolysis is suspected, and the necessity of a multimodal approach.
Limitations of the study
We used MARS MRI as the “gold standard” test for soft tissue disease. We did not perform surgical validation, but have previously shown close correlation using the same imaging sequence (Sabah et al. 2011).
In addition, we did not calculate inter-rater statistics. In our practice, all cross-sectional images of MOM hips are evaluated in a multidisciplinary meeting with radiologists and surgeons. We feel that consensus reporting closely reflects clinical practice.
We used low-dose CT for soft tissue assessment. These scans were routinely performed in our center, with assessment of component position being the primary outcome measure. We acknowledge that higher-dose, soft tissue protocol CT might be expected to perform better. However, there is a need for repeat scans in the follow-up of MOM hip patients. It is not acceptable to subject young patients—who might become parents—to repeated irradiation of the gonads; especially as our study has shown that this modality does not perform adequately in visualization of periprosthetic complications. Low-dose protocols have been refined to minimize the effective radiation dose while still producing images in which soft tissue and bony interfaces remain well defined (Henckel et al. 2006), but we have not observed adequate diagnostic performance in the scans performed in our center for detection of periprosthetic changes.
To summarize, this is the first direct comparison of the diagnostic performance of CT and MARS MRI for the evaluation of symptomatic MOM-HA patients. While both modalities have been used successfully to evaluate the soft tissues of such patients, we have found that MARS MRI is superior to CT for the diagnosis and characterization of pseudotumor and muscle atrophy with a view to patient management and operative planning.
In addition, we found that CT is a more effective modality for detecting periprosthetic bony changes in MOM hips than MARS MRI. Based on our findings, we advocate a multimodal assessment of symptomatic patients to detect the full spectrum of MOM-associated pathologies in the most sensitive way. For these patients, a single-modality algorithm would prove to be less useful.
Metal artifact encountered in the use of these 2 modified modalities still prevents accurate diagnosis in some cases. The promise of new MRI sequences may alter some of the conclusions made here about the comparative performance of each modality.
Acknowledgments
ER: initial conception and design of the project, writing of the paper, and analysis and interpretation of data. JH: initial conception, and editing of the paper. SS and KS: collection of data, input in study design, and editing of the paper. JS: provision of resources and data. AH: ensuring the scientific integrity of the work, and editing of the paper.
No competing interests declared.
References
- Anderson H, Toms AP, Cahir JG, Goodwin RW, Wimhurst J, Nolan JF. Grading the severity of soft tissue changes associated with metal-on-metal hip replacements: reliability of an MR grading system . Skeletal Radiol. 2011;40:303–7. doi: 10.1007/s00256-010-1000-7. [DOI] [PubMed] [Google Scholar]
- Bal BS, Lowe JA. Muscle damage in minimally invasive total hip arthroplasty: MRI evidence that it is not significant . Instr Course Lect. 2008;57:223–9. [PubMed] [Google Scholar]
- Bosker BH, Ettema HB, Boomsma MF, Kollen BJ, Maas M, Verheyen CC. High incidence of pseudotumour formation after large-diameter metal-on-metal total hip replacement: a prospective cohort study . J Bone Joint Surg (Br) 2012;94:755–61. doi: 10.1302/0301-620X.94B6.28373. [DOI] [PubMed] [Google Scholar]
- Cahir JG, Toms AP, Marshall TJ, Wimhurst J, Nolan J. CT and MRI of hip arthroplasty . Clin Radiol. 2007;62:1163–71. doi: 10.1016/j.crad.2007.04.018. discussion 1172-3. [DOI] [PubMed] [Google Scholar]
- Carr AM, Desteiger R. Osteolysis in patients with a metal-on-metal hip arthroplasty . ANZ J Surg. 2008;78:144–7. doi: 10.1111/j.1445-2197.2007.04390.x. [DOI] [PubMed] [Google Scholar]
- Chang EY, Mcanally JL, Van Horne JR, Statum S, Wolfson T, Gamst A, Chung CB. Metal-on-metal total hip arthroplasty: do symptoms correlate with MR imaging findings? . Radiology. 2012;265:848–57. doi: 10.1148/radiol.12120852. [DOI] [PubMed] [Google Scholar]
- Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements . J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- Grammatopolous G, Pandit H, Kwon YM, Gundle R, Mclardy-Smith P, Beard DJ, Murray DW, Gill HS. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome . J Bone Joint Surg (Br) 2009;91:1019–24. doi: 10.1302/0301-620X.91B8.22562. [DOI] [PubMed] [Google Scholar]
- Hannemann F, Hartmann A, Schmitt J, et al. European multidisciplinary consensus statement on the use and monitoring of metal-on-metal bearings for total hip replacement and hip resurfacing . Orthop Traumatol Surg Res. 2013;99:263–71. doi: 10.1016/j.otsr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Sabah S, Henckel J, Lewis A, Cobb J, Sampson B, Mitchell A, Skinner JA. The painful metal-on-metal hip resurfacing . J Bone Joint Surg (Br) 2009;91:738–44. doi: 10.1302/0301-620X.91B6.21682. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Satchithananda K, Liddle AD, Sabah SA, Mcrobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell AW. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging . J Bone Joint Surg Am. 2012;94:317–25. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- Hauptfleisch J, Pandit H, Grammatopoulos G, Gill HS, Murray DW, Ostlere S. A MRI classification of periprosthetic soft tissue masses (pseudotumours) associated with metal-on-metal resurfacing hip arthroplasty . Skeletal Radiol. 2012;41:149–55. doi: 10.1007/s00256-011-1329-6. [DOI] [PubMed] [Google Scholar]
- Hayter CL, Potter HG, Su EP. Imaging of metal-on-metal hip resurfacing . Orthop Clin North Am. 2011a;42:195–205. doi: 10.1016/j.ocl.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Hayter CL, Koff MF, Shah P, Koch KM, Miller TT, Potter HG. MRI after arthroplasty: comparison of MAVRIC and conventional fast spin-echo techniques . AJR Am J Roentgenol. 2011b;197:W405–11. doi: 10.2214/AJR.11.6659. [DOI] [PubMed] [Google Scholar]
- Hayter CL, Gold SL, Koff MF, Perino G, Nawabi DH, Miller TT, Potter HG. MRI findings in painful metal-on-metal hip arthroplasty . AJR Am J Roentgenol. 2012a;199:884–93. doi: 10.2214/AJR.11.8203. [DOI] [PubMed] [Google Scholar]
- Hayter CL, Koff MF, Potter HG. Magnetic resonance imaging of the postoperative hip . J Magn Reson Imaging. 2012b;35:1013–25. doi: 10.1002/jmri.23523. [DOI] [PubMed] [Google Scholar]
- Henckel J, Richards R, Lozhkin K, Harris S, Rodriguez Y, Baena FM, Barrett AR, Cobb JP. Very low-dose computed tomography for planning and outcome measurement in knee replacement. The imperial knee protocol . J Bone Joint Surg (Br) 2006;88:1513–8. doi: 10.1302/0301-620X.88B11.17986. [DOI] [PubMed] [Google Scholar]
- Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up . J Bone Joint Surg Am. 2006;88:1183–91. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data . Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- McMinn D. McMinn Centre: MHRA Advice for Patients. [Online] [Cited 2014 April 01]. Available from: http://www.mcminncentre.co.uk/mhra-advice-patients.html . [Google Scholar]
- MHRA (Medicines and Healthcare products Regulatory Agency). Medical Device Alert: All metal-on-metal (MoM) hip replacements (MDA/2012/036) [Online] 2012 [Cited 2014 April 01]. Available from: http://www.mhra.gov.uk/home/groups/dts-bs/documents/medicaldevicealert/con155767.pdf .
- Milosev I, Trebse R, Kovac S, Cor A, Pisot V. Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years . J Bone Joint Surg Am. 2006;88:1173–82. doi: 10.2106/JBJS.E.00604. [DOI] [PubMed] [Google Scholar]
- Munro JT, Masri BA, Duncan CP, Garbuz DS. High complication rate after revision of large-head metal-on-metal total hip arthroplasty . Clin Orthop Relat Res. 2014;472:523–8. doi: 10.1007/s11999-013-2979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2013;472:471–81. doi: 10.1007/s11999-013-2788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit H, Glyn-Jones S, Mclardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings . J Bone Joint Surg (Br) 2008;90:847–51. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- Park JS, Ryu KN, Hong HP, Park YK, Chun YS, Yoo MC. Focal osteolysis in total hip replacement: CT findings . Skeletal Radiol. 2004;33:632–40. doi: 10.1007/s00256-004-0812-8. [DOI] [PubMed] [Google Scholar]
- Park YS, Moon YW, Lim SJ, Yang JM, Ahn G, Choi YL. Early osteolysis following second-generation metal-on-metal hip replacement . J Bone Joint Surg Am. 2005;87:1515–21. doi: 10.2106/JBJS.D.02641. [DOI] [PubMed] [Google Scholar]
- Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty . J Bone Joint Surg Am. 2002;84-A:609–14. doi: 10.2106/00004623-200204000-00016. [DOI] [PubMed] [Google Scholar]
- Roth TD, Maertz NA, Parr JA, Buckwalter KA, Choplin RH. CT of the hip prosthesis: appearance of components, fixation, and complications . Radiographics. 2012;32:1089–107. doi: 10.1148/rg.324115183. [DOI] [PubMed] [Google Scholar]
- Sabah SA, Mitchell AW, Henckel J, Sandison A, Skinner JA, Hart AJ. Magnetic resonance imaging findings in painful metal-on-metal hips: a prospective study . J Arthroplasty. 2011;26(1):71–6. doi: 10.1016/j.arth.2009.11.008. [DOI] [PubMed] [Google Scholar]
- MHRA (Medicines and Healthcare products Regulatory Agency) Report of the Expert Advisory Group looking at soft tissue reactions associated with metal-on-metal hip replacements [Online]. 2012 [Cited 2014 April 01]. Available from: http://www.mhra.gov.uk/home/groups/dts-bi/documents/websiteresources/con097079.pdf . [Google Scholar]
- Thomas MS, Wimhurst JA, Nolan JF, Toms AP. Imaging metal-on-metal hip replacements: the Norwich experience. HSS J. 2013;9:247–256. doi: 10.1007/s11420-013-9357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms AP, Marshall TJ, Cahir J, Darrah C, Nolan J, Donell ST, Barker T, Tucker JK. MRI of early symptomatic metal-on-metal total hip arthroplasty: a retrospective review of radiological findings in 20 hips. Clin Radiol. 2008;63:49–58. doi: 10.1016/j.crad.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Walde TA, Weiland DE, Leung SB, Kitamura N, Sychterz CJ, Engh C A, Jr, Claus AM, Potter HG, Engh C A Sr. Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study . Clin Orthop Relat Res. 2005;437:138–44. doi: 10.1097/01.blo.0000164028.14504.46. [DOI] [PubMed] [Google Scholar]