Abstract
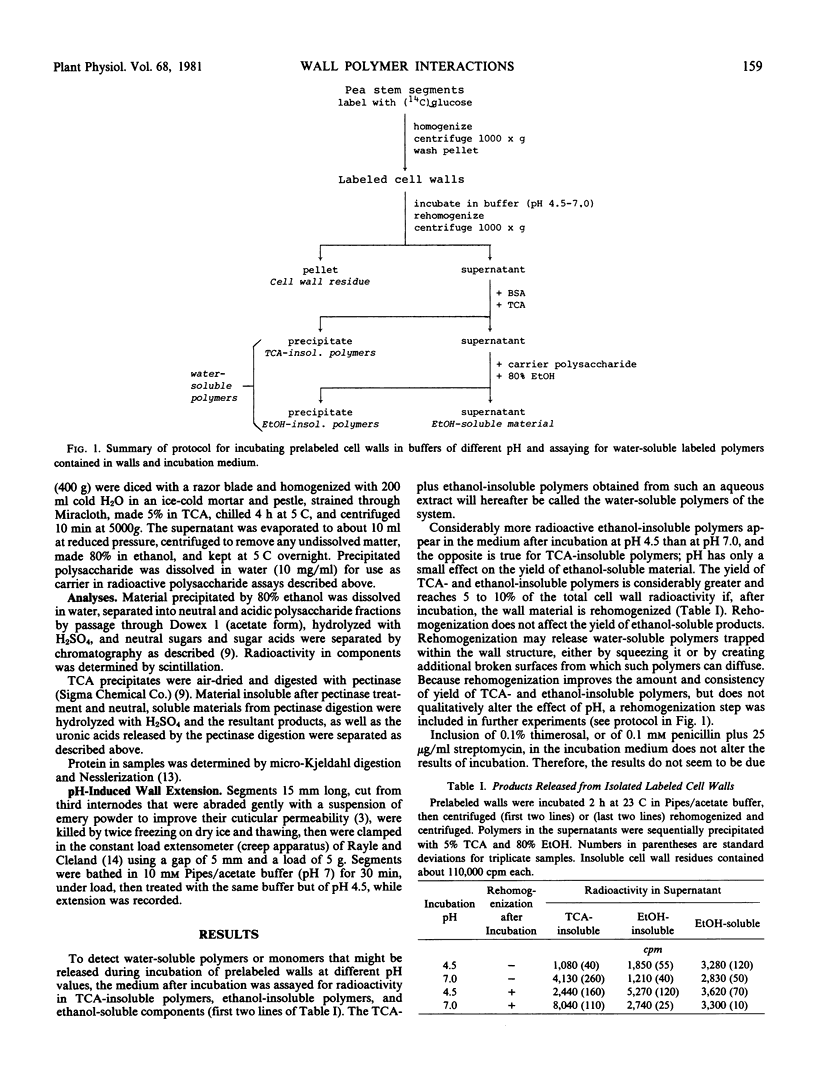
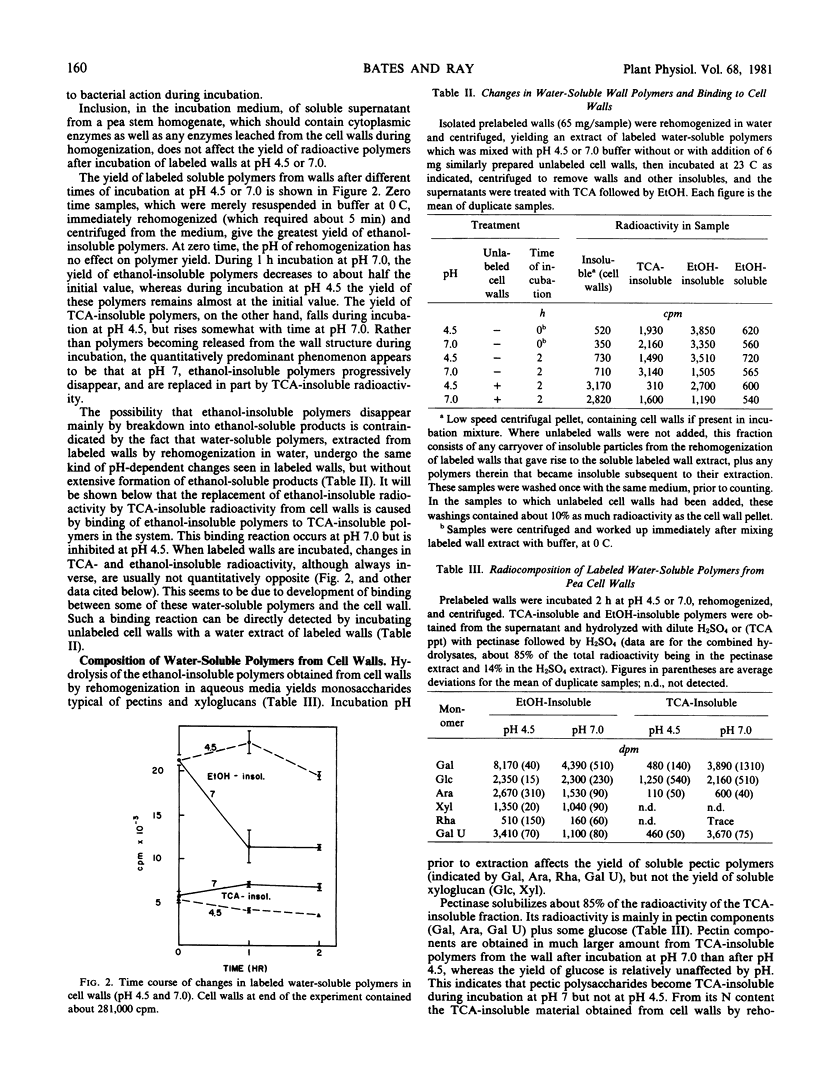
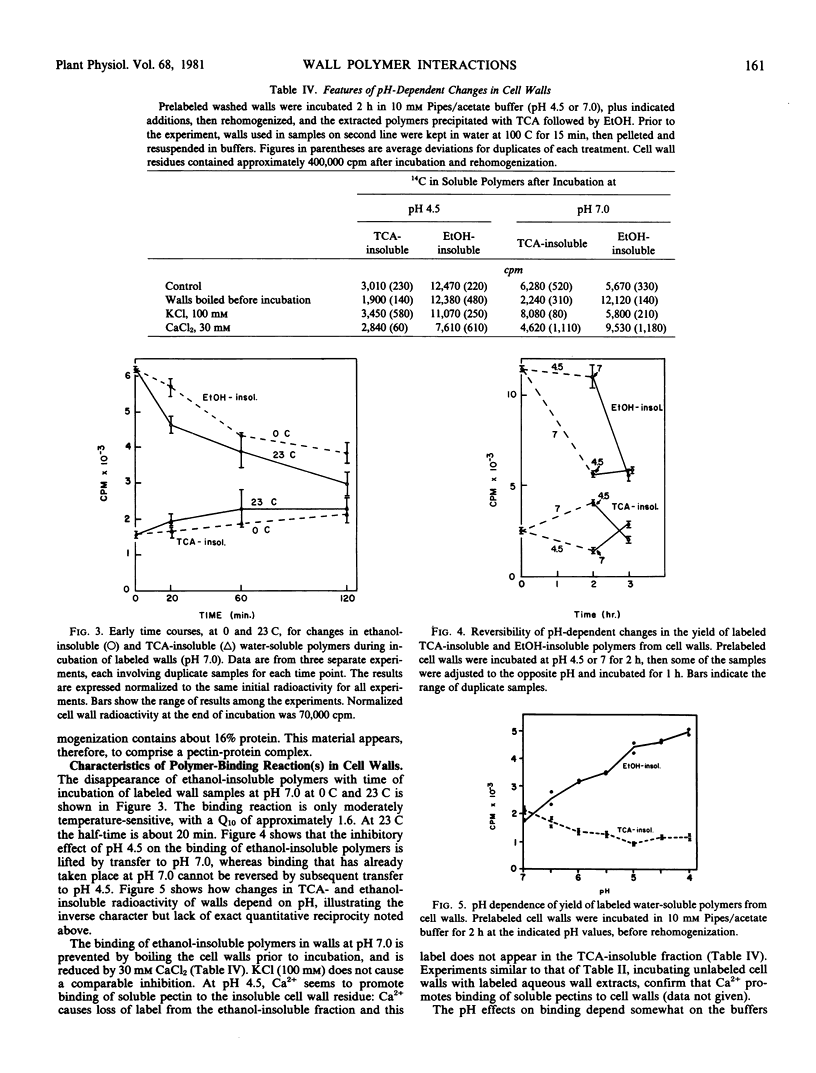
In an effort to detect a pH-dependent release of polymers such as xyloglucans, thought to be involved in auxin-induced cell wall expansion during growth, radioactively labeled cell walls from pea stem tissue were incubated at different pH values, and changes in water-soluble, ethanol- or trichloroacetic acid-insoluble components were determined. This revealed the occurrence, at neutral pH, of a time- and pH-dependent binding of soluble pectin, in the walls, to a heat-labile, presumably protein, wall component, yielding a trichloroacetic acid-insoluble pectin-protein complex. This reaction, which can also be observed between polymers in water extracts of cell walls, is inhibited at low pH and by Ca2+, and appears to be of a physical, possibly lectin-like, nature. Progressive binding of pectin or of the pectin-protein complex to the insoluble wall structure is also observed. These reactions may be involved in wall assembly during its deposition, and may participate in, or be analogous to pH-dependent physical interactions that participate in, wall extension during cell growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland R. E. Hydrogen Ion Entry as a Controlling Factor in the Acid-growth Response of Green Pea Stem Sections. Plant Physiol. 1975 Mar;55(3):547–549. doi: 10.1104/pp.55.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler M. J., Rayle D. L. Auxin Does Not Alter the Permeability of Pea Segments to Tritium-labeled Water. Plant Physiol. 1974 Feb;53(2):229–232. doi: 10.1104/pp.53.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Promotion of Xyloglucan Metabolism by Acid pH. Plant Physiol. 1975 Sep;56(3):373–376. doi: 10.1104/pp.56.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H., Glaser C. Carbohydrate-binding proteins from plant cell walls and their possible involvement in extension growth. FEBS Lett. 1974 Sep 1;45(1):304–307. doi: 10.1016/0014-5793(74)80867-7. [DOI] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Turnover of cell wall polysaccharides in elongating pea stem segments. Plant Physiol. 1974 May;53(5):669–673. doi: 10.1104/pp.53.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J. P., Taiz L. Cell wall extension in Nitella as influenced by acids and ions. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1565–1569. doi: 10.1073/pnas.74.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J. P., Taiz L. Transverse Viscoelastic Extension in Nitella: II. Effects of Acid and Ions. Plant Physiol. 1979 Apr;63(4):657–659. doi: 10.1104/pp.63.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Tepfer M., Cleland R. E. A Comparison of Acid-induced Cell Wall Loosening in Valonia ventricosa and in Oat Coleoptiles. Plant Physiol. 1979 May;63(5):898–902. doi: 10.1104/pp.63.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry M. E., Bonner B. A. An Examination of Centrifugation as a Method of Extracting an Extracellular Solution from Peas, and Its Use for the Study of Indoleacetic Acid-induced Growth. Plant Physiol. 1980 Aug;66(2):321–325. doi: 10.1104/pp.66.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]


