Abstract
We examined the effects of neurotrophins nerve growth factor (NGF) and neurotrophin-3 (NT-3) on trigeminal axon growth patterns. Embryonic (E13–15) wholemount explants of the rat trigeminal pathway including the whisker pads, trigeminal ganglia, and brainstem were cultured in serum-free medium (SFM) or SFM supplemented with NGF or NT-3 for 3 days. Trigeminal axon growth patterns were analyzed with the use of lipophilic tracer DiI. In wholemount cultures grown in SFM, trigeminal axon projections, growth patterns, and differentiation of peripheral and central targets are similar to in vivo conditions. We show that in the presence of NGF, central trigeminal axons leave the tract and grow into the surrounding brainstem regions in the elongation phase without any branching. On the other hand, NT-3 promotes precocious development of short axon collaterals endowed with focal arbors along the sides of the central trigeminal tract. These neurotrophins also affect trigeminal axon growth within the whisker pad. Additionally, we cultured dissociated trigeminal ganglion cells in the presence of NGF, NT-3, or NGF+NT-3. The number of trigeminal ganglion cells, their size distribution under each condition were charted, and axon growth was analyzed following immunohistochemical labeling with TrkA and parvalbumin antibodies. In these cultures too, NGF led to axon elongation and NT-3 to axon arborization. Our in vitro analyses suggest that aside from their survival promoting effects, NGF and NT-3 can differentially influence axon growth patterns of embryonic trigeminal neurons.
Indexing terms: axon elongation, axon arborization, neurotrophins, rat trigeminal system, trigeminal ganglion, primary sensory neurons
Axons first elongate without branching during targetdirected navigation and pathway formation; later, they emit branches along their course, or develop collaterals into target regions, and form synaptic terminal arbors (Nakamura and O’Leary, 1989; Heffner et al., 1990; Simon and O’Leary, 1990, 1992; Bhide and Frost, 1991; Jhaveri et al., 1991). Elongation, collateralization, and arborization phases are readily recognized in the projections of rodent trigeminal ganglion (TG) as these neurons bridge the sensory periphery to the central nervous system (Erzurumlu and Killackey, 1983; Stainier and Gilbert, 1990, 1991; Erzurumlu and Jhaveri, 1992). In the rat, central trigeminal axons enter the hindbrain around embryonic day (E)13 and grow in the elongation phase while they lay down the ascending and descending components of the central trigeminal tract. Around E17, axons in the tract emit radially oriented collaterals into the brainstem trigeminal nuclear complex, and develop terminal arbors. In explant cocultures of E15 TG with isochronic brainstem slices, axons grow in the elongation phase; when cocultured with older (E20) brainstem slices, they collateralize and form arbors (Erzurumlu and Jhaveri, 1995). Furthermore, older (E20) TG cells, which have already developed arbors in the brainstem, revert to elongation when cocultured with E15 brainstem slices (Erzurumlu and Jhaveri, 1995). These results indicate that target-derived cues play a major role in mediating axon growth patterns in this sensory system. Presently, molecular and cellular mechanisms underlying shifts in trigeminal axon growth phases are not well understood. Among other candidates, neurotrophins are one family of target-derived cues that could play a role in axon elongation and arborization.
Members of the nerve growth factor (NGF) family of neurotrophic factors, NGF (Levi-Montalcini, 1987), brain-derived neurotrophic factor (BDNF; Barde et al., 1982), neurotrophin-3 (NT-3; Ernfors et al., 1990; Jones and Reichardt, 1990; Rosenthal et al., 1990), and neurotrophin-4/5 (NT-4/5; Berkemeier et al., 1991; Ip et al., 1992) elicit a variety of biological responses during wiring of the nervous system. Recent evidence suggests that they also play a role in axonal patterning. Exogenous applications of neurotrophins promote axon collateral branch formation in vivo (Schnell et al., 1994; Zhang et al., 1994). Brain-derived neurotrophic factor mediates optic axon arborization in the developing Xenopus tectum (Cohen-Corey and Fraser, 1995). Genetic alterations in mice indicate that NGF and NT-3 are necessary for the development of proper sympathetic axon branching in target tissues (Hoyle et al., 1993; ElShamy et al., 1996). Localized source of neurotrophins can initiate collateral formation and filopodial sprouting along the shaft of cultured dorsal root ganglion (DRG) axons (Gallo and Letourneau, 1998), and NT-3 supports terminal arborization, whereas NGF produces axon elongation (Lentz et al., 1999). Finally, NT-3 regulates branching and targeting of cortical axons in vitro (Castellani and Bolz, 1999).
In this study, we examined the effects of NGF and NT-3 on morphological differentiation of embryonic trigeminal axons. We used a simple in vitro assay by culturing wholemounts of the trigeminal pathway from the whisker pad to the brainstem in the absence or presence of the two neurotrophins. These explant cultures provide a unique means to study the effects of a variety of axon growth-regulatory molecules in an intact, in vitro sensory system. In addition, we prepared low-density dissociated cell cultures to examine the effects of these neurotrophins on trigeminal axons in the absence of any targets. We show that both in wholemount explant and dissociated cell cultures, exogenous NGF promotes trigeminal axon elongation, and NT-3 induces arborization. We also tested the effects of BDNF on wholemount cultures, but the results were not consistent to derive meaningful interpretations. These data are not presented, and the effects of NT4/5, another member of the NGF family, were not examined.
MATERIALS AND METHODS
Preparation of wholemount cultures of the trigeminal pathway
Trigeminal pathway wholemount cultures were prepared from embryonic rats at a time when the peripheral and central pathways are laid down. As illustrated in Figure 1, these cultures included the whisker pad, the TG, and the brainstem from the pontine flexure to the upper cervical levels. In these explants, both the peripheral and central trajectories, and targets of the ganglion cells remained intact. In some wholemount cultures (where indicated in the text and figures) the whisker pad was omitted (see dotted lines in Fig. 1 schematic drawing of the trigeminal pathway wholemount explant). Embryos from 13-, 15-, and 17-day pregnant Sprague-Dawley rats were removed by caesarean section following euthanasia of the dam by intraperitoneal administration of a lethal dose of sodium pentobarbital (50 mg/kg body weight). The day of sperm-positivity was designated as E0. All of the protocols described below were approved by the LSU IACUC and conformed to the NIH guidelines for the use of animals. Dissections were made in ice-cold Gey’s balanced salt solution (GIBCO, Gaithersburg, MD) supplemented with sucrose (GBSS). Connective tissues around the central nervous system (CNS) were removed to expose the brain. A transverse cut was made through the pontine flexure; both hemispheres and the midbrain were dissected out. Next, the hindbrain including the upper cervical spinal cord was dissected free of connective tissues and meninges. Care was taken not to damage the trigeminal ganglia on both side, or their projections. Maxillary process/whisker pad was trimmed from the rest of the head, without damaging the infraorbital (IO) nerve. The IO nerve crosses into the whisker pad just below the eye through the developing infraorbital foramen. In order to avoid damage to the IO nerve, the eyecup was left intact. In another series of cultures, wholemount explants were prepared without the whisker pad. The wholemounts were then placed on a microporous Millicell membrane (Millipore, Bedford, MA) with the ventral side down and grown in serum-free medium (SFM, Collazo et al., 1992) supplemented with NGF or NT-3 (at a final concentration of 50 ng/ml; Collaborative Biomedical Products, Bedford, MA, and Regeneron Pharmaceuticals, Tarrytown, NY). Two types of controls were performed. In one series, wholemount explants were cultured for the same duration (3 days) in SFM only. In another series, wholemount explants from E13 and E15 embryos were placed on Millicell membranes and fixed immediately with 4% buffered paraformaldehyde. Wholemounts from E17 embryos (without the whisker pad) were also prepared (n = 4) and fixed immediately to visualize the central trigeminal axon differentiation at this stage. For each condition, 15 cultures were prepared from E13 and E15 embryos.
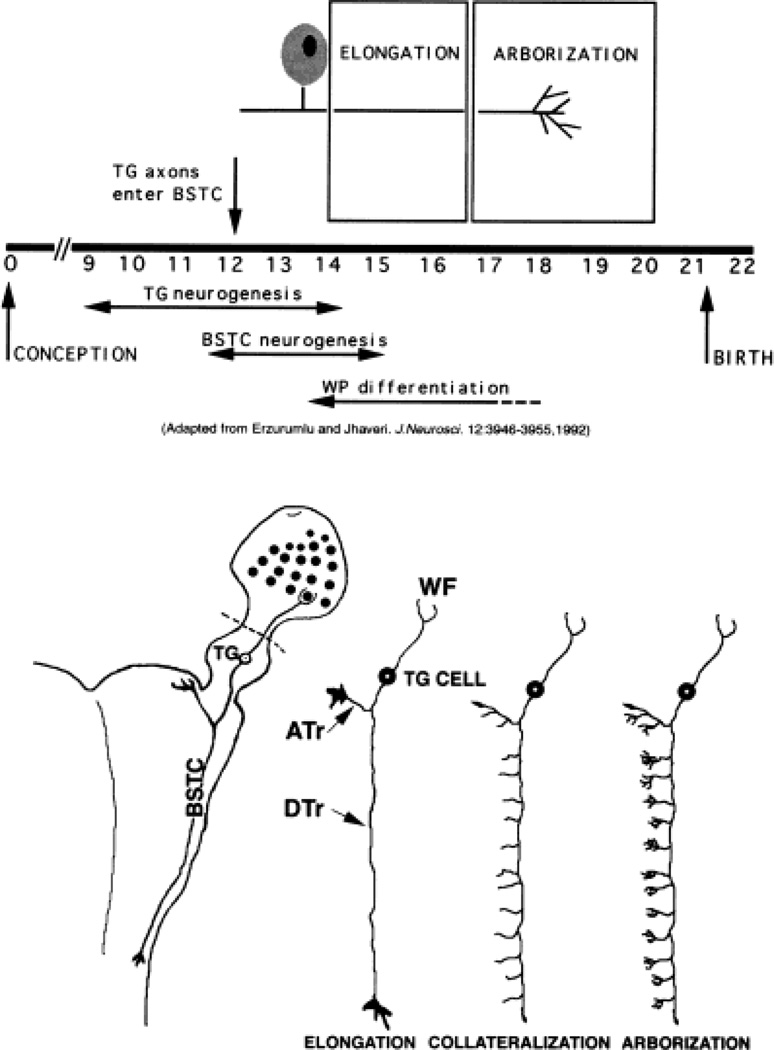
Fig. 1.
Developmental history of the rat trigeminal pathway. Significant developmental events for the rat trigeminal pathway are schematized in the top panel. Diagrammatic illustrations of axon growth phases along the central trigeminal pathway during embryonic development are shown in the bottom panel (drawings not to scale). On the left, wholemount explant preparation of intact trigeminal pathway (whisker pad, trigeminal ganglion, and brainstem) is sketched. Dashed lines in bottom panel indicate where the whisker pad was left out in some cultures. WP, whisker pad. For other abbreviations, see list. Adapted with permission from Erzurumlu and Jhaveri (1992). J Neurosci 12:3946–3955.
Preparation of dissociated cell cultures
Trigeminal ganglia from E15 embryos were collected into ice-cold GBSS. This time point was chosen primarily because it is the time when central and peripheral TG axons have begun invading their central and peripheral targets. At this stage, peripheral axons are developing arbors around the whisker follicles, and central axons are still elongating within the central trigeminal tract (Erzurumlu and Jhaveri, 1992). All of the dissections were performed under sterile conditions. For each culture run, six ganglia were dissected and connective tissues around the TG were removed with electrolytically sharpened tungsten needles. Ganglia were rinsed twice (5 minutes each) with calcium-magnesium-free Hank’s balanced salt solution (CMF-H, GIBCO). Ganglia were then treated with 0.05% trypsin (GIBCO) in CMF-H at 37°C for 20 minutes. Rinsing the ganglia twice in 5 ml of culture medium containing 10% fetal calf serum stopped enzymatic activity. After a brief centrifugation, the ganglia were resuspended in 5 ml SFM, and gently triturated with a flame-polished Pasteur pipette for about 30 times. A drop of the cell suspension was placed on a hemocytometer to determine the density of cells in the suspension. The surfaces of six-well plates or 35-mm tissue culture dishes were coated with 0.5 mg/ml polyornithine (Sigma, St. Louis, MO) in borate buffer (pH 8.6) overnight. Next day, the plates were rinsed three times with sterile dH2O and coated with 20 µg/ml laminin (GIBCO) for 4–6 hours (Scott and Davies, 1993). For morphologic documentation of individual neurons, 35-mm tissue culture plastic dishes were coated by applying 200 µl laminin into the middle of the dish. Just before plating, laminin was aspirated, plates were rinsed with SFM, and the neurons were plated at a density of approximately 1,000 cells per well. In experimental conditions, growth factors NGF, NT-3, or a mixture of NGF and NT-3 were added to the culture medium at a final concentration of 50 ng/ml. The cultures were maintained at 33°C in a humidified incubator containing 5% of CO2, for 3 days.
Previous studies have charted dose response curves for primary sensory axons and reported that NGF and NT-3 exert their effects between 10 and 50 ng/ml medium range (e.g., see Lentz et al., 1999). In the present study, such dose response assays were not performed, and for all neurotrophin treatment conditions, 50 ng/ml medium was used to achieve maximal effects.
Labeling with the lipophilic tracer DiI and immunohistochemistry
Wholemount cultures were fixed with 4% buffered paraformaldehyde (pH 7.4, 0.1 M) and labeled by inserting small crystals of the fluorescent lipophilic tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) into the ganglion explants using a stereo microscope. Cultures were kept in a warm incubator for 2–3 weeks to allow for the diffusion of the dye. Labeled specimens were later photoconverted (Sandell and Masland, 1988) in the presence of 0.15% diaminobenzidine (Sigma) in 0.1 M Tris buffer (pH 8.2). Cellular and axonal profiles were analyzed following extensive photographic documentation.
The dissociated cell cultures supplemented with NGF, NT-3, or a mixture of NGF and NT-3 were fixed with 2% paraformaldehyde in phosphate buffer (pH 7.4, 0.1 M) after 3 days in vitro. For parvalbumin immunohistochemistry, cultures were first incubated in 2% horse serum containing 0.1% Triton X-100 for 30 minutes, then incubated in primary antibody (1:500, mouse monoclonal antiparvalbumin, Sigma) made up in phosphate-buffered saline (PBS, pH 7.4 0.1 M), overnight at 4°C. The next day, they were rinsed, and incubated in biotinylated horse anti-mouse antibody (1:200, Vector Laboratories, Burlingame, CA) for 2 hours. For TrkA immunohistochemistry, cultures were first treated with 5% normal goat serum in Tris-buffered saline (TBS, pH 7.4, 0.1 M) containing 0.1% Triton X-100 for 30 minutes at room temperature. Cultures were incubated in rabbit anti-TrkA antibody (1: 2,000, gift of Dr. L. Reichardt) in TBS overnight at 4°C. Following TBS washes, biotinylated goat anti-rabbit anti-body (1:400, Sigma) was applied. After the secondary antibody incubation, all of the cultures were treated with avidin-biotin-peroxidase complex (Vectastain Elite, Vector Laboratories) for 1 hour at room temperature, then placed in 0.025% 3,3′diaminobenzidine tetrahydrochloride (DAB, Sigma) and 0.3% hydrogen peroxidase in TBS or PBS for 10 minutes. For all immunohistochemical staining procedures, control sections were processed as above except that the primary antibody was omitted.
Analyses of axon growth in dissociated TG cultures
Immunohistochemically labeled cellular and axonal elements were analyzed with light microscopy and drawn with the aid of a drawing tube using a 20× objective. For each experimental condition, neurons that were located in the middle one-third of each culture dish were drawn. The neurons whose neurites crossed one another were excluded from the quantitative analysis. For neurotrophin (NGF, NT-3, or mixture of NGF and NT-3)-supplemented culture experiments, a total of 200 neurons was drawn for each case, from four separate experiments. Camera lucida drawings were then scanned and downloaded to a Power Macintosh. The following three parameters of neurite growth were measured using NIH Image (1.60) Analyzer Computer Program: the area of cell bodies (µm2), total length of primary neurites (µm), and numbers of branches from primary neurites.
Neuron survival in dissociated cell cultures
The cells that survive in culture can be identified by their translucent appearance with round soma and processes emerging from the cell body within hours after plating. To determine the number of viable cells, a translucent paper containing a grid composed of 3-mm squares was placed below each culture dish. After 3 hours, the number of neurons located in three different squares, from three different plates were recounted. The means of these numbers were considered as the initial number of surviving neurons. After 24, 48, and 72 hours in culture, the number of neurons located in the same grids was counted. The estimated number of neurons treated with different neurotrophins was expressed as the percentage of the initial number of plated neurons.
All of the photographic documentation presented in this study was done with the use of a Kodak digital camera attached to a Nikon Microphot-SA or a Nikon Diaphot inverted microscope. Digital images were transferred to a power PC or Macintosh G3 computer. The contrast and brightness of the images were adjusted using the ADOBE Photoshop program, and none of the images were modified in any other way. These images were grouped into figures, labeled, and printed on photographic film or paper by using an Epson inkjet printer.
Statistical analysis
The percentage values presented in Figure 7 were compared by one-way analysis of variance (ANOVA) with Tukey-HSD (honestly significant difference) test. Values for NGF- or NT-3-treated cultures were compared by using a χ2 test to determine the statistical difference of the percentage values presented in Table 1A–D. Differences between conditions presented in Figure 12 were tested by using a two-tailed t-test. The data in the graphs represent the means and S.E.M. (n values are indicated in the text).
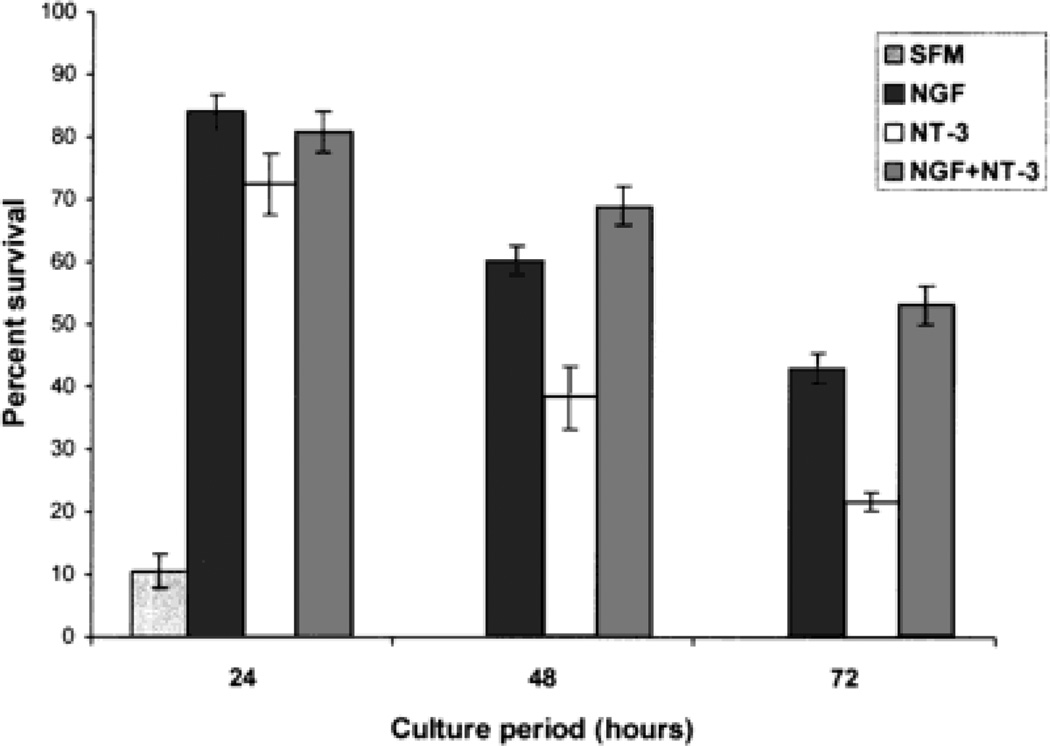
Fig. 7.
Bar graph showing the survival of embryonic day (E)15 trigeminal ganglion (TG) neurons cultured in serum-free culture medium (SFM) supplemented with 10% fetal calf serum, nerve growth factor (NGF) alone (50 ng/ml in SFM), neurotrophin-3 (NT-3) alone (50 ng/ml in SFM), and in NGF+NT-3 (50 ng/ml each in SFM). Percent survival of neurons in each case was determined as described in Materials and Methods. Note that TG neurons survive the best in the presence of both neurotrophins or NGF alone, moderately in the presence of NT-3 alone, and completely die off in SFM supplemented with fetal calf serum after 24 hours in culture. Differences in percentages of surviving neurons under different conditions were highly significant (P < 0.001).
TABLE 1.
Differentiation of TrkA and Parvalbumin-Positive Cells in Cultures Treated With Nerve Growth Factor (NGF) or Neurotrophin-3 (NT-3) Alone*
| A. TrkA | % of neurons with no branches from the primary neurites |
% of neurons with 1–3 branches from the primary neurites |
% of neurons with ≥4 branches from the primary neurites |
| NGF | 57 | 36 | 7 |
| NT-3 | 34 | 15 | 51 |
| B. Parvalbumin | % of neurons with no branches from the primary neurites |
% of neurons with 1–3 branches from the primary neurites |
% of neurons with ≥4 branches from the primary neurites |
| NGF | 44 | 39 | 17 |
| NT-3 | 9 | 20 | 71 |
| C. TrkA | % of neurons with total length of the primary neurites 0–250 µm |
% of neurons with total length of the primary neurites 250–500 µm |
% of neurons with total length of the primary neurites >500 µm |
| NGF | 4 | 24 | 72 |
| NT-3 | 18 | 47 | 35 |
| D. Parvalbumin | % of neurons with total length of the primary neurites 0–250 µm |
% of neurons with total length of the primary neurites 250– 500 µm |
% of neurons with total length of the primary neurites >500 µm |
| NGF | 3 | 31 | 66 |
| NT-3 | 40 | 45 | 15 |
P < 0.001.
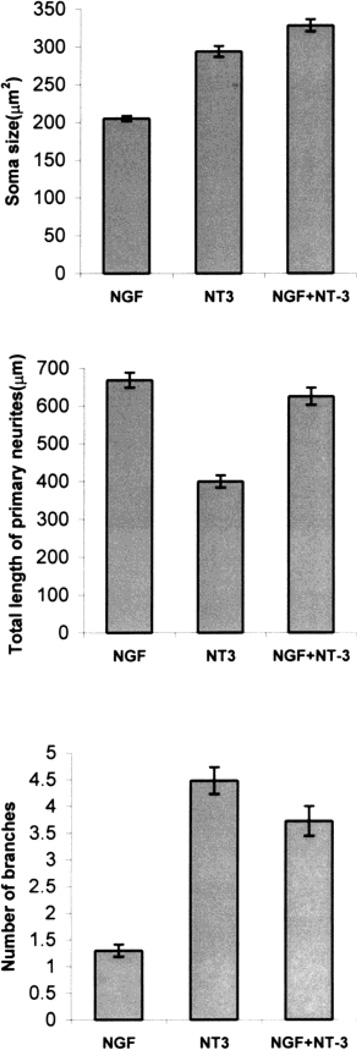
Fig. 12.
Bar graphs illustrating soma size, primary neurite length, and number of branches from the primary neurites for each of the experimental conditions used in this study. Soma size was the smallest in nerve growth factor (NGF)-treated cultures, but largest in neurotrophin-3 (NT-3)- or NT-3 + NGF-treated cultures. Total length of primary neurites was the highest in NGF-treated cultures (P < 0.001), lowest in NT-3-treated cultures. Number of branches from the primary neurites was the highest in NT-3-treated cultures (P < 0.001) and lowest in NGF-treated cultures. The values in the graph represent means ± S.E.M. of analysis of 200 neurons for each condition.
RESULTS
Morphogenetic events during the rat trigeminal pathway development
Specific events in development of the rat trigeminal pathway are schematically illustrated in Figure 1. Trigeminal ganglion cells are born between E9.5 and E14.5 (Rhoades et al., 1991). Soon after their differentiation, trigeminal ganglion cells assume a bipolar shape with one axonal process directed toward the sensory periphery and the other toward the brainstem. Peripheral and central trigeminal axons arrive near their prospective targets by E12 (Stainier and Gilbert, 1990, 1991; Erzurumlu and Jhaveri, 1992). At these early stages, both sets of axons are topographically organized with respect to the dorsoventral axis of the snout (Erzurumlu and Killackey, 1983; Erzurumlu and Jhaveri, 1992), and the scaffold of the trigeminal pathway between the whisker pad and the brainstem is built by E15. In the whisker pad, axon fascicles from the infraorbital nerve establish the whisker row nerves, and branch around newly emerging whisker follicles. Central trigeminal axons bifurcate upon entry into the hindbrain, and lay down the ascending and descending components of the trigeminal tract (Erzurumlu and Jhaveri, 1992). This pathway is located along a fairly restricted route within the lateral brainstem. Between E13 and E16, central trigeminal axons grow in the elongation phase without any branching or collateral formation (Fig. 1, bottom panel). They emit radially oriented collaterals into the trigeminal nuclear complex at E17, and begin forming terminal arbors. Within the topographically aligned central trigeminal projection zone, axons conveying information from the mystacial vibrissae and perioral sinus hairs form discrete patches. The distribution of these patches corresponds to the spatial alignment of five rows of vibrissae, and sinus hairs on the ipsilateral snout (Erzurumlu and Killackey, 1983; Erzurumlu and Jhaveri, 1992).
General characteristics of the rat trigeminal pathway grown in vitro
The above-described morphological features of the developing trigeminal pathway were confirmed in wholemount explants fixed shortly after preparation. Normal distribution of peripheral and central projections of TG are shown in Figure 2A. The central trigeminal tract is located as a restricted axonal pathway laterally in the brainstem. Peripherally, the IO nerve approaches the caudal edge of the whisker pad, and from its point of entry, whisker row nerves are dispersed. Deep and superficial follicular nerves leaving their parent fascicles form a dense cup-shaped plexus at the base of each follicle (data not shown).
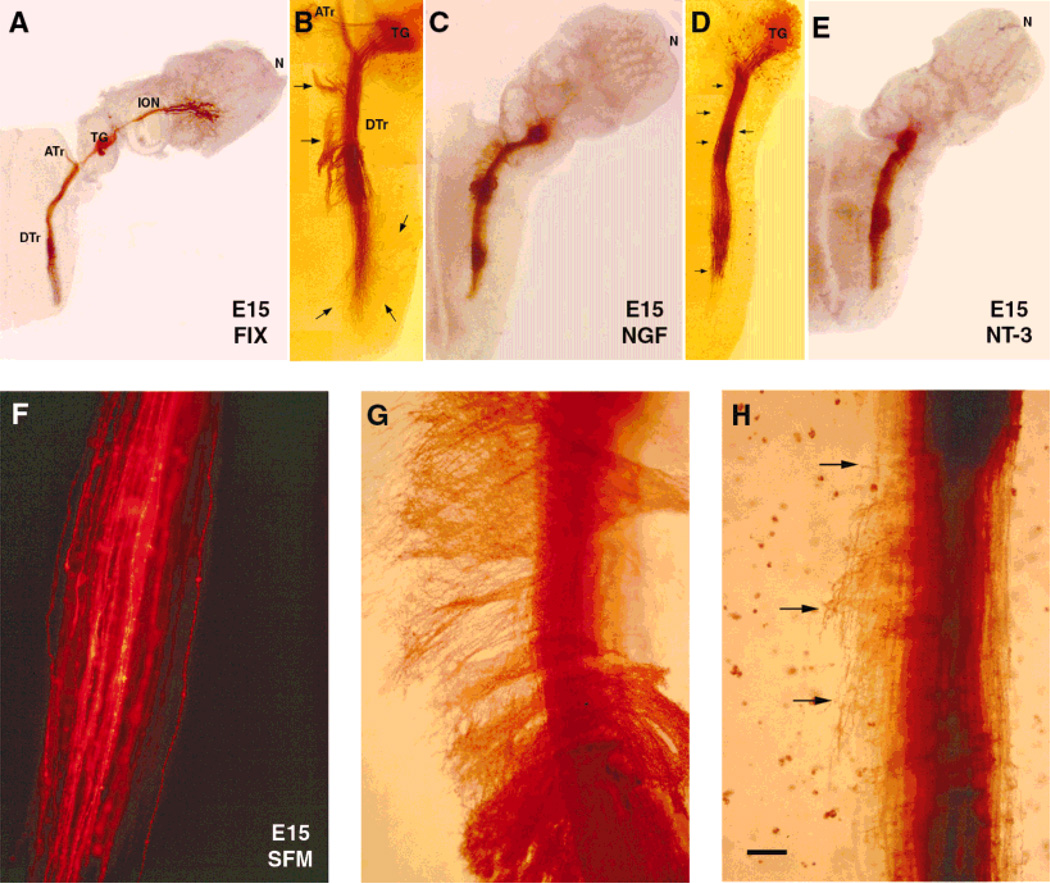
Fig. 2.
Effects of neurotrophins on embryonic day (E)15 central trigeminal axons. Low power photomicrographs of E15 of wholemount cultures of intact whisker pad-trigeminal ganglion (TG)-brainstem (A,C,E) or brainstem with TG (B,D) explants. Trigeminal axons are labeled by inserting 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) implants into the ganglion, and the fluorescent label is photoconverted. In control preparation (A), trigeminal pathway is fixed immediately after dissection. At this age, in cultures grown in serum-free culture medium (SFM), central trigeminal axons remain unbranched, and tightly fasciculated, as in normal control cases (F). In the presence of nerve growth factor (NGF; B,C), central trigeminal axons leave the tract, and extend medially or laterally into the brainstem (arrows). In the presence of neurotrophin-3 (NT-3; D,E), these axons emit collaterals and arborize along the trigeminal tract (arrows). Higher magnification views of descending trigeminal tract axons following NGF (G) and NT-3 (H) treatment. Scale bar = 600 µm in A,C,E; 300 µm in B,D; 75 µm in F–H.
In control wholemount cultures of the trigeminal pathway derived from E15 rats, both the whisker pad and the brainstem retain their tissue-specific characteristics much like that reported for the explant cocultures of this pathway (Erzurumlu et al., 1993; Erzurumlu and Jhaveri, 1995). For example, in the whisker pad, five curvilinear rows of whisker follicles and sinus hair follicles flanking these rows maintain their spatial organization. Follicles develop, and even rudimentary hairs form within the follicle cores. Centrally, the brainstem trigeminal nuclear complex is located medial to the trigeminal tract, and the cytoarchitecture of this region appears “normal” when assessed with histological stains such as Cresyl violet or bisbenzimide (data not shown). Trigeminal axons also maintain their integrity and well-defined projection patterns in the brainstem. When grown in SFM for 3 days, central trigeminal axons remain unbranched, and do not reach the developmental maturity of axons seen at E18 in vivo. Similarly, E13 trigeminal axons maintained in SFM for 3 days display morphological characteristics more similar to their E13–14 in vivo counterparts than E16 trigeminal axons. In cultures from E13 embryos, the presumptive whisker field did not differentiate as well as those seen in E15 embryos (compare Fig. 4A with Fig. 2A). Thus, in cultures maintained in SFM, trajectories and morphological characteristics of peripheral and central trigeminal axons are not compromised, but their maturation is arrested or slowed.
Fig. 4.
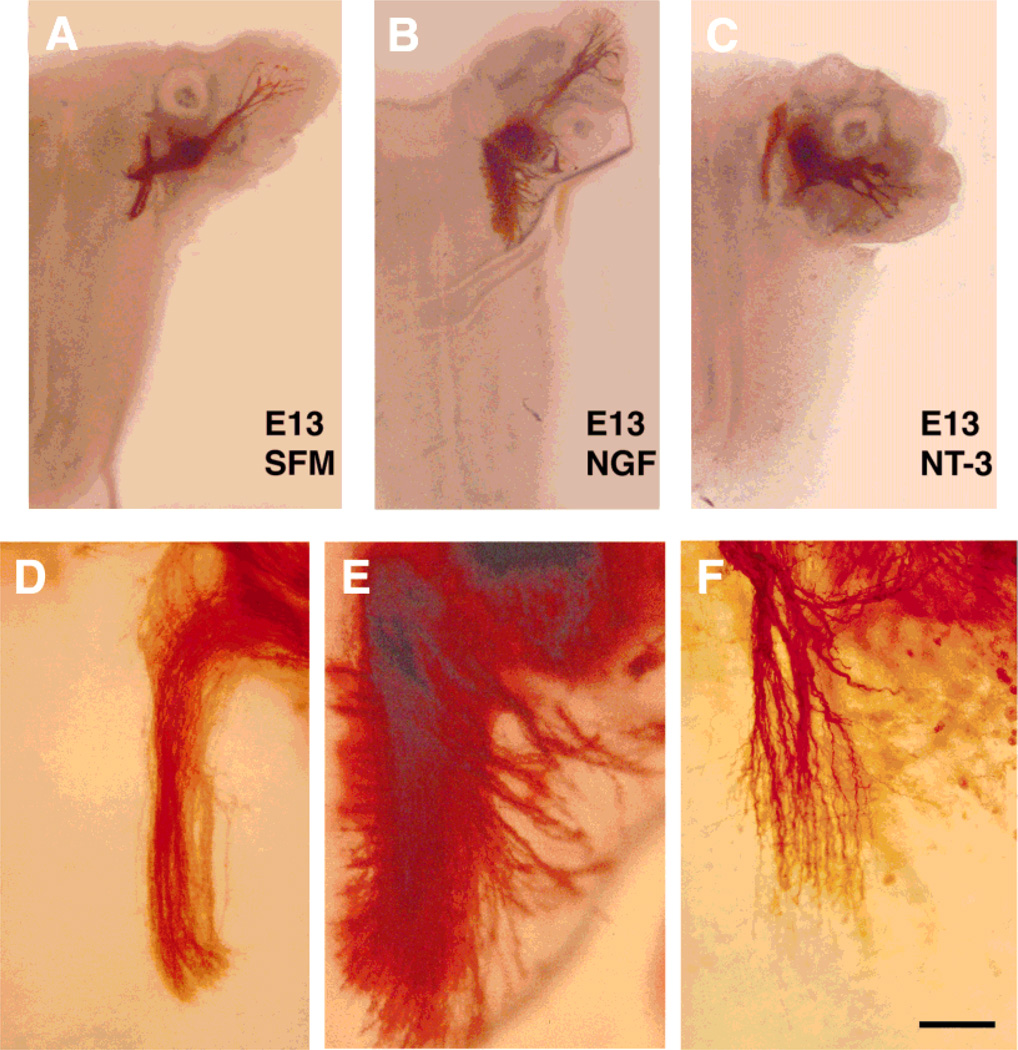
Effects of neurotrophins on embryonic day (E)13 central trigeminal axons. Low-power photomicrographs of E13 wholemount cultures of intact whisker pad-trigeminal ganglion (TG)-brainstem explants (A–C). As in E15 cases, central trigeminal axons remained unbranched in cultures grown in serum-free culture medium (SFM; A and D). In nerve growth factor (NGF)-treated cultures (B and E), descending trigeminal tract expanded and dense axon fascicles diverged from the central tract into the brainstem parenchyma. In neurotrophin-3 (NT-3)-treated cultures (C and F), axons formed short collateral branches some with rudimentary arbors. Scale bar = 500 µm in A–C; 150 µm in D–F.
Effects of neurotrophins on central trigeminal axons in wholemount cultures
Exogenous addition of 50 ng/ml NGF or NT-3 produced different effects on central trigeminal tract axon growth in wholemount cultures from E15 embryos (Fig. 2). Qualitatively, the effects of NGF or NT-3 on central trigeminal axon growth patterns were similar in wholemount cultures of the entire trigeminal pathway or in cultures without the whisker pad (compare Fig. 2B with C, and 2D with E).
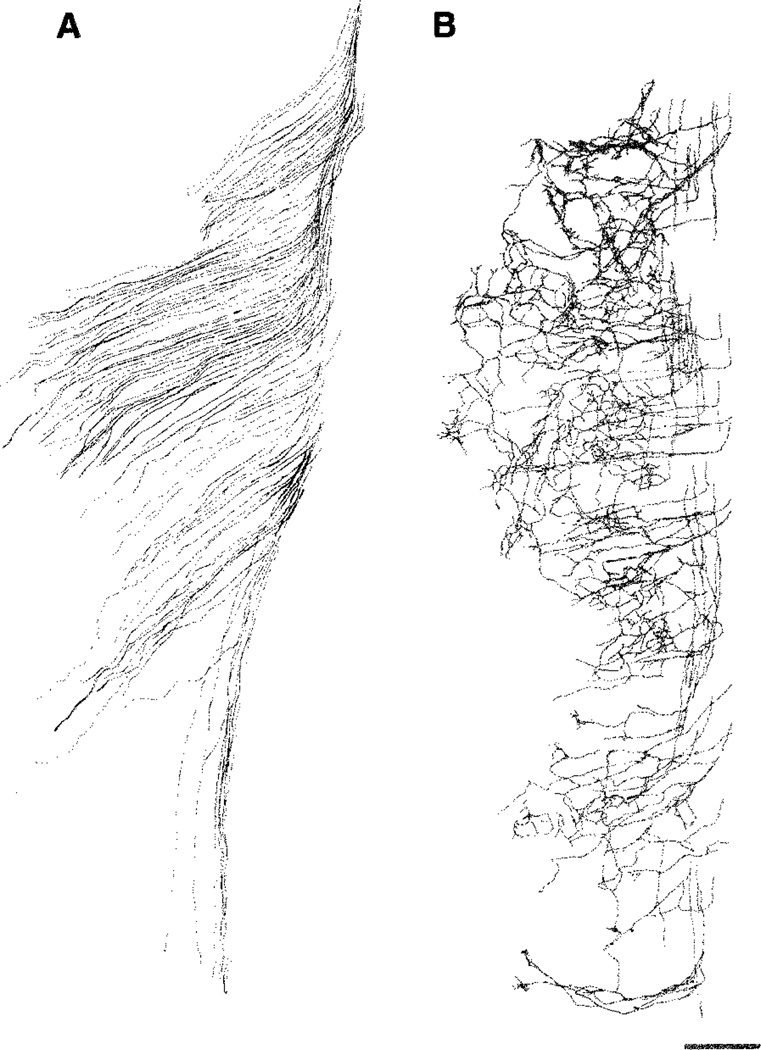
In all cases, addition of NGF into the culture medium caused central trigeminal axons to spread into the surrounding brainstem tissue, both medially and laterally. Several tightly bundled axon fascicles left the tract at irregular intervals, and after traveling some distance, took sharp rostral or caudal turns (Fig. 2B,C). These axons within the brainstem were unbranched (Fig. 2G). In contrast, exogenous addition of NT-3 into the culture medium caused precocious collateralization along the medial and lateral edges of the trigeminal tract (Fig. 2D,E). This effect was seen in all cultures from E15 embryos (Figs. 2H, 5D,E). Photographic documentation of the effects of neurotrophins on central trigeminal tract axons was difficult due to the thickness of the wholemount explants and the course of axons at various depths. Axonal differentiation in these explants could be better visualized by microscopic examination of the tissue at higher magnification at different depths of the tissue. Camera lucida drawings (using a 40× objective) from exemplary E15 cultures better illustrate neurotrophin effects on central trigeminal axons (Fig. 3).
Fig. 5.
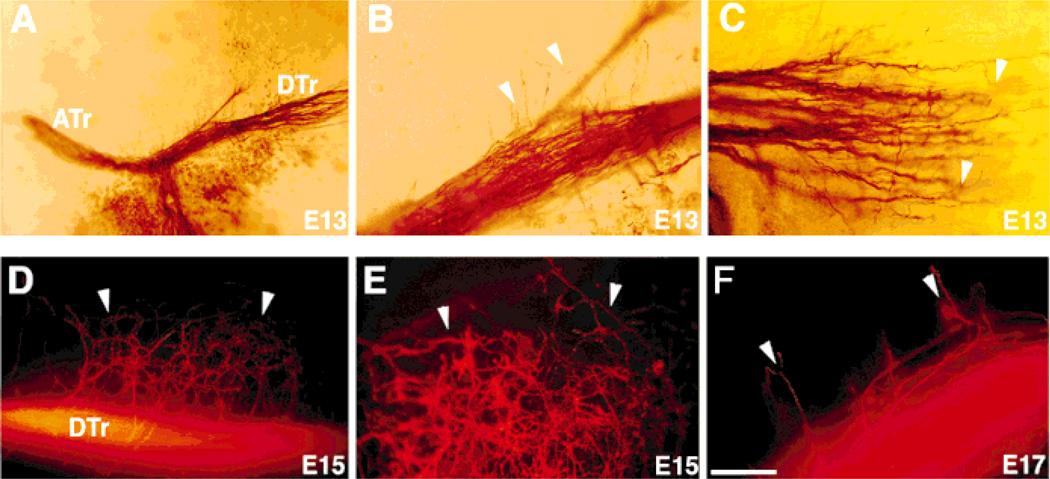
Arbor-inducing effects of neurotrophin-3 (NT-3) at embryonic day (E)13 and E15. Collateral inducing effects of NT-3 (arrowheads) can be seen as early as E13, the initial phase of trigeminal tract formation (A–C). B is the high magnification view of descending trigeminal tract shown in A. In some of the cultures from E13, defasciculation of central trigeminal arbors in the descending tract was also apparent (C). 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchloratn (DiI)-labeled central trigeminal axons from an E15 wholemount culture of brainstem explant with intact trigeminal ganglion (TG; D), and a higher-power view of dense collaterals and arbors is shown in E. Central trigeminal axons begin their collateralization (arrowheads) at E17 during normal development in vivo (F). For abbreviations, see list. Scale bar = 100 µm in B,C,E,F; 200 µm in D; 300 µm in A.
Fig. 3.
Camera lucida drawings of the central trigeminal tract axons following nerve growth factor (NGF; A) or neurotrophin-3 (NT-3; B) treatments from embryonic day (E)15 wholemount explant cultures. Scale bar = 100 µm.
We next prepared wholemount cultures of the entire trigeminal pathway from E13 embryos to investigate whether NGF and NT-3 could influence axon growth at earlier time points. At the time of dissection, central axons are entering the brainstem, and bifurcate to form the ascending and descending components of the trigeminal tract. In wholemount cultures, grown in serum-free culture medium for 3 days, these components remain intact (Fig. 4A,D).
Differential growth of central trigeminal axons treated with NGF or NT-3 was clear in E13 wholemount cultures (Fig. 4). In the presence of NGF, fascicles of axons left the tract from both the medial and lateral aspects. Nerve growth factor also led to a notable expansion of the central tract (Fig. 4B). In NT-3-treated cultures, many axons emitted short collaterals with several branches along the sides of the central tract. In most of the cultures, there was a notable defasciculation of the descending trigeminal tract (Fig. 4F, see also Fig. 5B,C). To further examine the arborization effects of NT-3, we prepared wholemount cultures, without the whisker pads, from E13–15 embryos and exposed them to NT-3 (Fig. 5). In every case, collateralization and arborization was more robust than that seen normally at E17 (Fig. 5F). Collectively, these observations indicate that NT-3 can induce premature and extensive arborization of central trigeminal axons.
Effects of neurotrophins on peripheral trigeminal axon growth patterns in E13 wholemount cultures
DiI-labeled peripheral axons could be discerned in the maxillary pad at E13 in wholemount trigeminal pathway preparations. In cultures grown in SFM, the IO nerve was identifiable as a tightly bundled fascicle of axons. Upon entering the developing whisker pad, it fanned out into presumptive whisker row nerves, and into finer branches from the parent axons (Fig. 6A). Axon growth in the maxillary process was robust in NGF-treated cultures. Instead of a tightly bundled IO nerve, thick fascicles of axons entered this peripheral target field, and extended towards the nasal pole (Fig. 6B). Often, intercrossing between “fascicles,” and a reticular meshwork of axonal processes could be seen. In NT-3-treated cultures, axon growth was also dense, but not as widespread as that seen in cases treated with NGF. We also observed entangling of thick bundles of axons, and neurites ending in “knots” in these cultures (Fig. 6C).
Fig. 6.
Effects of neurotrophins on embryonic day (E)13 peripheral trigeminal axons. 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)-labeled and photoconverted peripheral trigeminal axons in E13 whisker pad explants of intact wholemount cultures. In cultures grown in serum-free culture medium (SFM), infraorbital nerve (ION) remain tightly fasciculated, and after entering from the caudal edge of maxillary pad explants, breaks up into whisker row nerves (A). In nerve grwoth factor (NGF)- or neurotrophin-3 (NT-3)-treated cultures, axonal morphologies are notably different: several thick fascicles of peripheral trigeminal axons enter the maxillary pad (B,C). In NGF-treated cultures, dense fibers occupy a larger area in the maxillary pad explants in comparison to ones grown in the presence of NT-3 or SFM (B). Arrows indicate the “knots” seen in NT-3-treated cultures (C). Scale bar = 300 µm.
The effects of neurotrophin treatments in E15 whisker pad explants were apparent, but not as robust as those seen in E13 wholemount cultures (data not shown). This is perhaps due to the thickness of the whisker pads, where access to exogenous neurotrophins by peripheral axons may be limited. Whisker row nerves could be barely discerned with DiI labeling in E15 cultures.
We do not know whether specific subpopulations of neurons survive and exhibit different axon growth patterns in cultures treated with different neurotrophins. A variety of cell types are present in the E15 rat TG which can be distinguished by soma size, and expression of a variety of histochemical and molecular markers such as receptors for different neurotrophins. In some of the wholemount cultures treated with NGF or NT-3, TG neurons were retrogradely labeled with DiI. Most ganglion cells retained their bipolar shape in the presence of either neurotrophin (data not shown) after 3 days in culture. Both small- and large-diameter neurons could be readily identified in these cultures. In a preliminary analysis, we could not discern major differences in the soma sizes of neurons grown in SFM or in the presence of different neurotrophins (data not shown). Quantitative analyses of cell types, and their frequencies should shed light into the specific responsiveness of different classes of TG neurons to different neurotrophin treatments.
Neurotrophin effects on trigeminal axon growth in dissociated cell cultures
Our focus was on axon growth parameters in response to exogenous NGF or NT-3, but we also documented survival of neurons under various conditions (Fig. 7). When E15 TG neurons were cultured in medium supplemented with 10% fetal calf serum, survival was minimal. After 24 hours in culture, only 11% of the neurons survived, and all neurons were dead by 48 hours. Survivals of TG neurons were similar in cultures supplemented with NGF alone (84%), mixture of NGF and NT-3 (80%), or NT-3 alone (72%) after 24 hours. However, there was a significant decrease in the survival of neurons supplemented with NT-3 after 48 hours (P < 0.001). At the end of the 3-day culture period, differences in the percentages of surviving neurons treated with the mixture of NGF and NT-3 (53%), NGF (43%), or NT-3 alone (21%) were highly significant (P < 0.001). We were not able to examine trigeminal axon development in culture medium supplemented with normal serum. Although axon outgrowth patterns in the absence of NGF or NT-3 would be an important control, we could not perform this due to neurotrophin dependence of embryonic TG cells for survival. Nevertheless, the results described below underscore the differential effects of NGF and NT-3 on axon growth phases in cells grown without their normal targets.
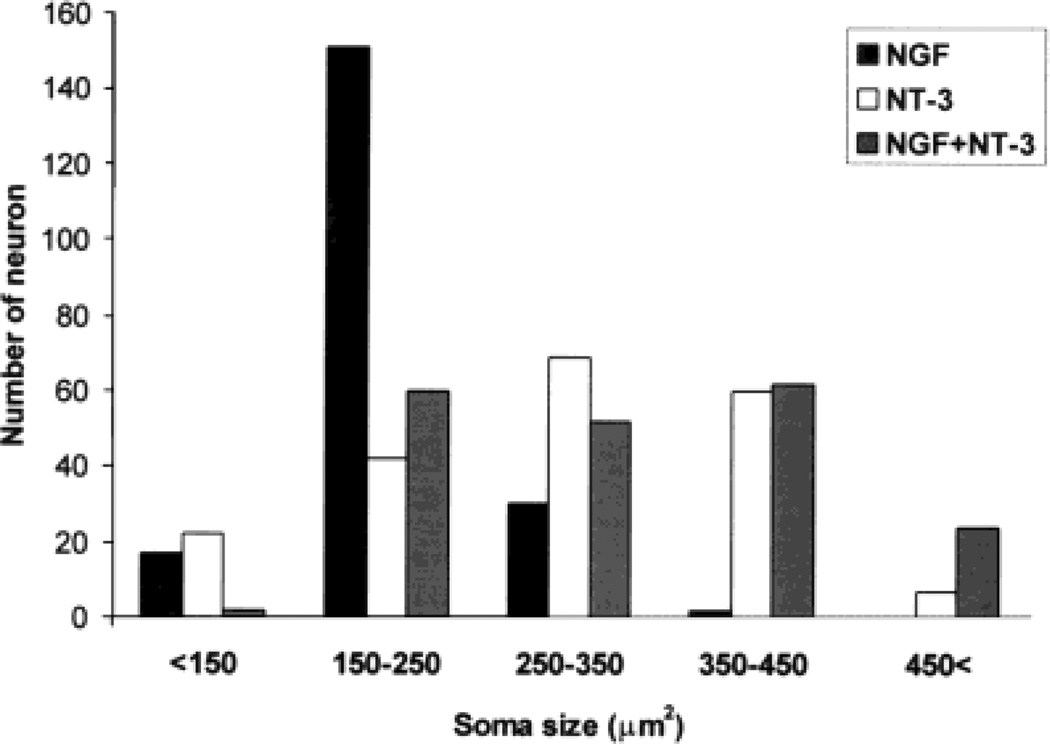
Previous studies classified primary sensory neurons on the basis of their soma sizes (reviewed in Lawson, 1992). Analyses in mice with null mutations of NGF or NT-3 revealed differential loss of small, medium, or large trigeminal neurons in these animals (Ruit et al., 1992; Crowley et al., 1994; Ernfors et al. 1994; Fariñas et al., 1994). During our survey, we noted differences between the soma size of neurons under different neurotrophin treatment conditions. As illustrated in Figure 8, most trigeminal neurons (n = 151) had a soma size between 150 and 250 µm2 in NGF supplemented cultures. In the presence of NT-3, 65% of neurons (n = 129) were large cells with soma size between 250 and 450 µm2. Addition of both neurotrophins led to a wider range in soma size, with higher percentages between 150 and 250 µm2 (30%), and 350– 450 µm2 (31%) ranges. In addition, 12% of neurons were larger than 450 µm2. Only 3.5% of cells treated with NT-3 and none of the cells treated with NGF measured above 450 µm2. These results suggest that, although it is possible to select for small neurons in the presence of NGF alone, wider range of neurons (in terms of soma size) survive in the presence of NT-3 alone or NGF plus NT-3.
Fig. 8.
Distribution of embryonic day (E)15 trigeminal ganglion (TG) neurons based on their soma size in the presence of nerve growth factor (NGF), neurotrophin-3 (NT-3), or mixture of both neurotrophins. Note that the vast majority of neurons are small cells in NGF-treated cultures. In NT-3-treated cultures, there is a wider range of soma size with a distinct tendency towards larger neurons. In the presence of both neurotrophins, soma size of neurons also show a wide distribution with peaks in the small and large cell body ranges.
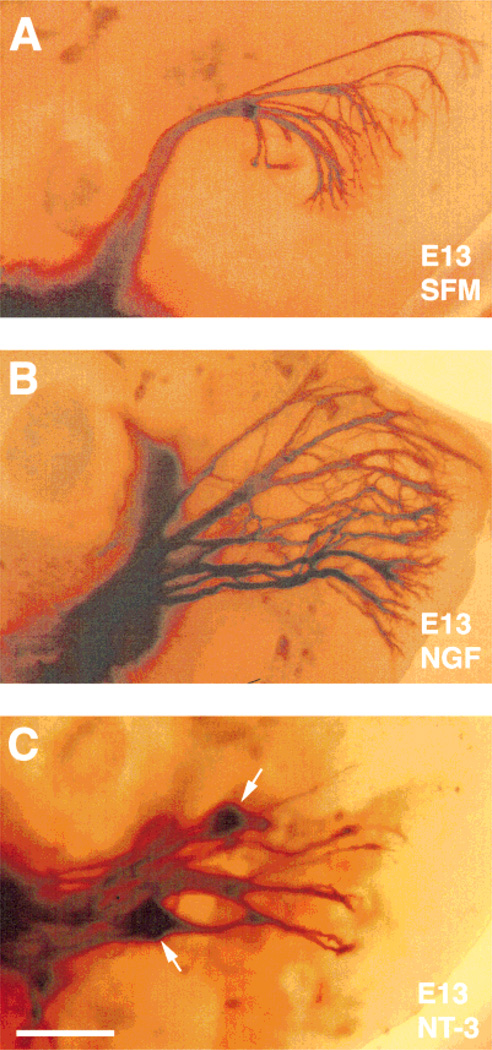
Qualitatively, NGF and NT-3 elicited different patterns of neurite growth from E15 trigeminal neurons. As illustrated in Figures 9 and 10, NGF-treated neurons grew long, unbranched axons, and NT-3-treated neurons grew short neurites with arbors. Quantitative analyses of axonal responses to neurotrophins revealed that the number of branches was significantly higher in NT-3-supplemented cultures (P < 0.001) than those grown in NGF-supplemented cultures (Fig. 12). In contrast, the total length of primary arbors was significantly longer in the presence of NGF than those grown in the presence of NT-3 (P < 0.001; Fig. 12). These results provide further evidence in support of a role for NGF and NT-3 in modulating axon elongation and branching/arborization of embryonic trigeminal neurons in culture. It is possible that NGF promotes the survival of small soma size neurons that would normally develop long neurites with minimal arborization, and conversely NT-3 promotes the survival of larger neurons, which are programmed to form short neurites with extensive arbors. To elucidate this issue, we grew cells in the presence of both neurotrophins, and analyzed the morphological features of surviving population of neurons. In such cultures, most cells extended long neurites, some with branches and arbors (Fig. 11). Soma size-frequency histograms (Fig. 8) showed that the number of large size neurons (soma size > 250 µm2) was more than twofold higher (n = 138) than small cells (soma size < 250 µm2, n = 62). The length of the primary neurites in these cultures was comparable to those grown in NGF alone (Fig. 12), but significantly longer than those seen with NT-3 alone (P < 0.001). The number of branches arising from the primary neurites was significantly higher than those seen with NGF alone (P < 0.001), but lower than those seen in cultures treated with NT-3 alone (P < 0.05).
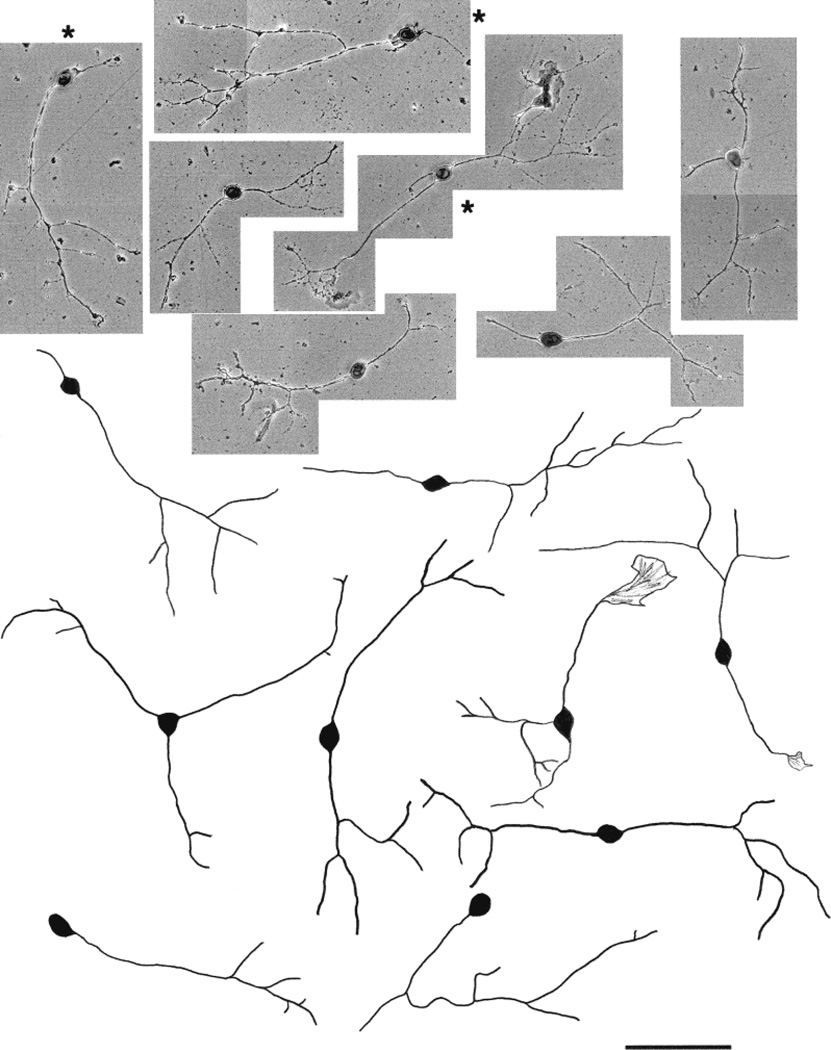
Fig. 9.
Photomicrographs and camera lucida drawings of embryonic day (E)15 trigeminal ganglion (TG) neurons cultured in the presence of nerve growth factor (NGF) for 3 days. These neurons are mostly small cells with very long, unbranched primary neurites. Note the presence of both parvalbumin immunopositive (marked with asterisks) and TrkA immunopositive (unmarked) cells. Scale bar = 100 µm.
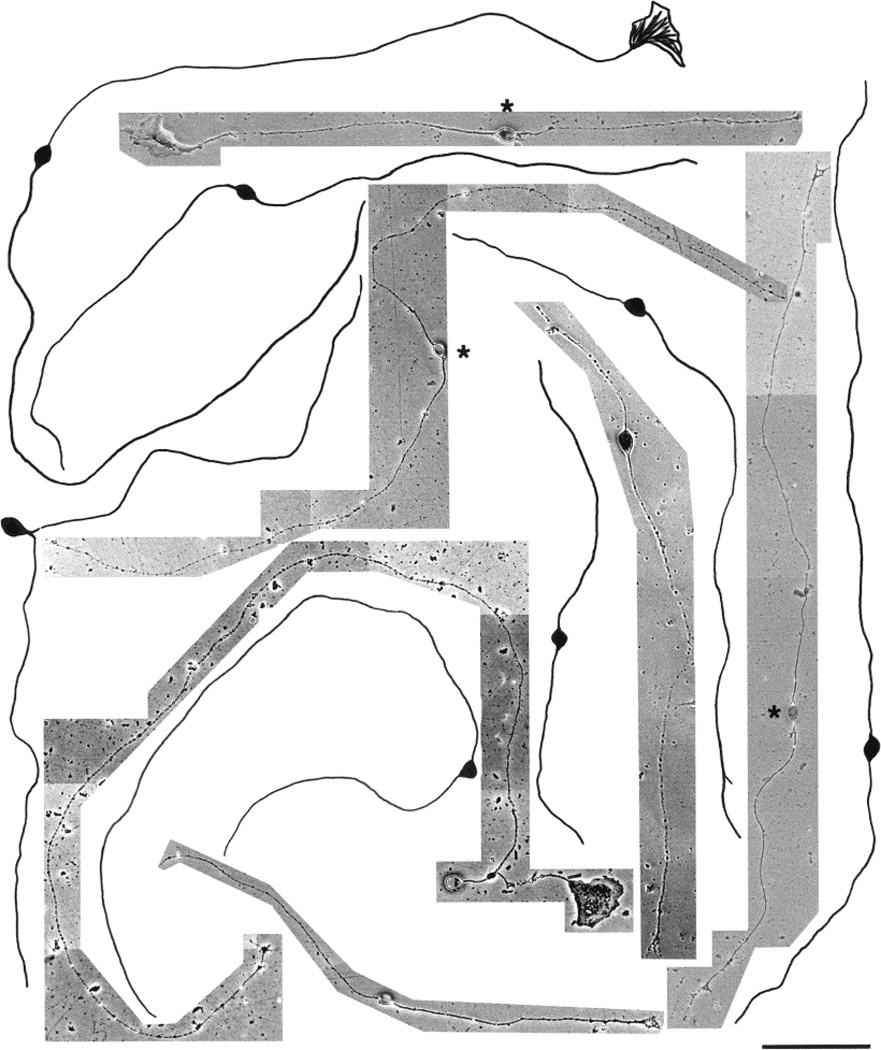
Fig. 10.
Photomicrographs and camera lucida drawings of embryonic day (E)15 trigeminal ganglion (TG) neurons cultured in the presence of neurotrophin-3 (NT-3) for 3 days. These neurons are mostly large cells with short primary neurites and focal arbors. Note the presence of both parvalbumin immunopositive (marked with asterisks) and TrkA immunopositive (unmarked) cells. Scale bar = 100 µm.
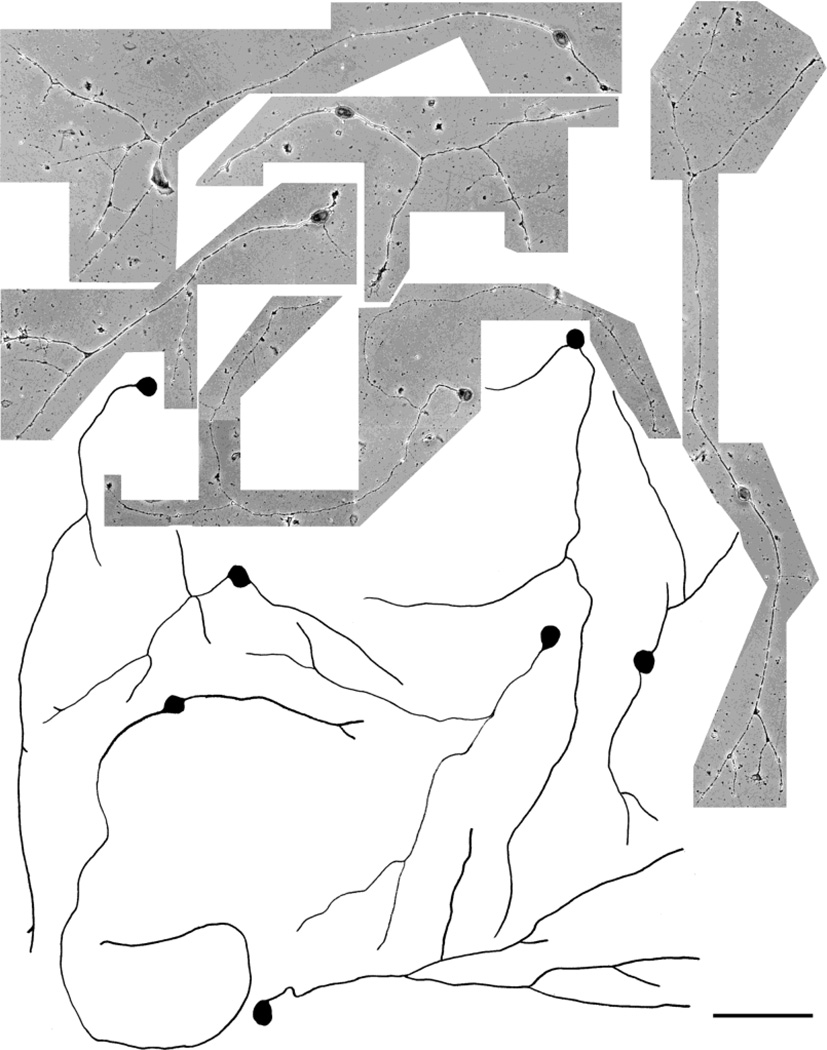
Fig. 11.
Photomicrographs and camera lucida drawings of embryonic day (E)15 trigeminal ganglion (TG) neurons (immunostained with TrkA) cultured in the presence of nerve growth factor (NGF) + neurotrophin-3 (NT-3) for 3 days. Note that these neurons vary in size, extend longer primary neurites, and develop branches with arbors. Scale bar = 100 µm.
In summary, E15 TG neurons emit long, unbranched neurites in the presence of NGF, and develop short primary neurites with arbors in the presence of NT-3. When both neurotrophins are present, primary neurite length increases significantly, but not as much as that seen with NGF alone. These treatments further lead to increased branching of primary neurites but not as much as those seen with NT-3 alone.
Expression of class-specific markers and axon growth parameters in dissociated cell cultures
Previous studies indicated that small-diameter, NGF-dependent, nociceptive neurons express TrkA receptors and calcitonin-gene-related peptide (CGRP). Large-diameter, NT-3-dependent proprioceptive neurons, on the other hand, express TrkC receptors and calcium binding protein parvalbumin (see Snider and Wright, 1996 for review). By using parvalbumin and TrkA antibodies, we wanted to selectively label NT-3- and NGF-responsive neurons, respectively, and examine their axonal differentiation under different neurotrophin treatments. However, we found that all neurons, small and large, were TrkA- or parvalbumin-positive, indicating that each cell expresses both under culture conditions. Table 1 summarizes percentages of TrkA- and parvalbumin-positive neurons (n = 100 cells for each condition) based on their neurite length and branching parameters following NGF or NT-3 treatments.
When we compared the growth response of Trk-A- or parvalbumin-expressing neurons to NGF or NT-3 treatment, we found a significant difference in their primary neurite length and number of branches from their primary neurites (P < 0.001; Table 1). In cultures grown in NGF, 57% of the TrkA-positive neurons had no branches, 36% of them had 1–3 branches, and only 7% of them had more than 4 branches (Table 1A). However, in cultures grown in NT-3, 34% of the neurons had no branches, 15% of the neurons had 1–3 branches, and 51% of them had more than 4 branches (Table 1A). Parvalbumin-positive neurons also had significantly more branches in cultures treated with NT-3 (71%) than those treated with NGF (17%; P < 0.001, Table 1B). Although 44% of them had no branches in NGF supplemented cultures, this percentage was only 9 in NT-3-supplemented cultures (Table 1B).
Treatment with NGF resulted in 72% of the TrkA-positive, and 66% of parvalbumin-positive neurons having total length of the primary neurites longer than 500 µm (Table 1C,D). On the other hand, treatment with NT-3 resulted in 35% of TrkA-positive, and 15% of parvalbumin-positive neurons having neurites longer than 500 µm (Table 1C,D). In cultures grown in NGF, only 3% of the parvalbumin-positive neurons were shorter than 250 µm, but in cultures grown in NT-3, 40% of them were shorter than 250 µm (Table 1D). Similarly, TrkA-positive axons were also significantly shorter (P < 0.001) in cultures treated with NT-3 than those of treated with NGF (Table 1C). Thus, general trends in axonal responses of - or parvalbumin-expressing neurons to NGF or NT-3 treatment were in the form of elongation or arborization, respectively.
DISCUSSION
In the present study, we report that in wholemount trigeminal pathway cultures, addition of NGF to the serum-free culture medium led to a robust growth of trigeminal axons in the form of elongation. In the presence of NT-3, central TG axons developed discrete arbors. Neurotrophin-3 induced collateralization and arborization of the central trigeminal axons as early as E13 (whereas in vivo arborization begins at E17). Primary sensory neurons depend on NGF family of neurotrophins for survival, and express different receptor tyrosine kinases (Trks) which bind these ligands (Kalcheim et al., 1987; Hohn et al., 1990; Maisonpierre et al., 1990a,b; Vogel and Davies, 1991; Davies, 1992; Mu et al., 1993; Lamballe et al., 1994; Bothwell, 1995; White et al., 1996). Both peripheral and central targets express neurotrophins coincident with neurogenesis of sensory ganglia and before the first axons reach their prospective target fields (Lindsay et al., 1985; Davies et al., 1987; Ernfors and Persson 1991; Schechterson and Bothwell, 1992). The developing rodent whisker field expresses NGF, NT-3, NT-4, and BDNF mRNA with differing distributions around whisker follicles (Davies et al., 1987; Ernfors et al., 1992; Ibañez et al., 1993). Centrally, not much is known about neurotrophin expression and regulation in the brainstem trigeminal nuclei. High levels of mRNA expression for the neurotrophins NT-3 and NGF have been reported in the developing rat spinal cord and brainstem (Maisonpierre et al., 1990b; Elkabes et al., 1994). Examination of NT-3 expression by using lacZ histochemistry in wholemounts of embryos provides greater resolution than in situ hybridization studies, and using this approach,Wilkinson et al. (1996) did not observe NT-3 expression in the central targets of TG neurons between E10.5 and E13.5. However, this period is before the onset of arborization of the trigeminal tract axons. Presently, we do not know whether endogenous expression of neurotrophins in central trigeminal targets play a regulatory role in axon growth and branching patterns during in vivo development. Observations from mice with null mutations of NGF or NT-3 do not indicate major developmental abnormalities of the central pathways for primary sensory neurons of DRG and TG (Ernfors et al., 1994; Fariñas et al., 1994; see Snider, 1994 for a review). In these mice, compensatory mechanisms might be operational, and perhaps more information could be gleaned from double knockouts. It is also possible that in vivo spatiotemporal regulation of neurotrophin expression in the whisker pad (Davies et al., 1987; Ernfors et al., 1990, 1992; Ibañez et al., 1993) might influence peripheral TG axons in such a way that their central counterparts undergo developmental changes in their structural differentiation. This possibility could be studied in mice with overexpression of different neurotrophins only in the peripheral targets of TG neurons. Although such mice have been generated by using a keratin promoter (Albers et al., 1994, 1996; Davis et al., 1997), details of central trigeminal axon differentiation have not been charted. The results reported in the present study cannot be interpreted to mean that similar mechanisms are operational during in utero development of the rodent trigeminal pathway. However, they clearly show that exogenous application of neurotrophins lead to dramatic shifts in axon growth patterns. This response of embryonic trigeminal neurons could be further exploited in studies aimed at restoring damaged sensory pathways during development.
Differential effects of NGF and NT-3 on embryonic trigeminal axons could be seen in dissociated cell cultures as well. It is important to note that these cells grow in the absence of peripheral and central target influences. Qualitative and quantitative analyses consistently revealed that axon growth was in the form of elongation in the presence of NGF, and in the form of short growth with pronounced branching and arborization in the presence of NT-3. However, we could not attribute these axon growth patterns to specific classes of neurons. Results from parvalbumin and TrkA immunohistochemistry did not yield insights into this issue. It might be argued that NGF and NT-3 selectively promoted the survival of small neurons programmed to emit long and unbranched axons and large neurons intrinsically programmed to become short and branched. However, in cultures with two neurotrophins, the neurite length and branching of both small and large neurons increased. This effect cannot be solely attributed to selective cell survival and intrinsic programming of different classes of cells in their axon growth parameters.
Recently Huang et al. (1999) reported limited coexpression of different Trk receptors at early ages in the mouse trigeminal ganglion, but at E13.5, each neuron was found to express only one Trk receptor in vivo. E15 rat TG neurons also do not coexpress TrkA and TrkC receptors (Genc and Erzurumlu, 2000). Under in vitro conditions, neurotrophin treatment may change the expression patterns of Trk receptors, as well as neuropeptides and calcium-binding proteins, and there is precedence for this in the avian sensory ganglia (Friedel et al., 1997). In situ hybridization studies in adult rat DRGs also indicate that different percentages of ganglion cells coexpress mRNA for high- and low-affinity neurotrophin receptors (Kashiba et al., 1995; Karchewski et al., 1999). However it is not clear whether these receptors are expressed at the protein level. New preliminary results from our laboratory indicate that under culture conditions, dissociated E15 TG cells coexpress TrkA and TrkC proteins few hours after plating in serum-free medium, or medium supplemented with neurotrophins (Genc and Erzurumlu, 2000). In the present study, immunohistochemical labeling of neurons with anti-TrkA antibody showed that half of the neurons (51%) had more than four branches in cultures treated with NT-3. This percentage was only 7% in NGF treated cultures. On the other hand, in the presence of NGF, 57% of TrkA-positive cells, and in the presence of NT-3, 34% of TrkA-positive cells had unbranched primary neurites. Thus, these results are consistent with the idea that NGF and NT-3 can differentially affect axon growth parameters of the same or overlapping populations of TG neurons. At the present time, we do not know whether NGF and NT-3 effects on axon growth parameters are mediated via binding of these ligands to specific high-affinity Trk receptors or low-affinity p75 receptors. Ongoing studies using tissues from p75 null mice might shed light to this issue.
Our current observations are also consistent with those reported by Lentz et al. (1999). These authors took advantage of the BAX knockout mice, in which the absence of this apoptosis regulator gene allows sensory neurons to survive independently of neurotrophins. By using DRG cells from these mice as baseline controls, they demonstrated differential effects of NGF and NT-3 on axon elongation and branching, respectively. The present results from cultured embryonic rat TG neurons complement their observations from BAX-deficient mouse DRG cells. Thus, neurotrophin effects on axon growth patterns of sensory neurons of dorsal root and cranial ganglia may be a shared phenomenon in the peripheral nervous system. Our results from explant cultures also demonstrate these effects in an intact sensory pathway maintained in vitro. Furthermore, wholemount explant cultures allowed us to examine the effects of neurotrophins on peripheral versus central axons of the ganglion cells, which is impossible to differentiate in dissociated cell cultures.
Exogenous addition of NGF and NT-3 into wholemount cultures affected axon growth in the whisker pad as in the central trigeminal tract. Unlike central axons, peripheral trigeminal axons give rise to collateral branches as soon as they enter the whisker pad. In cultures grown in SFM, the IO nerve and its tightly bundled fascicle of axons are readily identifiable. Within the whisker pad, IO nerve branches (presumptive whisker row nerves) fan out towards the nasal pole. Axon growth in these cultures was similar to that seen in E13 embryos fixed immediately after dissection and labeled with DiI (data not shown). In NGF-treated cultures, axon growth in the maxillary process was robust. Instead of a tightly bundled IO nerve, thick fascicles of axons entered this peripheral target field. Many axons crisscrossed between thick fascicles. In NT-3-treated cultures, axon growth was also dense, but these axons did not span long distances from a caudal to rostral direction. They often formed entangled bundles and neuritic knots. These results are in agreement with in vivo studies in which NGF or NT-3 transgene is expressed at high levels in keratinized epithelium (Albers et al., 1994, 1996; Davis et al., 1997). In these transgenic mice, neurotrophin overexpression in the peripheral target tissues leads to increase in skin innervation density, and in the number of trigeminal sensory neurons. Ongoing studies are aimed at determining changes in the numbers of cells and their size-specific distribution in the TG of wholemount explants grown under different conditions.
The innervation of the whisker pad fur between the vibrissal follicle sinus complex (FSC) of normal mice is similar to that reported for normal rats (Rice et al., 1993, 1997; Fundin et al., 1997). Rice and colleagues showed that axons derived from the infraorbital nerve fascicles form a dense plexus composed of four tiers, each with distinct classes of sensory endings (Rice and Munger, 1986; Rice et al., 1986, 1993). A detailed analysis of these endings following neurotrophin treatments in wholemount cultures was beyond the scope of this study. However, studies on neurotrophin or Trk receptor knockout mice clearly show that neurotrophins do regulate this aspect of trigeminal axon-target interactions as well (Fundin et al., 1997; Rice et al., 1998). These studies indicate that NGF/TrkA signaling plays a major role in the outgrowth and proliferation of sensory axons, whereas NT-3/TrkC signaling plays a major role in the formation of sensory endings. Most types of afferents are dependent on different combinations of neurotrophins and receptors for their survival (Fundin et al., 1997).
The findings reported here suggest a role for neurotrophins NGF and NT-3 as potential regulators of trigeminal axon growth. However, other molecules most likely play a role in determining trigeminal axon growth patterns. For example, glial cell line-derived neurotrophic factor (GDNF) and neurturin (NTN) have been shown to influence trigeminal neurons (Fundin et al., 1999; Rosenthal, 1999). Recently, Slit 2, mammalian homologue of Drosophila Slit, has been identified as an axon elongationand branch-promoting factor for embryonic rat DRG neurons (Wang et al., 1999). The relative contributions of these molecules to sensory axon growth phases remain to be determined in vivo.
What are the cellular mechanisms that mediate different axonal responses to NGF and NT-3? Distinguishing between growth-permissive (i.e., survival-promoting), and instructive (i.e., axon growth patterns) effects of neurotrophins have been a difficult task. For cell survival and differentiation, related intracellular signaling pathways have been detailed (see Klesse and Parada, 1999, for a recent review). For neurotrophin-induced differentiation response, intracellular signals that couple growth factors to mitogen-activated protein kinase (MAPK) appear to be critical (Alessi et al., 1995; Skaper and Walsh, 1998). Cross-talk between different Trk receptors and their ligands may activate more than one signaling cascade, some leading to proliferation, some to cell survival, and others to finer aspects of neurite differentiation, elongation, and arbor formation. Furthermore, all these effects could be a dynamic interplay during the course of development, some mechanisms operating within a narrow window, others in an overlapping fashion. Discrepant numbers of cell losses between mice with null mutations for specific neurotrophins and their high-affinity receptors (e.g., NT-3 and TrkC knockout mice) underscore multiplicity of the signaling pathways involved (see Snider, 1994 for a review). Most likely, different classes of sensory neurons spatiotemporally regulate their expression of a variety of receptors giving them the option to interact with ever-changing supply of neurotrophins. This could then lead to a shifting balance at any given moment in development and optimize cell survival, generation of neurites, and maintenance of axon terminals in target fields. Such a strategy can also enable developing axons to better respond to and interact with a variety of target-derived axon growth regulatory molecules.
ACKNOWLEDGMENTS
The authors thank Dr. K. S. Vogel for help with primary cell cultures and discussion of some of the experiments, Dr. W. Snider for comments on an earlier version of the manuscript, Dr. L. Reichardt for providing TrkA antibody, T. Ulupinar and A. Haeberle for assistance with the figures and manuscript preparation. NT-3 was provided by Regeneron Pharmaceuticals. E.U. was supported by a predoctoral fellowship from the Higher Education Commision of Turkey and Osmangazi University. This work was submitted by E.U. in partial fulfillment of the requirements for the Doctor of Philosophy to the Department of Cell Biology and Anatomy of LSU Health Sciences Center in New Orleans. It was presented in preliminary form at the 1998 Society for Neuroscience Meetings.
Grant sponsor: N.I.H.; Grant numbers: NS32195, DE07734.
Abbreviations
- ATr
ascending trigeminal tract
- BDNF
brain-derived neurotrophic factor
- BSTC
brainstem trigeminal nuclear complex
- CGRP
calcitonin gene-related peptide
- CMF-H
calcium, magnesium-free Hank’s balanced salt solution
- CNS
central nervous system
- DAB
3,3′-diaminobenzidine tetrahydrochloride
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- DRG
dorsal root ganglion
- DTr
descending trigeminal tract
- E
embryonic day
- FSC
follicle-sinus complex
- GDNF
glial cell line-derived neurotrophic factor
- GBSS
Gey’s balanced salt solution supplemented with sucrose
- IO/ION
infraorbital nerve
- N
nasal pole
- NGF
nerve growth factor
- NT-3
neurotrophin-3
- NTN
neurturin
- PBS
phosphate-buffered saline
- SFM
serum-free culture medium
- TBS
tris-buffered saline
- TG
trigeminal ganglion
- Trks
receptor tyrosine kinases
- WF
whisker follicle
LITERATURE CITED
- Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers KM, Perrone TN, Goodness TP, Jones ME, Green MA, Davis BM. Cutaneous overexpression of NT-3 increases sensory and sympathetic neuron number and enhances touch dome and hair follicle innervation. J Cell Biol. 1996;134:487–497. doi: 10.1083/jcb.134.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkemeier L, Winslow J, Kaplan D, Nicolis K, Goeddel D, Rosental A. Neurotropin-5: a novel neurotropic factor tat activates trk and trkB. Neuron. 1991;7:857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Bhide PG, Frost DO. Stages of growth of hamster retinofugal axons: implications for developing axons with multiple targets. J Neurosci. 1991;11:485–504. doi: 10.1523/JNEUROSCI.11-02-00485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Castellani V, Bolz J. Opposing roles for neurotrophin-3 in targeting and collateral formation of distinct sets of developing cortical neurons. Development. 1999;126:3335–3345. doi: 10.1242/dev.126.15.3335. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- Collazo D, Takahashi H, McKay RDG. Cellular targets and trophic functions of neurotrophin-3 in the developing rat hippocampus. Neuron. 1992;9:643–656. doi: 10.1016/0896-6273(92)90028-c. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, Philips HS. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Davies AM. Cell death and tropic requirements of developing sensory neurons. In: Scott SA, editor. Sensory neurons: diversity and plasticity. New York: Oxford University Press; 1992. pp. 194–214. [Google Scholar]
- Davies AM, Bandtlow C, Heumann R, Korshing S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- Davis BM, Fundin BT, Albers KM, Goodness TP, Cronk KM, Rice FL. Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J Comp Neurol. 1997;387:489–506. doi: 10.1002/(sici)1096-9861(19971103)387:4<489::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Elkabes S, Dreyfus C, Schaar DG, Black I. Embryonic sensory development: local expression of neurotrophin-3 and target expression of nerve growth factor. J Comp Neurol. 1994;341:204–213. doi: 10.1002/cne.903410206. [DOI] [PubMed] [Google Scholar]
- ElShamy WM, Linnarsson S, Lee KF, Jaenisch R, Ernfors P. Prenatal and postnatal requirements of NT-3 for sympathetic neuroblast survival and innervation of specific targets. Development. 1996;122:491–500. doi: 10.1242/dev.122.2.491. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Persson H. Developmentally regulated expression of HDNT/NT-3 mRNA in rat spinal cord motor neurons and expression of BDNF mRNA in embryonic dorsal root ganglion. Eur J Neurosci. 1991;3:953–961. doi: 10.1111/j.1460-9568.1991.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–26. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J Neurosci. 1992;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Target maturity influences morphological patterning of trigeminal sensory axons. Exp Neurol. 1995;135:1–16. doi: 10.1006/exnr.1995.1061. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Killackey HP. Development of order in the rat trigeminal system. J Comp Neurol. 1983;213:365–380. doi: 10.1002/cne.902130402. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S, Takahashi H, McKay RDG. Targetderived influences on axon growth modes in explant cultures of trigeminal neurons. Proc Natl Acad Sci USA. 1993;90:7235–7239. doi: 10.1073/pnas.90.15.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Friedel RH, Schnurch H, Stubbusch J, Barde YA. Identification of genes differentially expressed by nerve growth factor- and neurotrophin-3-dependent sensory neurons. Proc Natl Acad Sci USA. 1997;94:12670–12675. doi: 10.1073/pnas.94.23.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL. Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR. Dev Biol. 1997;190:94–116. doi: 10.1006/dbio.1997.8658. [DOI] [PubMed] [Google Scholar]
- Fundin BT, Mikaels A, Westphal H, Ernfors P. A rapid and dynamic regulation of GDNF-family ligands and receptors correlate with the developmental dependency of cutaneous sensory innervation. Development. 1999;126:2597–2610. doi: 10.1242/dev.126.12.2597. [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Localized sources of nurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc B, Erzurumlu RS. TrkA and TrkC co-expression in dissociated trigeminal ganglion cell cultures and axonal responses to NGF and NT-3. Soc Neurosci Abstr. 2000 (in press) [Google Scholar]
- Heffner CD, Lumsden AGS, O’Leary DDM. Target control of collateral extension and directional axon growth in the mammalian brain. Science. 1990;247:217–220. doi: 10.1126/science.2294603. [DOI] [PubMed] [Google Scholar]
- Hohn A, Leibrock J, Bailey K, Barde YA. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Mercer EH, Palmitier RD, Brinster RL. Expression of NGF in sympathetic neurons leads to excessive axon outgrowth from ganglia but decreased terminal innervation within tissues. Neuron. 1993;10:1019–1034. doi: 10.1016/0896-6273(93)90051-r. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Wilkinson GA, Fariñas I, Backus C, Zang K, Wong SL, Reichardt LF. Expression of trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to trkC. Development. 1999;126:2191–2203. doi: 10.1242/dev.126.10.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez CF, Ernfors P, Timmusk T, Ip NY, Arenas E, Yancopoulos GD, Persson H. Neurotrophin-4 is a target-derived neurotrophic factor for neurons of the trigeminal ganglion. Development. 1993;117:1345–1353. doi: 10.1242/dev.117.4.1345. [DOI] [PubMed] [Google Scholar]
- Ip NY, Ibáñez CF, Nye SH, McClain J, Jones PF, Gies DR, Belluscio L, LeBeau MM, Espinosa R, Squinto SP. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci USA. 1992;89:3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, Stitt TN, Tapley P, Klein R, Glass DJ, Fandl J, Greene LA, Barbacid M, Yancopoulos GD. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- Jhaveri S, Edwards MA, Schneider GE. Initial stages of retinofugal axon development in the hamster: evidence for two distinct modes of growth. Exp Brain Res. 1991;87:371–382. doi: 10.1007/BF00231854. [DOI] [PubMed] [Google Scholar]
- Jones KR, Reichardt LF. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci USA. 1990;87:8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheim C, Barde YA, Thoenen H, LeDourain NM. In vivo effect of brain-derived neurotrophic factor on the survival of developing dorsal root ganglion cells. EMBO J. 1987;6:2871–2873. doi: 10.1002/j.1460-2075.1987.tb02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchewski LA, Kim FA, Johnston J, McKnight RM, Verge VMK. Anatomical evidence supporting the potential for modulation by multiple neurotrophins in the majority of adult lumbar sensory neurons. J Comp Neurol. 1999;413:327–341. doi: 10.1002/(sici)1096-9861(19991018)413:2<327::aid-cne11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Noguchi K, Ueda Y, Senba E. Coexpression of Trk family members and low-affinity neurotrophin receptors in rat dorsal root ganglion neurons. Brain Res Mol Brain Res. 1995;30:158–164. doi: 10.1016/0169-328x(94)00249-e. [DOI] [PubMed] [Google Scholar]
- Klesse LJ, Parada LF. Trks: Signal transduction and intracellular pathways. Microscopy Res Tech. 1999;45:210–216. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<210::AID-JEMT4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Lamballe F, Smeyne RJ, Barbacid M. Developmental expression of trkC, the neurotrophin-3 receptor, in the mammalian nervous system. J Neurosci. 1994;14:14–28. doi: 10.1523/JNEUROSCI.14-01-00014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN. Morphological and biochemical cell types of sensory neurons. In: Scott SA, editor. Sensory neurons. Diversity, development and plasticity. New York: Oxford University Press; 1992. pp. 27–59. [Google Scholar]
- Lentz SI, Knudson CM, Korsmeyer SJ, Snider WD. Neurotrophins support the development of diverse sensory axon morphologies. J Neurosci. 1999;19:1038–1048. doi: 10.1523/JNEUROSCI.19-03-01038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Thoenen H, Barde YA. Placode and neural crest-derived sensory neurons are responsive at early development stages to brain-derived neurotrophic factor. Dev Biol. 1985;112:319–328. doi: 10.1016/0012-1606(85)90402-6. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990a;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990b;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J Neurosci. 1993;13:4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, O’Leary DDM. Inaccuracies in initial growth and arborization of chick retinotectal axons followed by course corrections and axon remodeling to develop topographic order. J Neurosci. 1989;9:3776–3795. doi: 10.1523/JNEUROSCI.09-11-03776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Enfiejian HL, Chiaia NL, MacDonald GJ, Miller MW, Mc- Cann P, Goddard CM. Birthdates of trigeminal ganglion cells contributing to the infraorbital nerve and specific vibrissa follicles in the rat. J Comp Neurol. 1991;307:163–175. doi: 10.1002/cne.903070114. [DOI] [PubMed] [Google Scholar]
- Rice FL, Munger BL. A comparative light microscopic analysis of the sensory innervation of the mystacial pad. II. The common fur between the vibrissae. J Comp Neurol. 1986;252:186–205. doi: 10.1002/cne.902520205. [DOI] [PubMed] [Google Scholar]
- Rice FL, Mance A, Munger BL. A comparative light microscopic analysis of the sensory innervation of the mystacial padIInnervation of vibrissal follicle-sinus complexes. J Comp Neurol. 1986;252:154–174. doi: 10.1002/cne.902520203. [DOI] [PubMed] [Google Scholar]
- Rice FL, Kinnman E, Aldskogius H, Johansson O, Arvidsson J. The innervation of the mystacial pad of the rat as revealed by PGP 9.5 immunofluorescence. J Comp Neurol. 1993;337:366–385. doi: 10.1002/cne.903370303. [DOI] [PubMed] [Google Scholar]
- Rice FL, Fundin BT, Arvidsson J, Aldskogius H, Johansson O. Comprehensive immunofluorescence and lectin binding analysis of vibrissal follicle sinus complex innervation in the mystacial pad of the rat. J Comp Neurol. 1997;385:149–184. [PubMed] [Google Scholar]
- Rice FL, Albers KM, Davis BM, Silos-Santiago I, Wilkinson GA, LeMaster AM, Ernfors P, Smeyne RJ, Aldskogius H, Phillips HS, Barbacid M, DeChiara TM, Yancopoulos GD, Dunne CE, Fundin BT. Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophins, trk receptors, and p75LNGFR. Dev Biol. 1998;198:57–81. [PubMed] [Google Scholar]
- Rosenthal A. The GDNF protein family: gene ablation studies reveal what they really do and how. Neuron. 1999;22:201–203. doi: 10.1016/s0896-6273(00)81077-6. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Goeddel DV, Nguyen T, Lewis M, Shih A, Laramee GR, Nikolics K, Winslow JWE. Primary structure and biological activity of a novel human neurotrophic factor. Neuron. 1990;4:767–773. doi: 10.1016/0896-6273(90)90203-r. [DOI] [PubMed] [Google Scholar]
- Ruit KG, Elliott JL, Osborne PA, Yan Q, Snider WD. Selective dependence of mammalian dorsal root ganglion neurons on nerve growth factor during embryonic development. Neuron. 1992;8:573–587. doi: 10.1016/0896-6273(92)90284-k. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Masland RH. Photoconversion of some fluorescent markers to a diaminobenzidine reaction product. J Histochem Cytochem. 1988;36:555–559. doi: 10.1177/36.5.3356898. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Scott SA, Davies AM. Age-related effects of nerve growth factor on the morphology of embryonic neurons in vitro. J Comp Neurol. 1993;337:277–285. doi: 10.1002/cne.903370208. [DOI] [PubMed] [Google Scholar]
- Simon DK, O’Leary DDM. Limited topographic specificity in the targeting and branching of mammalian retinal axons. Dev Biol. 1990;137:125–134. doi: 10.1016/0012-1606(90)90013-9. [DOI] [PubMed] [Google Scholar]
- Simon DK, O’Leary DDM. Development of topographic order in the mammalian retinocollicular projection. J Neurosci. 1992;12:1212–1232. doi: 10.1523/JNEUROSCI.12-04-01212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Walsh FS. Neurotrophic molecules: strategies for designing effective therapeutic molecules in neurodegeneration. Mol Cell Neurosci. 1998;12:179–193. doi: 10.1006/mcne.1998.0714. [DOI] [PubMed] [Google Scholar]
- Snider WD. Functions of neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Snider WD, Wright DE. Neurotrophins cause a new sensation. Neuron. 1996;16:229–232. doi: 10.1016/s0896-6273(00)80039-2. [DOI] [PubMed] [Google Scholar]
- Stainier DYR, Gilbert W. Pioneer neurons in the mouse trigeminal sensory system. Proc Natl Acad Sci USA. 1990;87:923–927. doi: 10.1073/pnas.87.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DYR, Gilbert W. Neuronal differentiation and maturation in the mouse trigeminal sensory system, in vivo and in vitro. J Comp Neurol. 1991;311:300–312. doi: 10.1002/cne.903110210. [DOI] [PubMed] [Google Scholar]
- Vogel KS, Davies AM. The duration of neurotrophic factor independence in early sensory neurons is matched to the time course of target field innervation. Neuron. 1991;7:819–830. doi: 10.1016/0896-6273(91)90284-7. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- White FA, Silos-Santiago I, Molliver DC, Nishimura M, Phillips H, Barbacid M, Snider WD. Synchronous onset of NGF and trk A survival dependence in developing dorsal root ganglia. J Neurosci. 1996;16:4662–4672. doi: 10.1523/JNEUROSCI.16-15-04662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GA, Fariñas I, Backus C, Yoshida CK, Reichardt LF. Neurotroophin-3 is a survival factor in vivo for early mouse trigeminal neurons. J Neurosci. 1996;16:7661–7669. doi: 10.1523/JNEUROSCI.16-23-07661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Schmidt RE, Yan Q, Snider WD. NGF and NT-3 have differing effects on the growth of dorsal root axons in developing mammalian spinal cord. J Neurosci. 1994;14:5187–5201. doi: 10.1523/JNEUROSCI.14-09-05187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]