Abstract
Avian and rodent trigeminal ganglion (TG) neurons share common features in their neurotrophin requirements and axonal projections between the sensory periphery and the brainstem. In rodents, the whisker pad (WP) is a major peripheral target of the infraorbital (IO) nerve component of the TG. The chick IO nerve is much smaller and innervates the maxillary process (MP). In the embryonic WP, IO axons course in fascicles from a caudal to rostral direction and form terminal plexuses around follicles. In the chick, IO axons travel as a thin bundle to the MP and branch out with no specific patterning. We cocultured E15 rat TG with E5–6 chick MP or chick TG with rat WP explants to examine target influences on trigeminal axon growth patterns as visualized with DiI labeling or neurofilament immunohistochemistry. Chick TG axons showed robust growth into WP explants, and the ganglion increased in size. Thick bundles of axons traveled between rows of follicles and formed a distinct pattern as they developed terminal arbors around individual follicles. In contrast, rat TG axon growth was sparse in chick MP explants and the ganglion size reduced over time. Furthermore, rat TG axons did not show any patterning in the chick MP. Similar target-specific growth patterns were observed when TG explants were given a choice between chick MP and rat WP explants. Collectively these results indicate that both the chick and rat TG cells respond to similar target-specific peripheral cues in the establishment of innervation density and patterning in peripheral orofacial targets.
Keywords: Chick trigeminal pathway, Rat trigeminal pathway, Primary sensory neuron, Whiskerpad, Maxillary process
1. Introduction
Sensory neurons of the trigeminal ganglion have been extensively studied in both the rodent and avian species. Neurogenesis, development of axon outgrowth into peripheral and central targets, and neurotrophin dependence for cell survival have been charted [1, 5, 7–9, 20, 23, 24, 43, 51]. While there are notable similarities in the development of the trigeminal pathway between the two species, histological differentiation of their peripheral target fields exhibits major differences. In rodents, the major component of the trigeminal pathway, the infraorbital (IO) branch of the maxillary division of the trigeminal nerve, is mostly devoted to the innervation of the whisker and sinus hair follicles on the snout In contrast, the maxillary (MP) peripheral field in the chick is a relatively small target without any patterned epidermal specializations.
In the rat, the trigeminal neurons are born between embryonic day (E) 9–14 [1, 45], and the earliest fibers emerge from the ganglion soon after neurogenesis [19, 24]. Peripheral trigeminal axons invade the WP from a caudal to rostral direction and begin arborizing around the developing whisker follicles. The follicle-related patterning of innervation emerges at E15 [20].
In the chick, trigeminal ganglion neurons are generated between E2 and E5 (Hamburger and Hamilton stage 12–24), and are both placode and neural crest-derived cells [5, 33, 34, 38, 45]. Ophthalmic and maxillomandibular primordia of the ganglion fuse and form a bilobed structure [38]. On E4 (HH stage 23) the TG is well formed and easily visible and the ophthalmic projection is more advanced than the maxillomandibular projections [43]. Immunohistochemical labeling with the neuron-specific β-tubulin antibody showed that the IO component of the maxillary nerve is detectable below the eye at stage 18 and extends into the maxillary process at stage 21 [43]. Thus, the peripheral trigeminal projections to the maxillary process are laid down during E3–E5 (HH stages 18–25) [5, 43].
Organotypic explant cocultures of the embryonic rat TG with peripheral and central targets revealed many aspects of axon-target interactions during the development of the trigeminal pathway [21, 25, 28, 53]. In the present study, we used rat-chick chimeric explant cocultures to examine target-derived influences on peripheral trigeminal axon growth patterns. We show that chick and rat TG axons can invade cross-species peripheral targets, yet their growth density and patterning are dependent on the specific target. Chick TG explants flourish with rat WP explants and their axons form distinct, whisker follicle-specific patterns, while rat TG explants cocultured with chick MP explants diminish in size, and their axons invade this target much like that seen with in vivo development of the chick trigeminal projections.
2. Materials and methods
We used tissues from E15 rat embryos and E5 (HH stage 26) chick embryos based on the comparable developmental stage of the trigeminal pathway in each species [5, 20, 24, 43]. Embryos from 15 day pregnant Sprague–Dawley rats (day of sperm positivity=E0, Taconic Farms, Germantown, NY) were removed by cesarean section following euthanasia of the dam by intraperitoneal administration of a lethal dose of sodium pentobarbital (50 mg/kg body weight). Fertilized white Leghorn eggs were obtained from Charles River Spafas. The protocols described below were approved by the LSUHSC IACUC and conformed to the NIH guidelines for the use of animals. All of the dissections were made in ice-cold Gey's balanced salt solution (GIBCO, Gaithersburg, MD) supplemented with 0.6% D-galactose (Sigma; GBSS). Explants of TG, WP, and MP were dissected out in GBSS under a Nikon SMZ-U dissecting microscope with dark field illumination [53]. Different types of explants were collected in separate petri dishes containing cold GBSS.
2.1. Preparation of chimeric explant cocultures
TG explants from rat or chick embryos were placed abutting the caudal edge of the rat WP or chick MP. Details of dissection and explant coculture procedures were previously described [28, 53]. For controls rat and chick TGs were cocultured with their native targets (WP, and MP, respectively). In addition, five E5 chick and five E15 rat embryos were fixed immediately and processed for immunohistochemistry to visualize the trajectory of the trigeminal projections into the peripheral target fields at this developmental stage. In a third series of cultures, chick or rat TGs were placed in between chick MP and rat WP explants. Explant cocultures were aligned on Millicell inserts (Millipore, Bedford, MA) and cultured in serum free medium (SFM) [3] for 3 days in a water jacket incubator at 33°C and 5% CO2.
2.2. DiI labeling and immunohistochemistry
Cocultures were fixed by immersion in 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4) overnight. A crystal of the lipophillic tracer DiI (1,1′-dioctadecyl-3, 3,3′, 3′-tetramethylindocarbocyanine perchlorate; Molecular Probes, Eugene, Oregon) was placed in the TG and the cultures were left in an incubator for 2 weeks for the dye diffusion. TG axon growth patterns were visualized under epifluorescence using a rhodamine filter set. In some cases, labeled specimens were photoconverted [47] in the presence of 0.15% diaminobenzidine (Sigma) in 0.1 M Tris buffer (pH 8.2). In another series of experiments cultures were cryoprotected in buffered 30% sucrose, sectioned frozen (50 µm) and processed for immunohistochemistry using anti-neurofilament antibody (Chemicon, 1:200). These sections were first permeabilized with 0.3% TritonX in PBS, then blocked in 20% normal goat serum for 1 h. They were then incubated overnight in the primary antibody solution made up in phosphate buffered saline (PBS, pH: 7.4). The next day, following several rinses in PBS, they were incubated in FITC-conjugated goat anti rabbit IgG secondary antibody (1:200), rinsed and examined under epifluorescence using an FITC filter set. Cytoarchitecture was visualized by staining with the nuclear stain bisbenzimide. Cellular and axonal profiles were analyzed following extensive photographic documentation.
2.3. Analyses of axon growth, and ganglion size
DiI or immunohistochemically labeled axonal elements were analyzed with light microscopy. Extensive photographic documentation was made by the use of a Kodak digital camera, images were downloaded to a PC and figures were prepared using the ADOBE PhotoShop program. Only the brightness and contrast of the images were adjusted and no other alterations were made. We also quantified changes in the size of the ganglion explants at 0 and 48 h, using the Scion Image Beta 4.02.
3. Results
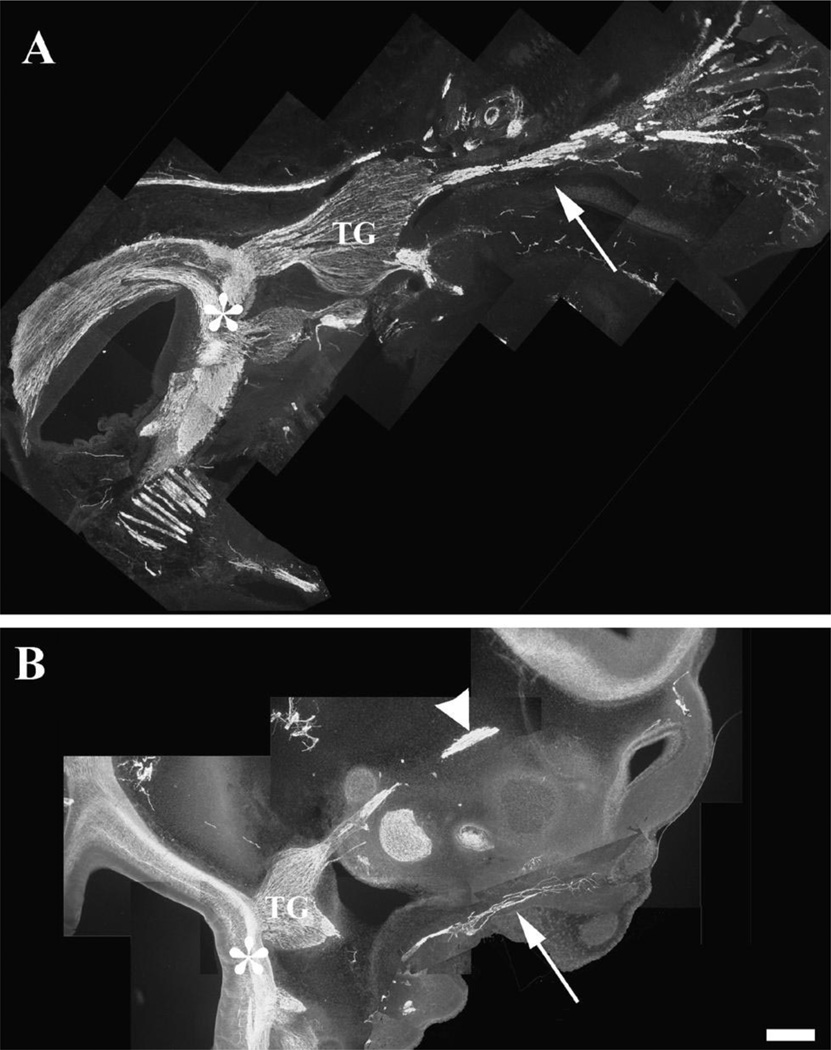
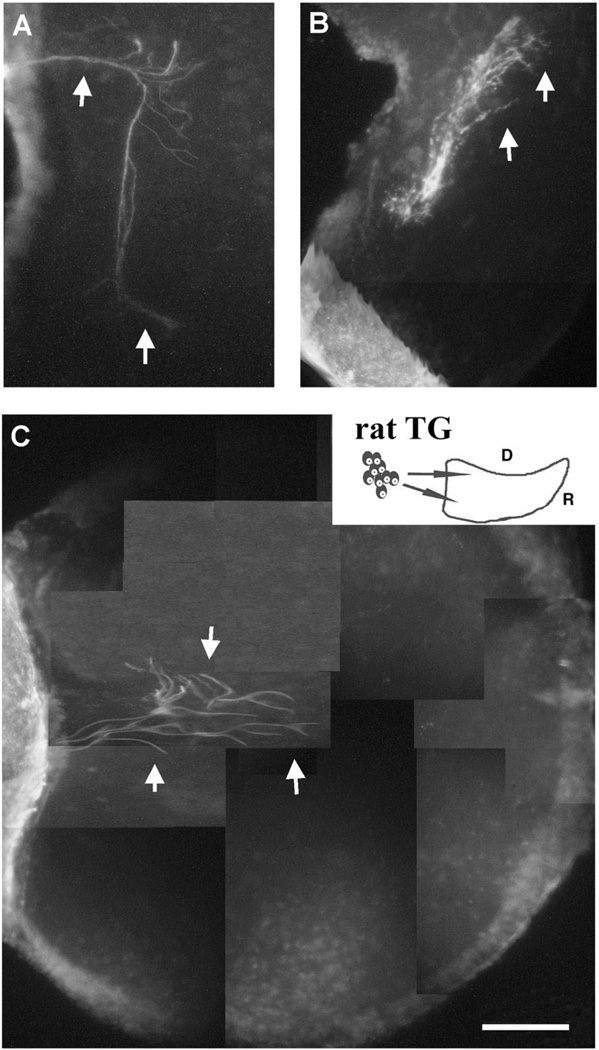
The trigeminal ganglion and its projections are clearly visible in both the E15 rat and E5 chick embryos following immunostaining with the neurofilament antibody. Fig. 1A shows the E15 rat TG as it bridges the WP to the brainstem. The IO component of the trigeminal nerve appears as a very large bundle of tightly fasciculated axons. As these axons enter the WP, they fan out into nerves innervating whisker rows. Their central counterparts are seen in the brainstem within the trigeminal tract. At a similar developmental stage for the chick (E5), the largest component of the peripheral trigeminal nerve is the ophthalmic branch (Fig. 1B). The infraorbital nerve component of the maxillomandibular branch is seen as a thin bundle within the developing MP. These axons take a narrow pathway within the MP and do not show any patterned distribution.
Fig. 1.
The trigeminal pathway in E15 rat and E5 chick embryos. Embryos were sectioned in the sagittal plane and immunostained with neurofilament antibody. (A) The trigeminal ganglion and its projections are clearly visible, including the infraorbital branch (arrow) innervating the whisker pad and the central projections into the brainstem (asterisk). The infraorbital branch is the largest branch of the trigeminal nerve in the rat. (B) In the chick, the trigeminal ganglion is bilobed and a major portion of it projects along the ophthalmic nerve (arrowhead), and the IO nerve (arrow) is very small and forms a few branches in the maxillary process. The brainstem is innervated by the central projection (asterisk). Also note the absence of axonal patterning in the chick MP. Scale bar=100 µm.
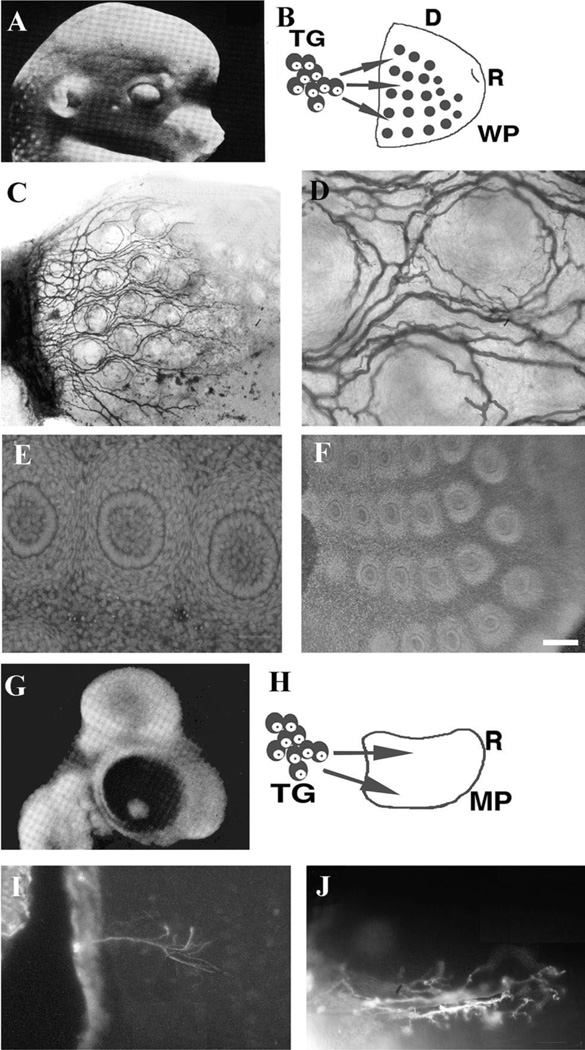
In the coculture model used in this study, trigeminal ganglia are placed abutting the caudal edge of the WP or MP explants (their normal orientation in vivo), and grown in SFM (Fig. 2). In cocultures of E15 rat trigeminal ganglia and WP explants, TG axons innervate the WP in a manner consistent with that seen in vivo (Fig. 2A–D). In such cultures the integrity of the WP explant is maintained and follicular organization is not compromised (Fig. 2E, F). Cocultures of E5 chick trigeminal ganglia paired with the same age chick maxillary process (Fig. 2G–J) also show TG axon growth into this peripheral target similar to that seen in vivo (compare with Fig. 1B).
Fig. 2.
The coculture model of the rat and chick TG with native peripheral targets. (A) Schematic diagram of the head of an E15 rat embryo and the peripheral projection patterns of TG cells into the whisker pad. (B) Schematic illustration of TG-WP cocultures, D=dorsal, R= rostral. (C,D) Rat trigeminal axon growth into WP explants and their patterning around the follicles as seen through DiI staining. (E,F) Bisbenzimide stained sections through the cocultured whiskerpad explants illustrating the morphological integrity of follicular organization after 3 days in vitro. (G) Schematic diagram of an E5 chick head and the projection of TG into the MP. (H) Schematic diagram of the coculture set up with chick tissues. (I,J) Chick TG axon growth into the MP as demonstrated by neurofilament (I) and DiI (F). Note the sparse projections and diffuse branching of axons. Scale bar=200 µm (C,F), 100 µm (I), 50 µm (D,E,J).
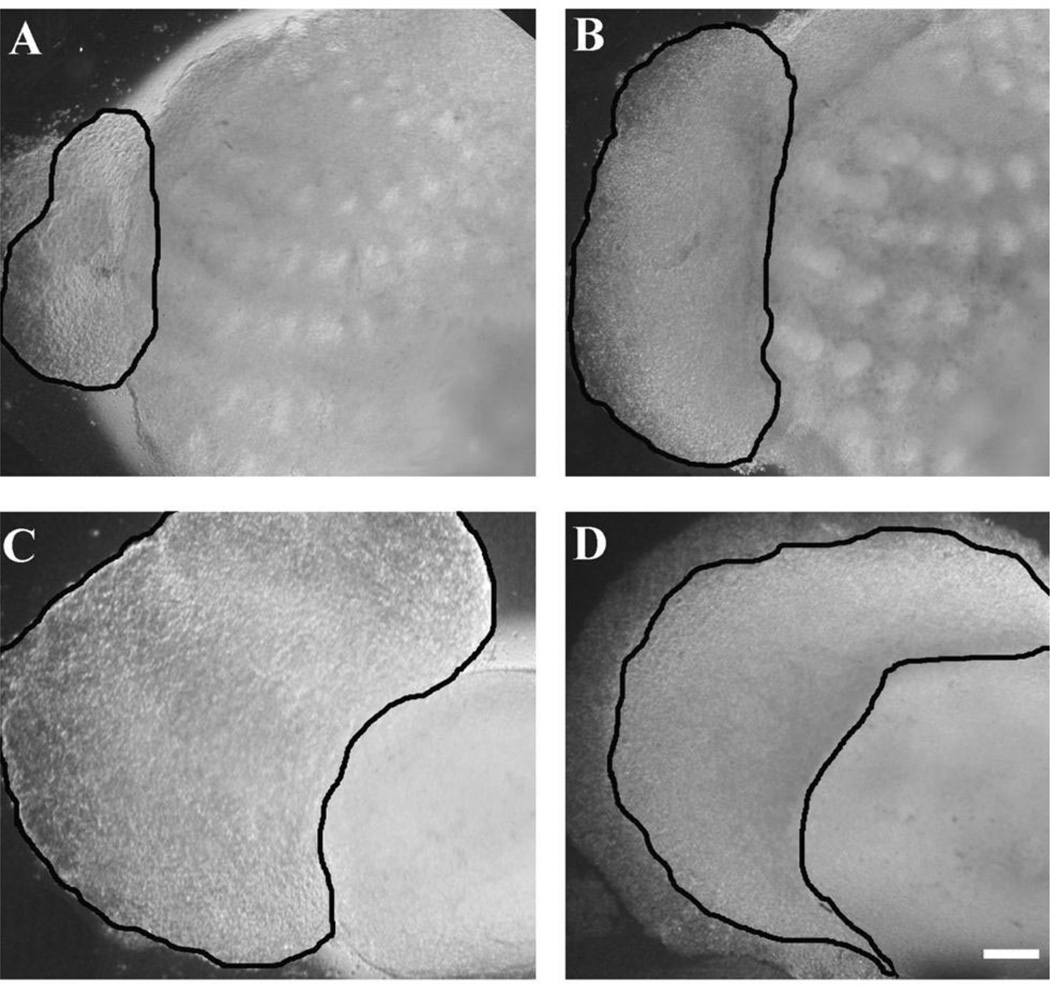
When rat TG explants are cocultured with WP explants there is no observable change in the size of the ganglia in their gross appearance. Similarly, E5 chick TGs cocultured with chick MP explants retain their shape and overall size in culture (not shown). However, when E5 chick TGs are placed in coculture with E15 rat WP, the overall area of the ganglia increased two to three times in 48 h, with differences visible after 12 h in culture (Fig. 3A and B). With chick TG and rat WP (n = 11) there was a percent area difference of 1.918 (or 191.8%, S.D.=0.335). In contrast, rat TG cocultured with chick MP significantly decreased in area, and many necrotic cells were visible under DIC optics (compare Fig. 3C and D). Measurements from rat TG and chick MP cocultures (n = 10) over a 48 h period indicated the difference in area to be 0.557 (or 55.7%, S.D.=0.0442). These results were consistently seen in all chimeric cocultures. The bilobed chick ganglia are initially much smaller than that of equivalent age rat ganglia, but increase in size. These results demonstrate that the rat WP can maintain and induce further growth of chick TG, whereas the health of the relatively large rat TG is not maintained when placed next to the MP in culture.
Fig. 3.
Role of target tissue in regulating TG size. E5 chick trigeminal ganglia triple in size in 48 h when cocultured with rat WP explants (A,B). Whereas, rat TG shrinks in size when cocultured with chick MP (C,D). The borders of the ganglion explants are outlined in each micrograph. Scale bar=200 µm.
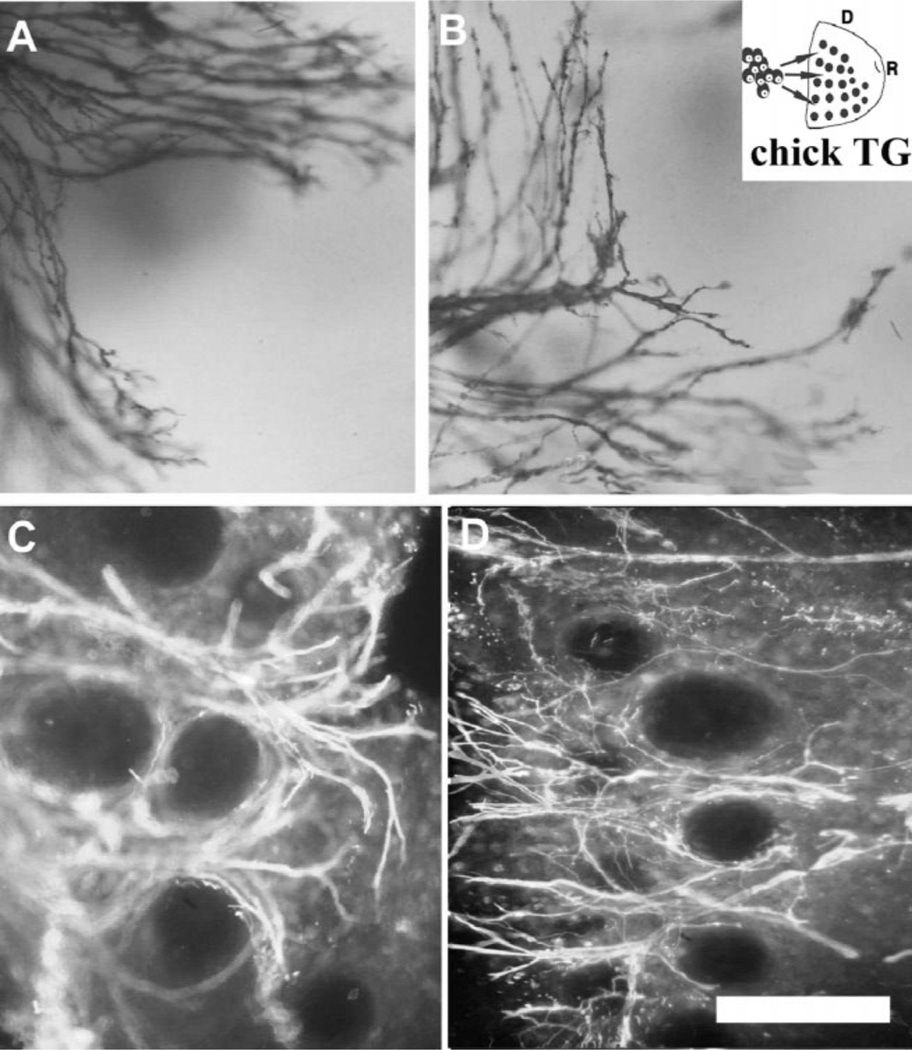
Next we examined TG axon growth patterns into the rat WP and chick MP. Surprisingly the growth patterns of chick TG axons into the WP were indistinguishable from rat TG axons, and rat TG axons into the chick MP from chick TG axons (Figs. 4 and 5). TG from both species showed a dense growth into the WP from a caudal to rostral direction. In all cases with either ganglion and a single target (n = 32), TG axons formed circular plexuses around individual follicles (Fig. 4). In contrast, TG axon growth into the chick MP was not as robust as that seen in WP but similar in density to that seen in normal E5 chick embryos (Fig. 5). Both rat and chick TG axons grew into the E5 MP as a thin bundle that branched in a narrow field along a caudal to rostral direction (compare Figs. 2I and J and Fig. 5).
Fig. 4.
E5 chick TG axon growth into E15 rat WP explants. A–D illustrate different examples of patterned ingrowth of E5 chick TG axons within the E15 rat WP explants as demonstrated by DiI labeling (A,B) and neurofilament staining (C,D). Note that the axon growth is similar to that seen with rat TG explants Neurofilament immunostaining. Scale bar=50 µm (A,B), 200 µm (C,D).
Fig. 5.
E15 rat TG axon growth into E5 chick MP explants. (A–C) illustrate different examples of rat TG axons within the chick MP explants. Note the axon growth (arrows) is very weak (but similar to that seen in vivo in E5 chick embryos) and no patterns are observed. Neurofilament immunostaining. Scale bar=200 µm.
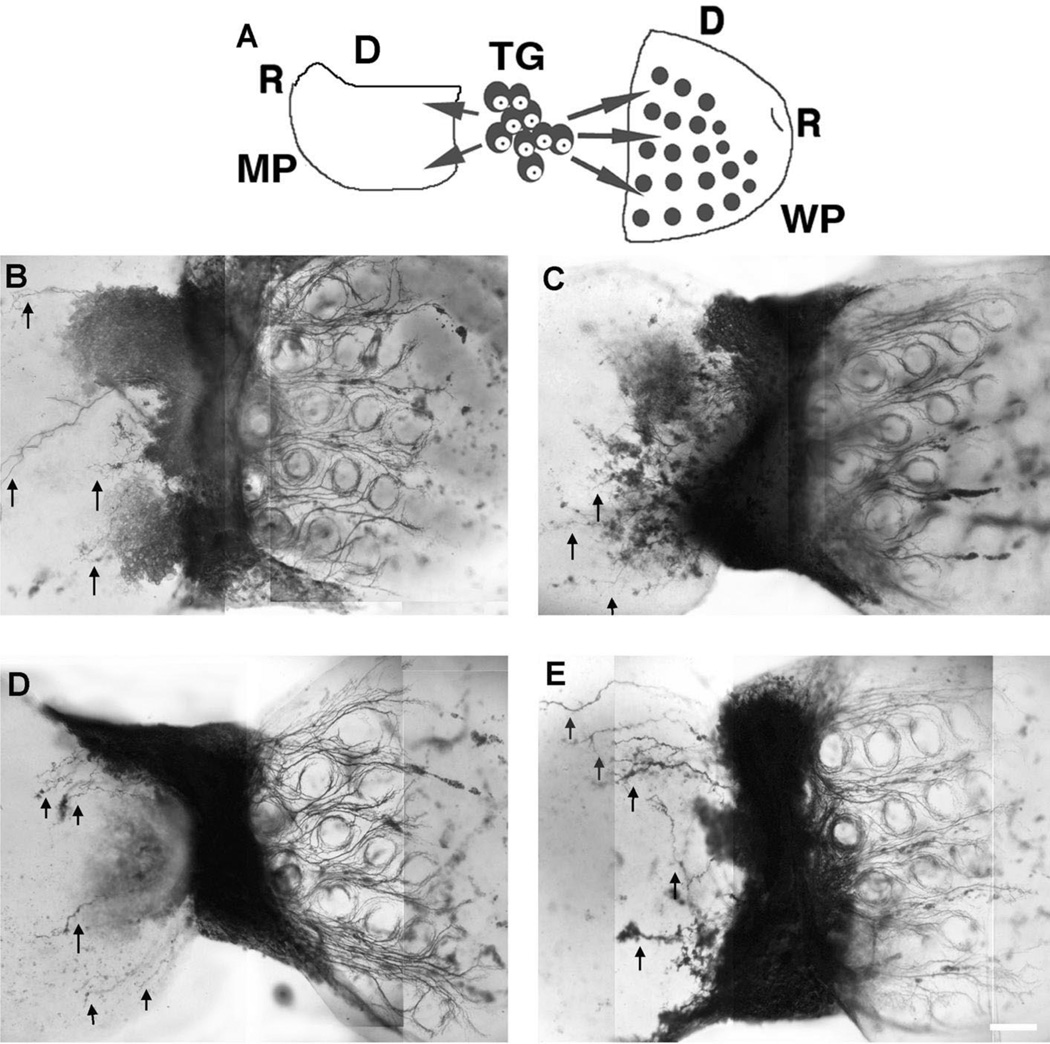
In the last series of experiments, we created a ‘choice’ paradigm by placing either chick or rat TG in between MP and WP explants. Previous studies showed that rat TG placed in between two WP explants, placed between peripheral and central targets or surrounded by WP, forepaw and heart explants show target-specific axon growth patterns [22, 25, 53]. Here we placed the caudal edges of the WP and MP on either side of a single TG derived from chick or rat embryos. In all such cocultures (n = 10), TG axon growth was again strictly target specific, and the behavior of TG axons from rat or chick were indistinguishable (Fig. 6). In all cases TG axons formed a patterned array in relation to the whisker follicles in WP explants and showed limited growth with no patterning in chick MP explants. Collectively, our observations suggest that similar trophic influences regulate the size of the chick and rat TG and axons from these two unrelated species respond in the same manner to target derived cues, which define their growth density and patterning.
Fig. 6.
TG axon growth patterns in a choice paradigm involving rat WP and chick MR (A) Schematic illustration of the culture set up with either chick or rat TG (R: rostral, D: dorsal). (B,C) Rat TG explants provide dense innervation to the WP (right side in each) and sparse innervation into the chick MP (arrows, left side in each). These axons form a distinct follicular pattern in the WP. (D,E) When given the same choice, chick TG axons behave much like rat TG axons, providing dense and patterned innervation to the WP and sparse innervation (arrows) to their own target, the MP. DiI labeling. Scale bar=200 µm.
4. Discussion
The present study demonstrates that the peripheral target tissues of the trigeminal ganglion of either rodent or avian species can attract both rat and chick TG axons, and regulate sensory innervation patterns in vitro. The results obtained here further indicate that specific patterning of TG axons in the rodent whiskerpad is not an intrinsic characteristic of the rodent trigeminal axons, but largely determined by target-derived axon-guidance cues.
4.1. Target regulation of sensory neuronal pools
Classical neuroembryology experiments of V Hamburger and R. Levi-Montalcini demonstrated that the size of the peripheral target tissues play a major role in regulation of sensory neuron pools [31, 32]. They showed that target limb bud ablation in chick embryos leads to atrophy of the sensory ganglia, due to the degeneration of many of the neurons that were present before the target removal. These studies paved the way to the identification of neurotrophins as potent regulators of developing sensory neuron pools. Nerve growth factor (NGF) was the first identified target-derived neurotrophic factor essential for the survival of several classes of neurons [39, 42]. The TG cells in both rodents and avian species express Trk receptors and depend on peripheral target-derived neurotrophin supplies for survival [2, 14, 35, 57]. It is well documented that peripheral target tissues regulate sensory neuron populations [4, 8, 9, 11, 50, 54]. Furthermore, animals with selective gene deletions of the NGF family of neurotrophins (specifically NGF, BDNF and NT-3) or the Trk receptors show drastically lowered numbers of sensory neurons [6, 16, 17, 36, 37, 49]. Developing trigeminal ganglion cells express Trk receptors, and their peripheral and central targets express the NGF family of neurotrophins even before the first trigeminal axons reach them [10, 30, 40, 48, 56, 57]. The whiskerpad in mice and rats is especially a rich source of neurotrophins. NGF, BDNF, NT-3, and NT-4 mRNA are all expressed with different distributions around developing whisker follicles [18, 35]. Detailed morphological analyses using immunohistochemistry and tissues derived from specific neurotrophin or Trk receptor knockout mice indicate that NGF family of neurotrophins regulate the innervation of whisker pad by different classes of sensory nerve endings [26, 46].
Our observations on the expansion of the chick TG explants cocultured with rat WP and shrinkage of rat TG explants cocultured with chick MP explants corroborate previous findings that target tissue plays a major role in differential growth of sensory ganglia that innervates it. However, it is quite surprising that peripheral targets from completely different species of animals can still regulate sensory ganglion size. While we do not have any direct evidence, the NGF family of neurotrophins is the most likely candidate molecules that play a major role in differential growth of the TG explants used in our cocultures. Future studies using WP tissues from single or double neurotrophin knockout or specific neurotrophin overexpressing transgenic mice [4, 12, 49] should provide clear insights. It also remains to be determined how chick TG axons can respond to trophic and axon guidance cues within a trigeminal peripheral target derived from a different species. NGF has been shown to have aproximately 85% homology between mouse and chick [13]. Additionally, hybridization probes from the transmembrane region of chick TrkA show high homology with the rat TrkA [15]. This homology may underlie the responsiveness of chick TG to rat WP and rat TG to chick MP.
Chick and rat trigeminal ganglia changed in size over a short period of time when cocultured with a homologous target tissue from a foreign species. This period in chick TG development does not correspond to an in vivo growth phase, and no similar increase in the size of the chick TG could be noted when cocultured with chick MP explants. Thus, the enlargement of chick TG explants cocultured with rat WP explants must be due to trophic effects from the WP explants and survival of many more neurons that are normally eliminated in the chick. In contrast, rat TG neurons have only a limited supply of trophic substances from the smaller chick MP explants and undergo large-scale reduction in size. Details of the specific increase and decrease in neuronal numbers and the level of cell death were beyond the scope of the present study. However, the xenotypic explant cocultures used in our study can be employed as a means to investigate these issues in future studies.
4.2. Target regulation of sensory axon growth parameters
Our results demonstrate that in vitro, chick trigeminal axons grow into rat whisker pad explants and form a patterned array around the developing whisker follicles. In contrast rat TG axons, which normally form whisker follicle-specific patterns in their normal target, fail to do so in chick MP explants and show limited growth into this peripheral target tissue. The density of axon growth from rat or chick TG explants into WP or MP explants were strikingly similar to that seen in normal chick or rat embryos in vivo. This clearly indicates that TG axon guidance and pattern formation in the periphery are based upon signals encountered upon entering the target tissue and that these signals are shared between species. Previous work from our laboratory showed that not only TG but also dorsal root, nodose, and superior cervical ganglion axons could invade the WP and develop follicular patterning [53]. In contrast, retinal axons also could grow into WP explants but they formed no patterns with respect to the follicles [53]. Collectively, these results suggest that a variety of primary sensory neurons (including those from different species) have the capacity to respond to axon patterning cues present in the rodent WP. Other sensory neurons from the central nervous system, such as the retinal ganglion cells, can be induced to grow into WP, but they are not responsive to axon patterning cues.
At the present time we do not know the precise nature of axon guidance signals that regulate similar patterning of rat and chick TG axons within the WP. However, our results indicate that embryonic chick and rat TG axons must be equipped with similar sets of receptors that bind a variety of axon guidance molecules present in the developing whisker pad. In recent years, differential distribution and spatiotemporal regulation of a variety of molecule families have been noted for the rodent whisker pad. Aside from neurotrophins discussed above, semaphorins [27, 52], slits [44, 58], B class ephrins [29], may also play a role in the patterning of TG axons within the WP. For example, developing mouse and rat TG cells transiently express the Sema3 receptor neuropilin, and in Sema3a knockout mice, trigeminal projections are severely disarrayed [52]. Collapsin, chick homologue of Sema3a shows similarities in structure [41]. Slits and their receptors Robos may play a role in the branching pattern of sensory axons in target tissue [55]. Preliminary studies from our laboratory show differential expression of slit 1–3 in the developing whiskerpad [44]. We have also seen differential distribution of Eph B1 and Eph B3 mRNA around the whisker follicles in embryonic mice [29]. It is highly likely that not any one but multiple axon guidance molecules expressed by the developing WP and their receptors present on the growth cones of incoming trigeminal axons sculpt the final patterning of trigeminal innervation. Within this context, it is interesting to note that embryonic chick trigeminal axons can respond to similar cues and develop innervation patterns in the whisker pad much like rat TG axons.
Acknowledgements
Grant sponsor: N.I.H./NIDCR; Grant number: DE07734
References
- 1.Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Adv. Anat. Embryol. Cell Biol. 1982;74:1–90. doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- 2.Barbacid M. The Trk family of neurotrophin receptors. J. Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 3.Collazo D, Takahashi H, McKay RD. Cellular targets and trophic functions of neurotrophin-3 in the developing rat hippocampus. Neuron. 1992;9:643–656. doi: 10.1016/0896-6273(92)90028-c. [DOI] [PubMed] [Google Scholar]
- 4.Conover JC, Yancopoulos GD. Neurotrophin regulation of the developing nervous system: analyses of knockout mice. Rev. Neurosci. 1997;8:13–27. doi: 10.1515/revneuro.1997.8.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Covell DA, Jr, Noden DM. Embryonic development of the chick primary trigeminal sensory-motor complex. J. Comp. Neurol. 1989;286:488–503. doi: 10.1002/cne.902860407. [DOI] [PubMed] [Google Scholar]
- 6.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, MacMahon SB, Shelton DL, Levinson AD, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 7.Davies AM. Neurotrophic factors. Switching neurotrophin dependence. Curr. Biol. 1994;4:273–276. doi: 10.1016/s0960-9822(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 8.Davies AM. The neurotrophic hypothesis: where does it stand? Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:389–394. doi: 10.1098/rstb.1996.0033. [DOI] [PubMed] [Google Scholar]
- 9.Davies AM. Studies of neurotrophin biology in the developing trigeminal system. J. Anat. 1997;191:483–491. doi: 10.1046/j.1469-7580.1997.19140483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies AM, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- 11.Davies AM, Thoenen H, Barde YA. Different factors from the central nervous system and periphery regulate the survival of sensory neurones. Nature. 1986;319:497–499. doi: 10.1038/319497a0. [DOI] [PubMed] [Google Scholar]
- 12.Davis BM, Fundin BT, Albers KM, Goodness TP, Cronk KM, Rice FL. Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J. Comp. Neurol. 1997;387:489–506. doi: 10.1002/(sici)1096-9861(19971103)387:4<489::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Ebendal T, Larhammar D, Persson H. Structure and expression of the chicken beta nerve growth factor gene. Embo J. 1986;5:1483–1487. doi: 10.1002/j.1460-2075.1986.tb04386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkabes S, Dreyfus CF, Schaar DG, Black IB. Embryonic sensory development: local expression of neurotrophin-3 and target expression of nerve growth factor. J. Comp. Neurol. 1994;341:204–213. doi: 10.1002/cne.903410206. [DOI] [PubMed] [Google Scholar]
- 15.Ernfors P, Hallbook F, Ebendal T, Shooter EM, Radeke MJ, Misko TP, Persson H. Developmental and regional expression of beta-nerve growth factor receptor mRNA in the chick and rat. Neuron. 1988;1:983–996. doi: 10.1016/0896-6273(88)90155-9. [DOI] [PubMed] [Google Scholar]
- 16.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 17.Ernfors P, Lee KF, Jaenisch R. Target derived and putative local actions of neurotrophins in the peripheral nervous system. Prog. Brain Res. 1994;103:43–54. doi: 10.1016/s0079-6123(08)61125-5. [DOI] [PubMed] [Google Scholar]
- 18.Ernfors P, Merlio J, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur. J. Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 19.Erzurumlu RS, Jhaveri S. Emergence of connectivity in the embryonic rat parietal cortex. Cereb. Cortex. 1992;2:336–352. doi: 10.1093/cercor/2.4.336. [DOI] [PubMed] [Google Scholar]
- 20.Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J. Neurosci. 1992;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erzurumlu RS, Jhaveri S. Target influences on the morphology of trigeminal axons. Exp. Neurol. 1995;135:1–16. doi: 10.1006/exnr.1995.1061. [DOI] [PubMed] [Google Scholar]
- 22.Erzurumlu RS, Jhaveri S, Takahashi H, McKay RD. Target-derived influences on axon growth modes in cultures of trigeminal neurons. Proc. Natl. Acad. Sci. USA. 1993;90:7235–7239. doi: 10.1073/pnas.90.15.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erzurumlu RS, Killackey HP. Order in the developing rat trigeminal nerve. Brain Res. 1982;255:305–310. doi: 10.1016/0165-3806(82)90030-x. [DOI] [PubMed] [Google Scholar]
- 24.Erzurumlu RS, Killackey HP. Development of order in the rat trigeminal system. J. Comp. Neurol. 1983;213:365–380. doi: 10.1002/cne.902130402. [DOI] [PubMed] [Google Scholar]
- 25.Erzurumlu RS, McKay RD, Jhaveri S. Morphological specification of trigeminal neurites depends on target fields. Brain Res. Dev. Brain Res. 1994;83:132–137. doi: 10.1016/0165-3806(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 26.Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL. Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR. Dev. Biol. 1997;190:94–116. doi: 10.1006/dbio.1997.8658. [DOI] [PubMed] [Google Scholar]
- 27.Giger RJ, Wolfer DP, De Wit GM, Verhaagen J. Anatomy of rat semaphorin III/collapsin-1 mRNA expression and relationship to developing nerve tracts during neuroembryogenesis. J. Comp. Neurol. 1996;375:378–392. doi: 10.1002/(SICI)1096-9861(19961118)375:3<378::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Gunhan-Agar E, Haeberle A, Erzurumlu RS. Directional specificity and patterning of sensory axons in trigeminal ganglion-whisker pad cocultures. Brain Res. Dev. Brain Res. 2000;119:277–281. doi: 10.1016/s0165-3806(99)00107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haeberle AS, Erzurumlu RS. Society of Neuroscience Abstracts. New Orleans, LA: 2000. Localization of B class Eph receptors in the developing mouse trigeminal pathway. [Google Scholar]
- 30.Hallbook F, Ibanez CF, Ebendal T, Persson H. Cellular localization of brain-derived neurotrophic factor and neurotrophin-3 mRNA expression in the early chicken embryo. Eur. J. Neurosci. 1993;5:1–14. doi: 10.1111/j.1460-9568.1993.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 31.Hambuger V. Motor and sensory hyperplasia following limb-bud transplantations in chick embryos. Physiol. Zool. 1939;12:268–284. [Google Scholar]
- 32.Hambuger V, Levi-Montalcini R. Proliferation, differentiation and degeneration of spinal ganglia of the chick embryo under normal and experimental conditions. J. Exp. Zool. 1949;111:457–501. doi: 10.1002/jez.1401110308. [DOI] [PubMed] [Google Scholar]
- 33.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 34.Heaton MB, Moody SA. Early development and migration of the trigeminal motor nucleus in the chick embryo. J. Comp. Neurol. 1980;189:61–99. doi: 10.1002/cne.901890105. [DOI] [PubMed] [Google Scholar]
- 35.Ibanez CF, Ernfors P, Timmusk T, Ip NY, Arenas E, Yancopoulos GD, Persson H. Neurotrophin-4 is a target-derived neurotrophic factor for neurons of the trigeminal ganglion. Development. 1993;117:1345–1353. doi: 10.1242/dev.117.4.1345. [DOI] [PubMed] [Google Scholar]
- 36.Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- 37.Korsching S. The neurotrophic factor concept: a reexamination. J. Neurosci. 1993;13:2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuratani S, Tanaka S. Peripheral development of avian trigeminal nerves. Am. J. Anat. 1990;187:65–80. doi: 10.1002/aja.1001870108. [DOI] [PubMed] [Google Scholar]
- 39.Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol. Rev. 1968;48:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- 40.Lindsay RM, Barde YA, Davies AM, Rohrer H. Differences and similarities in the neurotrophic growth factor requirements of sensory neurons derived from neural crest and neural placode. J. Cell Sci. Suppl. 1985;3:115–129. doi: 10.1242/jcs.1985.supplement_3.12. [DOI] [PubMed] [Google Scholar]
- 41.Luo Y, Shepherd I, Li J, Renzi MJ, Chang S, Raper JA. A family of molecules related to collapsin in the embryonic chick nervous system. Neuron. 1995;14:1131–1140. doi: 10.1016/0896-6273(95)90261-9. [DOI] [PubMed] [Google Scholar]
- 42.Mobley WC, Server AC, Ishii DN, Riopelle RJ, Shooter EM. Nerve growth factor. N. Engl. J. Med. 1977;297:1096–1104. doi: 10.1056/NEJM197711172972005. [DOI] [PubMed] [Google Scholar]
- 43.Moody SA, Quigg MS, Frankfurter A. Development of the peripheral trigeminal system in the chick revealed by an isotype-specific anti-beta-tubulin monoclonal antibody. J. Comp. Neurol. 1989;279:567–580. doi: 10.1002/cne.902790406. [DOI] [PubMed] [Google Scholar]
- 44.Ozdinler PH, Erzurumlu RS. Society of Neuroscience Abstracts. New Orleans, LA: 2000. Slit expression in the developing mouse trigeminal pathway. [Google Scholar]
- 45.Rhoades RW, Enfiejian HL, Chiaia NL, Macdonald GJ, Miller MW, McCann P, Goddard CM. Birthdates of trigeminal ganglion cells contributing axons to the infraorbital nerve and specific vibrissal follicles in the rat. J. Comp. Neurol. 1991;307:163–175. doi: 10.1002/cne.903070114. [DOI] [PubMed] [Google Scholar]
- 46.Rice FL, Albers KM, Davis BM, Silos-Santiago I, Wilkinson GA, LeMaster AM, Ernfors P, Smeyne RJ, Aldskogius H, Phillips HS, et al. Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophins, trk receptors, and p75LNGFR. Dev. Biol. 1998;198:57–81. [PubMed] [Google Scholar]
- 47.Sandell JH, Masland RH. Photoconversion of some fluorescent markers to a diaminobenzidine product. J. Histochem. Cytochem. 1988;36:555–559. doi: 10.1177/36.5.3356898. [DOI] [PubMed] [Google Scholar]
- 48.Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- 49.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 50.Snider WD, Silos-Santiago I. Dorsal root ganglion neurons require functional neurotrophin receptors for survival during development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:395–403. doi: 10.1098/rstb.1996.0034. [DOI] [PubMed] [Google Scholar]
- 51.Stainier DY, Gilbert W. Pioneer neurons in the mouse trigeminal sensory system. Proc. Natl. Acad. Sci. USA. 1990;87:923–927. doi: 10.1073/pnas.87.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ulupinar E, Datwani A, Behar O, Fujisawa H, Erzurumlu R. Role of semaphorin III in the developing rodent trigeminal system. Mol. Cell. Neurosci. 1999;13:281–292. doi: 10.1006/mcne.1999.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulupinar E, Erzurumlu RS. Peripheral target-specific influences on embryonic neurite growth vigor and patterns. J. Comp. Neurol. 1998;399:427–439. doi: 10.1002/(sici)1096-9861(19981005)399:4<427::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 54.Vogel KS. Development of trophic interactions in the vertebrate peripheral nervous system. Mol. Neurobiol. 1993;7:363–382. doi: 10.1007/BF02769183. [DOI] [PubMed] [Google Scholar]
- 55.Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 56.Williams R, Backstrom A, Ebendal T, Hallbook F. Molecular cloning and cellular localization of trkC in the chicken embryo. Brain Res. Dev. Brain Res. 1993;75:235–252. doi: 10.1016/0165-3806(93)90028-9. [DOI] [PubMed] [Google Scholar]
- 57.Williams R, Backstrom A, Kullander K, Hallbook F, Ebendal T. Developmentally regulated expression of mRNA for neurotrophin high-affinity (trk) receptors within chick trigeminal sensory neurons. Eur. J. Neurosci. 1995;7:116–128. doi: 10.1111/j.1460-9568.1995.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 58.Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev. Biol. 1999;212:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]