Abstract
Background and Purpose
Since the diagnosis and treatment of carotid artery disease may reduce the rate of stroke, the aim of this study was to determine whether a diet intervention was associated with incident carotid artery disease.
Methods
Participants were 48,835 postmenopausal women aged 50 to 79 years who were randomly assigned to either the intervention or comparison group in the WHI Diet Modification Trial. Incident carotid artery disease was defined as an overnight hospitalization with either symptoms or a surgical intervention to improve flow.
Results
After a mean follow-up of 8.3 years from 1994 – 2005, there were 297 (0.61%) incident carotid artery events. Contrasted to the comparison group, the risk of incident carotid disease did not differ from those assigned to the intervention group (HR: 1.08, 95% CI: 0.9 - 1.4). In secondary analysis, there was no significant effect of the intervention on the risk for incident carotid disease during the five years of post-intervention follow-up from 2005 to 2010 (1.24, 0.9 - 1.7) and no significant effect during cumulative follow-up from 1994 to 2010 (1.13, 0.9 – 1.4).
Conclusions
Among postmenopausal women, a dietary intervention aimed at reducing total fat intake and encouraging increased intake of fruit, vegetables and grains, did not significantly change the risk for incident carotid artery disease.
Keywords: diet, carotid artery disease, trial, women
INTRODUCTION
Carotid revascularization is frequently performed to prevent incident thromboembolic cerebrovascular accidents.Data from the North American Symptomatic Carotid Endarterectomy Trial and Asymptomatic Carotid Atherosclerosis Study indicate a significant reduction in fatal and non-fatal stroke among those undergoing surgical revascularization with significant stenosis of the carotid arteries. 1,2
To clarify the effect of diet changes on several chronic diseases, the Women’s Health Initiative (WHI) Dietary Modification trial (DMT)reported the effect of achieving adherence to a diet low in total fat and higher in fruit, vegetables and grains on the risk for incident CVD, among postmenopausal women between the age of 50 and 79 at baseline.3 After a mean follow-up of 8.1 years, the DMT intervention group did not have a significantly different rate of ischemic or hemorrhagic stroke (HR: 1.02; 95% CI: 0.90-1.15).4
Since a diagnosis of carotid artery disease, to include revascularization, influences the likelihood of future stroke, we conducted a study to test the hypothesis of a significant effect of DMT on the rates of incident carotid artery disease in the WHI cohort. A significant decrease in these rates would suggest that a low fat diet, which included higher intakes of fruits, vegetables and grains, may reduce the need for carotid revascularization and, therefore, provide an impetus for enhanced efforts to recommend such a diet in public health policy.
METHODS
Study population
The design of the WHI DMT has been described previously.3,4 In brief, 48,835 postmenopausal women aged 50–79 years were recruited from 40 sites around the United States from 1993 -1998. During enrollment 40% were randomly assigned to a low-fat (20% total kcal) dietary intervention group while the remaining 60% were allocated to the usual diet comparison group (Figure 1).5 Exclusion criteria for the DMT included type I diabetes mellitus, a history of cancer (except for nonmelanoma skin cancer in the previous 10 years), medical conditions predictive of a survival time of less than 3 years or a high risk of lack of retention or intervention nonadherence.6 Women were also excluded if they 1) reported consumption of less than 600 kcal/d or greater than 5,000 kcal/day; 2) consumed a diet with less than 32% of total energy from fat; or 3) reported consuming greater than or equal to 10 meals/week prepared outside of the home.6
Figure 1.
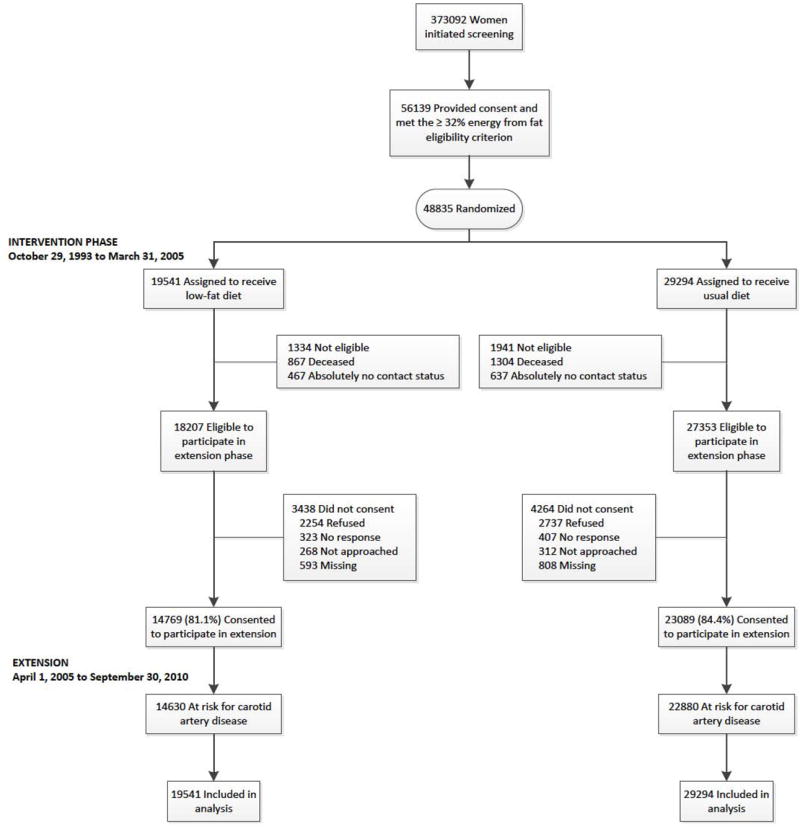
Participant Flow in the Dietary Modification Trial of the Women’s Health Initiative
At baseline, women could also be randomized to the postmenopausal hormone therapy trial. Details about eligibility and treatments for the hormone therapy trial have been published previously.7 After year 1, participants were invited to consider further randomization to Calcium and Vitamin D (CaD) trial where they would be randomly assigned to supplement (500 mg of calcium carbonate and 200 IU of Vitamin D3) or placebo. For the current report, a total of 8,050 women (16.5%) participated in the HT trial and 25,210 (51.6%) were in the CaD trial.
All participants provided written informed consent. The WHI protocol and consent forms were reviewed and approved by the Institutional Review Boards at all participating institutions.
Intervention
The primary goal of DMT intervention was to reduce risk of breast cancer by reducing total fat intake to 20% of total energy. Additional goals included increased vegetable and fruit intakes to greater than or equal to 5 servings per day and increased grain intake to greater than or equal to 6 servings per day.3 There were no additional diet intervention goals specific to cardiovascular risk reduction, nor was weight-loss advocated.
Women in the intervention group participated in an intensive behavioral modification program consisting of 18 group sessions in the first year and quarterly maintenance sessions until the trial ended in 2005.6 Women randomly assigned to the comparison group were given a copy of Nutrition and Your Health: Dietary Guidelines for Americans and asked to maintain their usual diet.8 Dietary intake data for all participants was assessed using the WHI food frequency questionnaire, which was administered at baseline, year 1 and thereafter on a rotating sample of one-third of participants every 3 years.
Data Collection
Demographic and personal characteristics, medication use, anthropometrics and self-reported medical history were collected at baseline.6 Type 2 diabetes mellitus was defined as the self-reported use of antidiabetic pills at any time or the use of injectable insulin. Hypertension was defined as self-report or use of an anti-hypertension medication. Smoking status was coded as former, current or never. Physical activity was calculated by using a standardized classification system9 and based on self reported physical activity data.
Outcome Ascertainment
Semiannually, participants reported emergency room visits, overnight hospitalizations and outpatient coronary revascularization procedures from their first follow-up to close-out in 2005. Medical records for potential reported outcomes were adjudicated by centrally trained physician adjudicators using standard criteria and blinded to randomization assignment.10
Incident carotid artery disease was defined as requiring an overnight hospitalization with either symptoms [relevant to carotid artery disease] or a surgical intervention to improve flow in the carotid arteries. The diagnosis was based on the presence of an overnight hospitalization and one or more of the following three criteria: 1. Symptomatic disease with carotid artery disease listed on the hospital discharge summary; 2. Symptomatic disease with abnormal findings (≥ 50% stenosis) on carotid angiogram, MRA, or Doppler flow study; 3. Vascular or surgical procedure to improve flow to the ipsilateral brain. A diagnosis of incident stroke of any type censored the participant for future diagnoses of carotid artery disease. Individuals with a history of transient ischemic attack resulting in or occurring during overnight hospitalization were included in the analysis, but only the first case of incident carotid artery disease was included.
Statistical methods
The analyses used time-to-event methods based on the intention-to-treat principle and included all randomized DMT participants, regardless of prior history of CVD. Follow-up time was censored at the time of a woman’s last documented follow-up contact, death or incident stroke. Hazard ratios were estimated using Cox proportional hazards models stratified by age, prior history of carotid revascularization, hysterectomy status, HT randomization group and CaD randomization group (time-dependent). Statistical significance by levels of 14 pre-specified characteristics was based on tests of interaction between randomization group and subgroup. Tests of proportionality did not yield any evidence against the assumption of proportional hazards either for the intervention (p=0.30) or combined follow-up (p=0.60) periods.
After the intervention period ended in 2005, follow-up of 83.1% of surviving participants who provided written consent continued through September 30, 2010. A secondary analysis, that combined the intervention and post-intervention periods, was conducted and was similar in design to the primary analysis. All statistical tests are two-sided and nominal p values of 0.05 or less regarded as significant.
RESULTS
Over a mean (SD) of 8.3 (1.8) years of follow-up, there were 297 incident carotid artery disease events among the 48,835 women enrolled in the DMT. Nearly all (277, 93%) were not associated with documented cerebral infarction. Of the 297 cases, 231 (85%) included the criteria of a vascular or surgical procedure to improve flow to the ipsilateral brain, while 129 (47%) included symptomatic disease with abnormal findings (i.e. ≥ 50% stenosis) on carotid angiogram, magnetic resonance angiogram or Doppler flow study, and 102 (37%) included symptomatic disease with carotid artery disease listed on the hospital discharge summary. Eight-five percent of cases had two of these criteria, while 24% had all three criteria.
The baseline characteristics of the participants by DMT arm assignment are presented in Table 1. There were no significant differences between those randomized to the intervention and comparison groups for all of the characteristics except mean systolic blood pressure (SBP).
TABLE 1. BASELINE CHARACTERISTICS OF THE WOMEN’S HEALTH INITIATIVE DIETARY MODIFICATION TRIAL PARTICIPANTS.
| Intervention (N=19541) |
Comparison (N=29294) |
P-Value1 | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age at screening | 62.3 | (6.9) | 62.3 | (6.9) | 0.99 |
| Race/ethnicity | 0.74 | ||||
| White | 15871 | 81.2 | 23891 | 81.6 | |
| Black | 2135 | 10.9 | 3127 | 10.7 | |
| Hispanic | 751 | 3.8 | 1094 | 3.7 | |
| American Indian | 88 | 0.5 | 114 | 0.4 | |
| Asian/Pacific Islander | 431 | 2.2 | 674 | 2.3 | |
| Unknown | 265 | 1.4 | 394 | 1.3 | |
| Body-mass index (kg/m2), baseline | 29.1 | (5.9) | 29.1 | (5.9) | 0.53 |
| Systolic BP (mm Hg), baseline | 127.5 | (17.2) | 127.9 | (17.2) | 0.02 |
| Diastolic BP (mm Hg), baseline | 75.9 | (9.1) | 76.0 | (9.1) | 0.07 |
| Antihypertensive medication use at Baseline | 6036 | 30.9 | 9230 | 31.5 | 0.15 |
| History of high cholesterol requiring pills | 2034 | 11.8 | 3138 | 12.1 | 0.29 |
| Treated diabetes (pills or shots) | 866 | 4.4 | 1337 | 4.6 | 0.49 |
| Pack years of smoking | 9.6 | (17.9) | 9.6 | (18.0) | 0.69 |
| Smoking status | 0.23 | ||||
| Never | 9918 | 51.4 | 15029 | 51.9 | |
| Past | 8121 | 42.1 | 11979 | 41.3 | |
| Current | 1273 | 6.6 | 1977 | 6.8 | |
| Family history of MI | 9722 | 52.5 | 14341 | 51.8 | 0.12 |
| History of carotid endarterectomy/angioplasty | 39 | 0.2 | 55 | 0.2 | 0.77 |
| History of CHD (MI/CABG/PTCA) | 482 | 2.5 | 709 | 2.5 | 0.75 |
| Total energy expenditure/wk from phys act, MET-hours | 10.0 | (11.7) | 10.1 | (12.0) | 0.44 |
| Percent Calories from Fat | 37.8 | (5.1) | 37.8 | (5.0) | 0.91 |
| Education | 0.65 | ||||
| ≤ High school/GED or less | 4267 | 22.0 | 6468 | 22.2 | |
| School after high school | 7712 | 39.7 | 11597 | 39.8 | |
| College degree or higher | 7446 | 38.3 | 11044 | 37.9 | |
| Family income | 0.36 | ||||
| <20K | 2774 | 15.1 | 4303 | 15.6 | |
| 20-<35K | 4501 | 24.4 | 6814 | 24.7 | |
| 35-<50K | 3955 | 21.5 | 5868 | 21.3 | |
| 50-<75K | 3887 | 21.1 | 5663 | 20.5 | |
| ≥75K | 3294 | 17.9 | 4949 | 17.9 | |
| Bilateral oophorectomy | 3884 | 20.3 | 5997 | 20.9 | 0.12 |
| HT trial arm | |||||
| CEE-Alone | 615 | 3.1 | 1039 | 3.5 | 0.412 |
| CEE-Alone Placebo | 670 | 3.4 | 1068 | 3.6 | |
| CEE+MPA | 972 | 5.0 | 1457 | 5.0 | 0.303 |
| CEE+MPA Placebo | 925 | 4.7 | 1304 | 4.5 | |
| Not randomized | 16359 | 83.7 | 24426 | 83.4 | |
| CaD trial arm | |||||
| Active | 4767 | 24.4 | 7827 | 26.7 | 0.184 |
| Placebo | 4878 | 25.0 | 7738 | 26.4 | |
| Not randomized | 9896 | 50.6 | 13729 | 46.9 | |
Based on chi-square test of association for categorical variables and t-test for continuous variables.
P-value corresponds to test of association between DMT group and CEE alone trial group.
P-value corresponds to test of association between DMT group and CEE+MPA trial group.
P-value corresponds to test of association between DMT group and CaD trial group.
Figure 2 displays the Kaplan-Meier plot for the rates of incident carotid artery disease for the intervention and comparison groups. Overall, there was no significant effect of the DMT intervention on the incidence of carotid artery disease (Hazard ratio: 1.08; 95% Confidence Interval: 0.85 – 1.36; p = 0.54).
Figure 2.
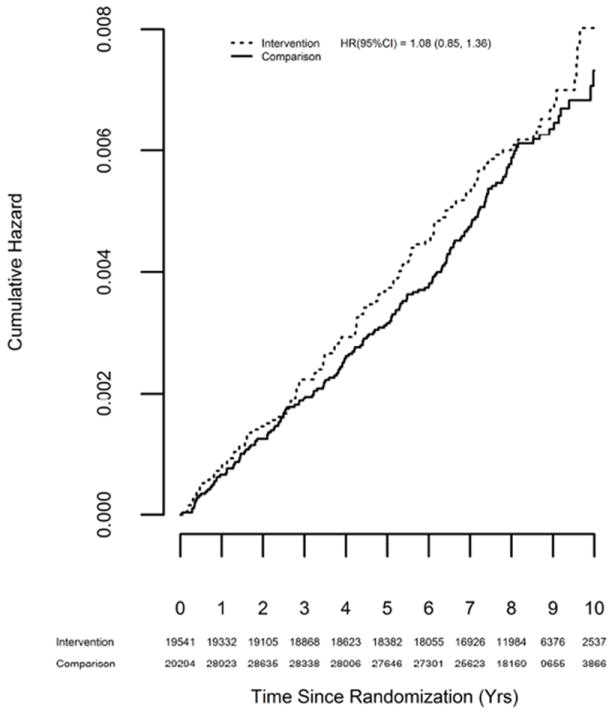
Kaplan-Meier Plot of Incident Carotid Artery Disease by Randomization Group
The effect of the DMT intervention on incident carotid artery disease by selected subgroups is provided in Table 2. There were significant differences by history of carotid revascularization (p=0.004) and hypertension (0.07). Specifically, the hazard ratio for women with a history of carotid revascularization was nearly 14-fold higher than who did not report such a history (HR’s: 13.95 vs. 1.02). The difference in hazard ratios was not as pronounced for a history of hypertension: HR = 1.29 for those who reported a history of HTN vs. HR = 0.76 for those without HTN.
TABLE 2. INCIDENCE OF CAROTID ARTERY DISEASE BY SELECTED SUBGROUPS.
| Subgroup | Intervention | Comparison | HR* | (CI 95%) | P-Value** | ||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
|
| |||||||
| Main Effect | 123 | (0.08) | 174 | (0.07) | 1.08 | (0.85, 1.36) | 0.54 |
| Age (years) | 0.19 | ||||||
| 50 to 59 | 20 | (0.03) | 26 | (0.03) | 1.16 | (0.65, 2.10) | |
| 60 to 69 | 70 | (0.10) | 86 | (0.08) | 1.25 | (0.91, 1.71) | |
| 70 to 79 | 33 | (0.13) | 62 | (0.16) | 0.80 | (0.52, 1.23) | |
| Race/ethnicity | 0.26 | ||||||
| White | 105 | (0.08) | 161 | (0.08) | 0.99 | (0.77, 1.27) | |
| Black | 12 | (0.07) | 9 | (0.04) | 1.68 | (0.69, 4.06) | |
| Other | 6 | (0.05) | 4 | (0.02) | 2.20 | (0.62, 7.82) | |
| BMI | 0.85 | ||||||
| < 25 | 22 | (0.05) | 30 | (0.05) | 1.00 | (0.57, 1.75) | |
| 25 -< 30 | 49 | (0.09) | 64 | (0.07) | 1.18 | (0.81, 1.73) | |
| >= 30 | 52 | (0.09) | 79 | (0.09) | 1.00 | (0.70, 1.42) | |
| CEE alone Trial | 0.80‡ | ||||||
| Active | 8 | (0.16) | 9 | (0.11) | 1.51 | (0.58, 3.93) | |
| Placebo | 5 | (0.09) | 7 | (0.08) | 1.25 | (0.38, 4.10) | |
| No Randomized | 57 | (0.10) | 70 | (0.08) | 1.22 | (0.86, 1.73) | |
| CEE+MPA Trial | 0.38‡ | ||||||
| Active | 3 | (0.04) | 7 | (0.06) | 0.68 | (0.17, 2.75) | |
| Placebo | 7 | (0.09) | 7 | (0.07) | 1.48 | (0.51, 4.26) | |
| Not randomized | 43 | (0.06) | 74 | (0.06) | 0.87 | (0.59, 1.27) | |
| CaD Trial | 0.67‡ | ||||||
| Active | 24 | (0.06) | 37 | (0.06) | 1.05 | (0.63, 1.77) | |
| Placebo | 34 | (0.09) | 47 | (0.07) | 1.22 | (0.78, 1.91) | |
| Not randomized | 65 | (0.08) | 90 | (0.08) | 1.02 | (0.74, 1.40) | |
| % energy from fat at baseline (tertiles) | 0.50 | ||||||
| < 34.8% | 34 | (0.06) | 51 | (0.06) | 1.02 | (0.65, 1.59) | |
| 34.8-<39.4% | 40 | (0.07) | 61 | (0.07) | 0.90 | (0.60, 1.36) | |
| >= 39.4% | 48 | (0.09) | 60 | (0.08) | 1.22 | (0.83, 1.79) | |
| History of Carotid endarterectomy/ angioplasty | 0.004 | ||||||
| No | 115 | (0.07) | 169 | (0.07) | 1.02 | (0.80, 1.29) | |
| Yes | 8 | (3.18) | 5 | (1.29) | 13.95 | (1.63, 119.0) | |
| History of hypertension† | 0.07 | ||||||
| No | 23 | (0.03) | 43 | (0.04) | 0.76 | (0.46, 1.27) | |
| Yes | 96 | (0.14) | 118 | (0.12) | 1.29 | (0.98, 1.69) | |
| Smoking status | 0.38 | ||||||
| Never | 43 | (0.05) | 48 | (0.04) | 1.29 | (0.85, 1.96) | |
| Past | 65 | (0.10) | 97 | (0.10) | 1.02 | (0.74, 1.40) | |
| Current | 13 | (0.13) | 27 | (0.17) | 0.75 | (0.38, 1.46) | |
| Diabetes (treated) | 0.63 | ||||||
| No | 101 | (0.07) | 148 | (0.06) | 1.04 | (0.80, 1.34) | |
| Yes | 22 | (0.34) | 26 | (0.26) | 1.21 | (0.67, 2.17) | |
| History of statin use | |||||||
| No | 104 | (0.07) | 139 | (0.06) | 1.14 | (0.88, 1.48) | 0.23 |
| Yes | 19 | (0.20) | 35 | (0.25) | 0.78 | (0.44, 1.38) | |
| History of CHD (MI/CABG/PTCA) | |||||||
| No | 105 | (0.07) | 150 | (0.06) | 1.06 | (0.82, 1.36) | 0.23 |
| Yes | 16 | (0.46) | 18 | (0.34) | 1.67 | (0.82, 3.40) | |
| History of stroke | 0.33 | ||||||
| No | 120 | (0.08) | 166 | (0.07) | 1.09 | (0.86, 1.38) | |
| Yes | 3 | (0.20) | 8 | (0.33) | 0.47 | (0.09, 2.56) | |
Hazard ratio (95%CI) from a proportional hazards model stratified by age, hysterectomy status, prior history of carotid endarterectomy/ angioplasty, HT trial randomization group, CaD trial randomization group (time-dependent) and subgroup.
Corresponds to a significance test of the main effect or test of interaction or trend.
Self-report of hypertension or high blood pressure at baseline.
Test of interaction does not include those not randomized.
We conducted secondary analyses using incident carotid artery disease data collected from 2005 to 2010. During this period 63 (0.08%) and 79 (0.07%) incident carotid artery disease events occurred in the intervention and comparison groups, respectively, which was not significant (1.24, 0.89 - 1.73). Of note, there was no compelling evidence (p=0.49) of differential risk between the intervention (HR=1.08) and post-intervention periods (1.24). Consequently an analysis combining these time periods was performed and revealed that the DMT intervention was not associated with a significant effect on incident carotid artery disease (1.13, 0.93 – 1.36, p = 0.22). As before, a history of carotid revascularization and hypertension were associated with differential risks of incident carotid artery disease for the combined time period (Table 3).
TABLE 3. INCIDENCE OF CAROTID ARTERY DISEASE BY SELECTED SUBGROUPS‡.
| Intervention | Comparison | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Subgroup | N | % | N | % | HR* | (CI 95%) | P-Value** |
|
| |||||||
| Main Effect | 186 | (0.08) | 253 | (0.07) | 1.13 | (0.93, 1.36) | 0.22 |
| Age (years) | 0.20 | ||||||
| 50 to 59 | 36 | (0.04) | 46 | (0.03) | 1.24 | (0.80, 1.93) | |
| 60 to 69 | 105 | (0.10) | 130 | (0.08) | 1.23 | (0.95, 1.60) | |
| 70 to 79 | 45 | (0.13) | 77 | (0.14) | 0.88 | (0.61, 1.28) | |
| Race/ethnicity | 0.67 | ||||||
| White | 164 | (0.08) | 231 | (0.08) | 1.08 | (0.89, 1.33) | |
| Black | 15 | (0.06) | 16 | (0.04) | 1.22 | (0.59, 2.51) | |
| Other | 7 | (0.04) | 6 | (0.02) | 1.75 | (0.59, 5.22) | |
| BMI | 0.39 | ||||||
| < 25 | 36 | (0.06) | 43 | (0.04) | 1.20 | (0.77, 1.88) | |
| 25 -< 30 | 73 | (0.09) | 91 | (0.07) | 1.25 | (0.91, 1.71) | |
| >= 30 | 77 | (0.09) | 118 | (0.09) | 1.00 | (0.75, 1.34) | |
| CEE alone Trial | 0.92 | ||||||
| Active | 10 | (0.14) | 14 | (0.12) | 1.09 | (0.47, 2.52) | |
| Placebo | 9 | (0.11) | 14 | (0.11) | 1.02 | (0.44, 2.41) | |
| No Randomized | 80 | (0.09) | 93 | (0.07) | 1.31 | (0.97, 1.77) | |
| CEE+MPA Trial | 0.40 | ||||||
| Active | 6 | (0.05) | 10 | (0.06) | 0.97 | (0.34, 2.73) | |
| Placebo | 13 | (0.12) | 11 | (0.07) | 1.71 | (0.76, 3.84) | |
| Not randomized | 68 | (0.06) | 111 | (0.07) | 0.94 | (0.69, 1.27) | |
| CaD Trial | 0.85 | ||||||
| Active | 43 | (0.07) | 62 | (0.06) | 1.13 | (0.76, 1.68) | |
| Placebo | 52 | (0.09) | 72 | (0.07) | 1.19 | (0.83, 1.71) | |
| Not randomized | 91 | (0.08) | 119 | (0.07) | 1.09 | (0.83, 1.43) | |
| % energy from fat at baseline (tertiles) | 0.73 | ||||||
| < 34.8% | 57 | (0.07) | 73 | (0.06) | 1.18 | (0.83, 1.68) | |
| 34.8-<39.4% | 64 | (0.08) | 87 | (0.07) | 1.06 | (0.77, 1.48) | |
| >= 39.4% | 64 | (0.08) | 91 | (0.08) | 1.08 | (0.78, 1.49) | |
| History of Carotid endarterectomy/ angioplasty | 0.005 | ||||||
| No | 177 | (0.08) | 247 | (0.07) | 1.09 | (0.90, 1.32) | |
| Yes | 9 | (2.55) | 6 | (1.18) | 13.95 | (1.63, 119.0) | |
| History of hypertension† | 0.07 | ||||||
| No | 42 | (0.04) | 71 | (0.04) | 0.87 | (0.59, 1.28) | |
| Yes | 134 | (0.14) | 162 | (0.11) | 1.31 | (1.04, 1.65) | |
| Smoking status | 0.69 | ||||||
| Never | 64 | (0.05) | 74 | (0.04) | 1.26 | (0.90, 1.77) | |
| Past | 97 | (0.10) | 137 | (0.09) | 1.07 | (0.83, 1.40) | |
| Current | 23 | (0.16) | 39 | (0.17) | 1.01 | (0.59, 1.70) | |
| Diabetes (treated) | 0.46 | ||||||
| No | 162 | (0.07) | 216 | (0.06) | 1.15 | (0.94, 1.41) | |
| Yes | 24 | (0.27) | 37 | (0.26) | 0.93 | (0.55, 1.58) | |
| History of statin use | 0.52 | ||||||
| No | 156 | (0.07) | 207 | (0.06) | 1.16 | (0.94, 1.43) | |
| Yes | 30 | (0.22) | 46 | (0.22) | 0.98 | (0.60, 1.57) | |
| History of CHD (MI/CABG/PTCA) | 0.10 | ||||||
| No | 159 | (0.07) | 223 | (0.06) | 1.10 | (0.89, 1.35) | |
| Yes | 24 | (0.52) | 24 | (0.33) | 1.89 | (1.02, 3.49) | |
| History of stroke | 0.61 | ||||||
| No | 181 | (0.08) | 244 | (0.07) | 1.13 | (0.93, 1.37) | |
| Yes | 5 | (0.24) | 9 | (0.26) | 0.77 | (0.18, 3.34) | |
Hazard ratio (95%CI) from a proportional hazards model stratified by age, hysterectomy status, prior history of carotid endarterectomy/ angioplasty, HT trial randomization group, CaD trial randomization group (time-dependent) and subgroup.
Corresponds to a significance test of the main effect or test of interaction or trend.
Self-report of hypertension or high blood pressure at baseline.
Includes intervention (1995 – 2005) and postintervention (2005 – 20100 periods).
We also examined whether the effect of the intervention varied by the participants’ dietary habits achieved with the intervention and for the combined follow-up time. In this analysis, there were significant interactions between DMT assignment status and quartile of total energy intake (p = 0.01), fruit and vegetable intake (p = 0.007) and fiber intake (p = 0.05) (Table 4). As shown, the associations between total energy, fruit and vegetable and fiber intakes, and the DMT diet for incident carotid artery disease were strongest in the lowest quartile and somewhat U-shaped. However, when the models for fruit and vegetable, as well as fiber intake, were adjusted for energy intake, these associations were no longer significant.
TABLE 4. INCIDENCE OF CAROTID ARTERY DISEASE BY QUARTILE OF DIETARY NUTRIENT/FOOD GROUP INTAKE.
| Dietary Variable | Quartile | Comparison Group* | DMT Intervention Group | Hazard Ratio | 95% CI | p-value† |
|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||
| Total Energy Intake (kcal/day) | <Q1 | 253 (0.07%) | 56 (0.11%) | 1.77 | 1.3 - 2.4 | 0.01 |
| Q1 - < Q2 | 39 (0.07%) | 1.00 | 0.7 - 1.4 | |||
| Q2 - < Q3 | 37 (0.07%) | 0.95 | 0.7- 1.4 | |||
| >= Q4 | 35 (0.08%) | 1.13 | 0.8 - 1.7 | |||
| Q1 = 1155.52, Q2 = 1458.56, Q3 = 1802.93 (kcal/day) | ||||||
| Fruit and Vegetable Intake (servings/day) | <Q1 | 253 (0.07%) | 56 (0.12%) | 1.73 | 1.3 - 2.4 | 0.07 |
| Q1 - < Q2 | 32 (0.06%) | 0.84 | 0.8 - 1.2 | |||
| Q2 - < Q3 | 39 (0.07%) | 0.99 | 0.7 - 1.4 | |||
| >= Q4 | 40 (0.08%) | 1.34 | 0.9 - 1.9 | |||
| Q1 = 3.4, Q2 = 4.9, Q3 = 6.5 (medium servings/day) | ||||||
| Fiber Intake (g/d) | <Q1 | 253 (0.07%) | 55 (0.11%) | 1.57 | 1.2 - 2.2 | 0.05 |
| Q1 - < Q2 | 39 (0.07%) | 1.03 | 0.7 - 1.5 | |||
| Q2 - < Q3 | 34 (0.06%) | 0.89 | 0.6 - 1.3 | |||
| >= Q4 | 39 (0.08%) | 1.38 | 1.0 - 2.0 | |||
| Q1 = 13.0, Q2 = 17.5, Q3 = 22.7 (grams/day) | ||||||
| Percent Energy from Fat (%) | <Q1 | 253 (0.07%) | 17 (0.05%) | 0.92 | 0.5 - 1.5 | 0.72 |
| Q1 - < Q2 | 38 (0.08%) | 1.29 | 0.9 - 1.9 | |||
| Q2 - < Q3 | 49 (0.08%) | 1.23 | 0.9 - 1.7 | |||
| >= Q4 | 63 (0.10%) | 1.23 | 0.9 - 1.7 | |||
| Q1 = 18.8, Q2 = 23.2, Q3 = 28.7 (%) | ||||||
| Polyunsaturated to Saturated Fat Intake | <Q1 | 253 (0.07%) | 43 (0.10%) | 1.40 | 1.0 - 2.0 | 0.44 |
| Q1 - < Q2 | 40 (0.07%) | 1.02 | 0.7 - 1.5 | |||
| Q2 - < Q3 | 52 (0.09%) | 1.31 | 1.0 - 1.8 | |||
| >= Q4 | 32 (0.07%) | 1.06 | 0.7 - 1.6 | |||
| Q1 = 0.54, Q2 = 0.65, Q3 = 0.79 | ||||||
| Percent Energy from Saturated Fat (%) | <Q1 | 253 (0.07%) | 18 (0.05%) | 0.95 | 0.6 - 1.6 | 0.68 |
| Q1 - < Q2 | 40 (0.08%) | 1.34 | 1.0 - 1.9 | |||
| Q2 - < Q3 | 47 (0.08%) | 1.15 | 0.8 - 1.6 | |||
| >= Q4 | 62 (0.10%) | 1.24 | 0.9 - 1.7 | |||
| Q1 = 6.1, Q2 = 7.6, Q3 = 9.6 (%) | ||||||
| Percent Energy from Polyunsaturated Fat (%) | <Q1 | 253 (0.07%) | 15 (0.04%) | 0.80 | 0.5 - 1.4 | 0.23 |
| Q1 - < Q2 | 44 (0.09%) | 1.47 | 1.1 - 2.1 | |||
| Q2 - < Q3 | 53 (0.08%) | 1.24 | 0.9 - 1.7 | |||
| >= Q4 | 55 (0.09%) | 1.14 | 0.8 - 1.6 | |||
| Q1 = 3.9, Q2 = 4.9, Q3 = 6.1 (%) |
Comparison is quartile of intervention group to the mean of the entire comparison group;
p-value for interaction
To determine whether the DMT intervention may have had differential effects on biomarkers relevant to incident carotid artery disease, we conducted analyses on a subset (5.8%) of randomly selected participants (n = 2815) that examined changes in these biomarkers by DMT randomization group at years 1, 3 and 6 of follow-up. Contrasted with the comparison group, factor VIIC levels were 2.7% lower in the DMT intervention group at year 1 and remained at a similar difference for the duration of the trial (p = 0.006). Likewise, LDL and HDL cholesterol levels were 2.4 and 1.1 mg/dL lower in the DMT intervention group at one year of follow-up (p < 0.01 for both). However, while the difference in LDL cholesterol levels persisted to the end of the trial period, that for HDL cholesterol diminished annually with no appreciable difference by year 6. For triglycerides and serum glucose, the direction of the effect at year 1 had reversed by year 6. There were no significant persistent differences for fibrinogen or Lp(a).
DISCUSSION
While the focus of the DMT was not on a diet intervention shown to reduce cardiovascular risk, it was anticipated that adherence to the low fat diet would have cardiovascular benefits.4 However, the results of our study showed no significant differences in the rate of incident carotid artery disease among the intervention and comparison groups. Moreover, assignment to the DMT intervention was suggestive of an unfavorable effect among those with a baseline history of CHD or hypertension and was substantially higher for those with both conditions. That is, among women who were both hypertensive and had a history of CHD, the DMT intervention resulted in more than a doubling of the risk for incident carotid disease (HR: 2.52, 95% CI: 1.26 - 5.04).
There are several potential reasons for this lack of effect. First, the DMT intervention was not designed to reduce risk of cardiovascular disease, nor was it a weight loss diet. Indeed, this intervention had mixed and modest effects on metabolic characteristics. Second, although women in the DMT intervention lost 2.2 kg more than the comparison group in the first year of the trial and maintained lower weight during the follow-up period,5 the difference in body fat was quite modest (<1%)11 and, by year 6, the mean percentage body fat increased in both groups. Finally, the DMT intervention achieved only 70% of the targeted reduction in total fat intake needed to obtain the effect designed in the trial.12 Taken together, the overall beneficial effects of the DMT intervention could be considered inadequate for changing the trajectory among those who may have already had significant carotid atherosclerosis.
Findings from this study are not entirely inconsistent with previous studies on the effect of diet on morbidity associated with atherosclerotic carotid artery disease. For instance, among men enrolled in the Framingham Heart study, the risk of incident ischemic stroke decreased across increasing quintiles of total, saturated and monounsaturated fats, but not polyunsaturated fat.13 Additionally, a recent comprehensive review found that adherence to the DASH, Mediterranean or “prudent” diet patterns was associated with a reduced risk of stroke, while a diet low in total fat was not found to have a protective effect.14 The finding of a protective effect from the Mediterranean diet may be particularly relevant to our findings since this diet does not recommend a substantial reduction in total fat intake.15 Indeed, the WHI DMT has reported a trend toward reducing incident CVD among those who reached the lowest intakes of either saturated or trans fat.4 Of note, the effect of carotid revascularization is likely only to affect ischemic stroke rates. As such, comparison to studies that used total (ischemic and hemorrhagic) stroke as the outcome are likely to be biased and probably not judicious.
On the other hand, the findings are somewhat disparate from many studies on the association between dietary factors and the extent of carotid atherosclerosis. In this regard, data from the Women’s Healthy Lifestyle Project showed that a lifestyle intervention that aimed to reduce dietary fat and caloric intake resulted in a significantly slower annual rate of progression of carotid intimal medial thickness (IMT) compared to controls. 16 Other trial and epidemiologic data support favorable associations of a higher fiber intake and a lower intake of total cholesterol on carotid atherosclerosis, as measured by IMT.17 Conversely, a dietary intervention, randomized controlled trial that resulted in significant weight loss and a greater decrease in systolic blood pressure led to a regression of carotid atherosclerosis.18 Moreover, there are studies that suggest an inverse association between specific fatty acids and carotid IMT19, which is consistent with the hypothesis of a protective effect of certain types dietary fat and a lower rate of stroke.20 More studies are needed to clarify the potential disparities between these findings.
An important gap in the literature is the effect of a dietary intervention on incident carotid artery disease, sans incident stroke or measures of subclinical carotid atherosclerosis. Indeed, while there have been studies on the effects of pharmacologic interventions on the risk for incident carotid revascularization21, a thorough review of the dietary literature did not provide any reports on this topic.
Strengths of this study include a very large sample size in a well-characterized cohort that was followed for an extended period of time after a randomized intervention. On the other hand, the women enrolled in the WHI were all post-menopausal and relatively healthy. The latter may have resulted in a smaller number of incident carotid artery disease cases than would be expected in other populations. As such, inferences to populations dissimilar to the WHI should be made with caution. Also, in addition to those who were asymptomatic and underwent carotid artery revascularization, the outcome definition may have included some individuals with transient ischemic attack who were hospitalized overnight and that may or may not have been accompanied by a revascularization procedure. As such, this definition is somewhat heterogeneous and likely different from other studies that have examined revascularizations only.
CONCLUSION
The results of our study are similar to those obtained for the WHI DMT intervention on incident cardiovascular disease, including stroke. As such, the findings of the current analysis indicate that the effect of the DMT intervention on rates of carotid artery revascularization is not a likely explanation for the null effect of the DMT intervention on incident stroke.
Acknowledgments
None
SOURCES OF FUNDING
The WHI program is funded by the National Institutes of Health through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
DISCLOSURES
The authors report no conflicts of interest for this manuscript.
References
- 1.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA: The Journal of the American Medical Association. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 2.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 3.Ritenbaugh C, Patterson RE, Chlebowski RT, Caan B, Fels-Tinker L, Howard B, et al. The Women’s Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S87–97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 4.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA: The Journal of the American Medical Association. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 5.Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, et al. Low-fat dietary pattern and weight change over 7 years: the Women’s Health Initiative Dietary Modification Trial. JAMA: The Journal of the American Medical Association. 2006;295:39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 6.Women’s Health Initiative Investigators. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Controlled clinical trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 7.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services. Dietary guidelines for Americans. 4. Washington, DC: USDA; 1995. [Google Scholar]
- 9.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 10.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–8. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 11.Carty CL, Kooperberg C, Neuhouser ML, Tinker L, Howard B, Wactawski-Wende J, et al. Low-fat dietary pattern and change in body-composition traits in the Women’s Health Initiative Dietary Modification Trial. American Journal of Clinical Nutrition. 2011;93:516–524. doi: 10.3945/ajcn.110.006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beresford SAA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA: The Journal of the American Medical Association. 2006;295:643–654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 13.Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA. Inverse association of dietary fat with development of ischemic stroke in men. JAMA: The Journal of the American Medical Association. 1997;278:2145–2150. [PubMed] [Google Scholar]
- 14.Sherzai A, Heim LT, Boothby C, Sherzai AD. Stroke, food groups, and dietary patterns: a systematic review. Nutr Rev. 2012;70:423–435. doi: 10.1111/j.1753-4887.2012.00490.x. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 16.Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. JAC. 2004;44:579–585. doi: 10.1016/j.jacc.2004.03.078. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Dwyer KM, Fan Z, Shircore A, Fan J, Dwyer JH. Dietary fiber and progression of atherosclerosis: the Los Angeles Atherosclerosis Study. Am J Clin Nutr. 2003;78:1085–1091. doi: 10.1093/ajcn/78.6.1085. [DOI] [PubMed] [Google Scholar]
- 18.Shai I, Spence JD, Schwarzfuchs D, Henkin Y, Parraga G, Rudich A, et al. Dietary intervention to reverse carotid atherosclerosis. Circulation. 2010;121:1200–1208. doi: 10.1161/CIRCULATIONAHA.109.879254. [DOI] [PubMed] [Google Scholar]
- 19.Hino A, Adachi H, Toyomasu K, Yoshida N, Enomoto M, Hiratsuka A, et al. Very long chain N-3 fatty acids intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis. 2004;176:145–149. doi: 10.1016/j.atherosclerosis.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, et al. Atorvastatin Reduces the Risk of Cardiovascular Events in Patients With Carotid Atherosclerosis: A Secondary Analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Trial. Stroke. 2008;39:3297–3302. doi: 10.1161/STROKEAHA.108.516450. [DOI] [PubMed] [Google Scholar]


