Abstract
We present a novel design for an endoscopic imaging catheter utilizing diffractive optics for ultrahigh resolution optical coherence tomography (OCT) imaging at 800 nm. A diffractive microlens was developed to alleviate severe chromatic aberration when a broadband light source was employed at the 800 nm wavelength range. Combined with a home-built fiber rotary joint and a broadband Ti:sapphire laser, the imaging catheter achieved a lateral resolution of 6.2 μm and an axial resolution of 3.0 μm in air. The performance of the catheter was demonstrated by three-dimensional-fullcircumferential endoscopic OCT imaging of guinea pig esophagus in vivo.
Optical coherence tomography (OCT) is a noninvasive imaging technology capable of assessing tissue micro-anatomy with micrometer-scale resolution and a few millimeters of imaging depth. Miniature imaging catheters/endoscopes are a critical component of OCT technology for enabling translational imaging of internal luminal organs, such as the gastrointestinal tract or airways. Most OCT catheters/endoscopes that have been developed so far were designed for working at the wave-length around 1300 nm, which provides an excellent axial resolution of about 7-20 μm and an imaging depth of about 2-3 mm [1-7]. A higher resolution is highly desired in order to resolve fine tissue structures such as airway smooth muscle, intestinal crypts, and structural changes associated with early stage diseases. Considering the quadratic dependence of the axial resolution on the center wavelength λc of the OCT light source (i.e., δz ∝ /Δλ, where Δλ is the 3 dB bandwidth of the source spectrum), it would be more convenient to achieve an ultrahigh axial resolution with a broadband light source at 800 nm compared to 1300 nm. In addition, an 800 nm source could also potentially provide improved image contrast owing to the increased light scattering and lower tissue absorption at 800 nm. Ultrahigh-resolution OCT imaging at 800 nm with excellent contrast has been demonstrated with a bench-top system [8]. However, ultrahigh-resolution endoscopic OCT imaging at 800 nm remains challenging due to the difficulties in managing chromatic aberration and polarization over a broadband spectrum, etc., and so far only a few achromatic endoscopic OCT setups have been reported [5,9]. The designs in those endoscopes are rather complicated and expensive, involving multielement achromatic microlenses. Besides, none of the endoscopic setups has the capability of performing high-speed three-dimensional (3D) circumferential imaging over a large volume.
In this Letter, we report a novel design for an imaging catheter that enables real-time ultrahigh-resolution circumferential spectral-domain OCT imaging to form a 3D volumetric dataset. The imaging catheter, hereby named “diffractive catheter,” includes a diffractive lens for chromatic aberration management. Different from those reported in [5,9], the diffractive lens can be readily applied to a conventional imaging catheter that uses a gradient-index (GRIN) lens [3,10], with almost no compromise to the catheter from factors such as diameter, weight, and rigid length.
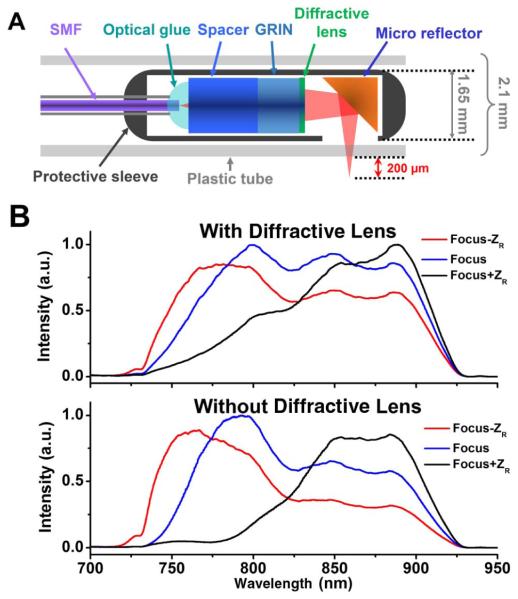
Figure 1.A illustrates the configuration of the diffractive catheter, which consists of a single-mode fiber (SMF), a 1 mm diameter glass rod, a 1 mm diameter GRIN lens, and a diffractive lens of ~1 mm diameter. The combination of a glass rod and a GRIN lens with the proper length and pitch number allowed for maximal use of the limited numerical aperture (NA) of the 1 mm optics, and enabled the beam to be focused to a designed focal point (1.9 mm in our case) with the highest lateral resolution (6.2 μm in our case) [10]. Due to the optical properties of the glass rod and GRIN lens, a significant amount of chromatic aberration is introduced into the imaging system (with a greater than 70 μm longitudinal focal shift for a wavelength range of 750-950 nm according to ray-tracing simulation). The diffractive lens was directly applied after the GRIN lens to alleviate the above-mentioned chromatic aberration over the broad spectrum. The diffractive lens had a very weak focusing power, causing only a small overall focal shift around 200 μm, which has been taken into account during the catheter design. At the distal end of the catheter, a 45° microreflector was attached to the end of the catheter to divert the focused beam 90° for side-view imaging. The total length of the diffractive catheter was measured to be ~1 m.
Fig. 1.
A, schematic of an ultrahigh-resolution OCT imaging catheter utilizing a diffractive lens to alleviate chromatic aberration. B, reflected spectra of the imaging catheters with a diffractive lens (upper panel) and without a diffractive lens (lower panel) when the mirror position was moved from the focal point by 1 Rayleigh range ZR by an optical spectrum analyzer.
To show the effect of the diffractive lens on compensating chromatic aberration, the spectrum reflected by a mirror at the focal point of the catheter was compared with those reflected at one Rayleigh length ZR away from the focal point, as shown in the top panel of Fig. 1B. As a comparison, the reflected spectra of a conventional imaging catheter with the same design but without a diffractive lens were also measured, as shown in the lower panel of Fig. 1.B. The results clearly demonstrated the reduction of chromatic aberration in the catheter with the aid of the diffractive lens.
To achieve 3D circumferential scanning, a fiber rotary joint is required to couple the source light to the rotating diffractive catheter (and the backreflected light detected by the catheter to the OCT interferometer) through a stationary SMF. Most commercially available fiber rotary joints involve a pair of lenses for coupling light between a stationary and rotating SMF and are generally designed for the 1310/1550 nm spectral range. At the spectral range of 800 nm the chromatic aberration of optical materials is much larger and the mode field diameter of an SMF is smaller. As a result, a standard rotary joint becomes very challenging to use for coupling light between a stationary and a rotating SMF. Here, a mechanical coupling scheme similar to the one reported in [11] was employed. In brief, a 126 μm capillary tube filled with an index-matching liquid was used to connect a stationary fiber and the rotating catheter with the catheter rotated by a DC motor through a timing belt. The whole rotary unit was then mounted on a translational stage to enable 3D circumferential imaging. The measured throughput of the tubular rotary joint was greater than 90% and the fluctuation of the double-pass coupling efficiency during 360° rotation was less than 6% at a rotation speed of 10 revolutions per second.
Figure 2 illustrates the endoscopic spectral-domain OCT system at 800 nm, which integrates the diffractive catheter with the home-built tubular fiber-optic rotary joint in the sample arm. A broadband Ti:sapphire laser with a central wavelength of 825 nm and a full width at half-maximum of ~150 nm was used as the light source, which is highly linearly polarized and significantly eases the polarization mode dispersion management in such an endoscopic OCT system. One polarizer (indicated by PL in Fig. 2) was used to control the total power sending to the interferometer, and the output power from the catheter tip was kept at about 4 mW. To match the dispersion between the sample and reference arms, a prism pair made of SF11 flint glass was inserted in the reference arm. The flint glass provides a much higher group velocity dispersion and third-order dispersion (TOD) than crown glass (e.g., BK7) and optical fiber around 800 nm, and therefore more effectively minimizes the dispersion mismatch between the two arms while keeping a considerably large air gap in the reference arm. To further optimize the axial resolution, the spectra returned from both arms (measured by the line scan CCD in the OCT system) were tuned separately to achieve a near-Gaussian shape while maintaining maximum spectral overlap between the two arms. The axial resolution of the diffractive-catheter-based endoscopic OCT system was able to reach as high as 3.0 μm in air, as shown in the inset of Fig. 2. This was slightly worse than what was afforded by the laser source due to the suboptimal spectral throughput bandwidth of the fiber coupler and the collimators in the OCT system. It is noted that the residual TOD mismatch between the two arms was almost negligible and can be further numerically compensated to slightly improve the sidelobe of the point spread function (see inset of Fig. 2).
Fig. 2.
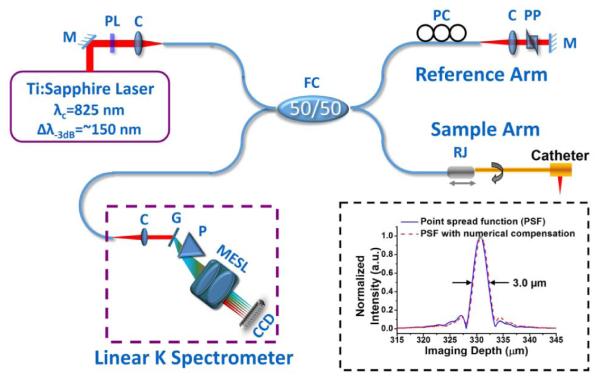
Schematic of the ultrahigh resolution spectral domain endoscopic OCT imaging system and the measured point spread function (inset). C, multielement achromatic collimator; CCD, line scan CCD; G, grating; M, mirror; MESL, multielement scan lens; P, linear K mapping prism; PC, polarization controller; PL, polarizer; PP, prism pair; R, fiber optic rotary joint.
For detection, a custom-designed, home-built broadband linear-in-wavenumber spectrometer was employed, which in principle is similar to the one reported in [12] but covers a much broader spectrum. A 1200 lines/mm grating was used in the spectrometer to disperse the detected light. A prism was inserted after the grating to achieve a linear wavenumber distribution over a broad spectrum (750-950 nm). A cusrom-designed multielement scan lens was used to focus the linearly dispersed light onto a line scan CCD camera. The CCD has 2048 pixels that cover a 250 nm wavelength range with a line scan rate of up to 70 k/s at a 12 bit resolution. The imaging depth was calibrated to be 1.23 mm corresponding to a measured 16 dB fall-off using a form of 20 log[A(z)], where A(z) is the intensity of the Fourier transformed interference signal at different depth z. The measured detection sensitivity of the endoscopic OCT system was around −105 dB when the incident power on the sample was about 4 mW. OCT images were acquired, processed, displayed, and stored in real time with each frame consisting of 2048 × 2048 (lateral × axial) pixels.
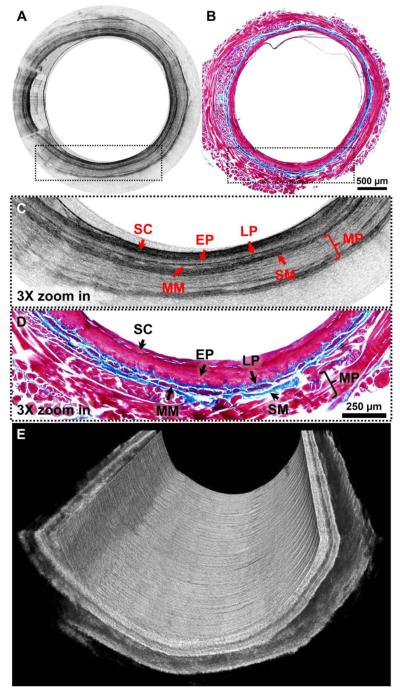
The performance of the ultrahigh-resolution diffractive imaging catheter was tested for 3D imaging of guinea pig esophagus in vivo under an imaging prorocol approved by the Animal Care and Use Committee at the Johns Hopkins University. For in vivo imaging, the A-scan rate of the line CCD was reduced to either 10or 20 kHz in order to match the rotation speed of the tubular fiber-optic rotary joint at 5 or 10 revolutions per second. A representative snapshot of 2D circumferential OCT images of a guinea pig esophagus in vivo is displayed in Fig. 3A, while the corresponding Masson's trichrome stained hisrology is shown in Fig. 3B. To better visualize the fine structures on the OCT images and correlate those with hisrology, a small region in the OCT image and the hisrology micrograph, as indicated by the dotted boxes in Figs. 3A and 3B are zoomed in by 3 times, as shown in Figs. 3C and 3D respectively. Good correlation between OCT images and corresponding hisrology is evident on these zoomed-in images where all the layered esophageal structures, such as stratum comeum, epithelium, lamina propria, muscularis mucosae,submucosa, and muscularis propria, can be clearly identified. It is noticed that a thick layer of stratum corneum on the tissue surface provides strong scattering on the OCT images due to its high keratin content,and it can be clearly differentiated from the stratified epithelium layer below, which exhibits less scattering. More interestingly, lamina propria and submucosa that contain abundant collagen fibers show very strong scattering signals on the zoomed-in OCT image (Fig. 3C), which is confirmed on the corresponding hisrology (Fig. 3D). Fine structures, such as a thin layer of muscularis mucosae that is embedded between lamina propria and submucosa, can also be easily identified on the OCT image. Such structures are very hard to differentiate on 1310 nm OCT images. Figure 3E shows a cutaway view of a 3D image reconstructed from a series of 2D images along the longitudinal axis of the esophagus with a pitch of 10 μm.
Fig. 3.
A, representative 2D circwnferential in vivo OCT im age of guinea pig esophagus and, B, its corresponding histology micrograph. C and D show 3 × zoomed-in regions of the dotted boxes inA and B, respectively. E is a cutaway view of a 3D OCT image of guinea pig esophagus. SC, stratum comeum; EP, epithelium; LP, lamina propria; MM, muscularis mucosae; SM, submucosa; MP, muscularis propria. Scale bars: 500 μm in A and B; 250 μm in Cand D.
In summary, we demonstrated a novel diffractive OCT imaging catheter that utilized a miniature diffractive lens to correct chromatic aberration for enabling ultrahigh-resolution spectral-domain endoscopic OCT imaging at 800 nm. The use of a diffractive lens makes the catheter design compact and cost-effective. Combined with a home-built broadband fiber-optic rotary joint, real-time circumferential OCT imaging became possible with the diffractive catheter. In vivo animal experiments demonstrated promising results in ultrahigh-resolution intraluminal imaging with such a catheter. The diffractive lens used in this Letter was originally designed for longer wavelengths, so it only alleviated the chromatic aberration instead of removing it Further optimization of the diffractive lens would accomplish better imaging quality. Moreover, the rotation speed of the home-built fiberoptic rotary joint is currently the bottleneck of the imaging speed. A faster rotation mechanism could unleash the potential of the high-speed endoscopic spectral-domain OCT system at 800 nm.
Acknowledgments
The authors would like to thank Jessica Mavadia for her technical assistance with the spectral-domain OCT system and helpful discussions. This research was supported in part by the National Institutes of Health under grants EB007636 and CA153023, and the Hartwell Foundation (XDL).
Footnotes
OCIS codes: (090.1970) Diffractive optics; (110.4500) Optical coherence tomography; (170.2150) Endoscopic imaging; (170.3880) Medical and biological imaging.
References
- 1.Adler DC, Zhou C, Tsai TH, Schmitt J, Huang Q, Mashimo H, Fujimoto JG. Opt. Express. 2009;17:784. doi: 10.1364/oe.17.000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouma BE, Tearney GJ, Compton CC, Nishioka NS. Gastrointest. Endosc. 2000;51:467. doi: 10.1016/s0016-5107(00)70449-4. [DOI] [PubMed] [Google Scholar]
- 3.Fu HL, Leng YX, Cobb MJ, Hsu K, Hwang JH, Li XD. J. Biomed. Opt. 2008;13:060502. doi: 10.1117/1.3037340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran PH, Mukai DS, Brenner M, Chen ZP. Opt. Lett. 2004;29:1236. doi: 10.1364/ol.29.001236. [DOI] [PubMed] [Google Scholar]
- 5.Tumlinson R, Povazay B, Hariri LP, McNally J, Unterhuber A, Hermann B, Sattmann H, Drexler W, Barton JK. J. Biomed. Opt. 2006;11:064003. doi: 10.1117/1.2399454. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Kang W, Carrigan T, Bishop A, Rosenthal N, Arruda M, Rollins AM. J. Biomed. Opt. 2011;16:110505. doi: 10.1117/1.3656966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huo L, Xi JF, Wu YC, Li XD. Opt. Express. 2010;18:14375. doi: 10.1364/OE.18.014375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drexler W, Morgner U, Ghanta RK, Kartner FX, Schuman JS, Fujimoto JG. Nat. Med. 2001;7:502. doi: 10.1038/86589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang DL, Hunter BV, Cobb MJ, Li XD. IEEE J. Sel. Top. Quantum Electron. 2007;13:1596. [Google Scholar]
- 10.Xi JF, Huo L, Wu YC, J. Cobb M, H. Hwang J, Li XD. Opt. Lett. 2009;34:1943. doi: 10.1364/ol.34.001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XD, Chudoba C, Ko T, Pitris C, Fujimoto JG. Opt. Lett. 2000;25:1520. doi: 10.1364/ol.25.001520. [DOI] [PubMed] [Google Scholar]
- 12.Hu Z, Rollins AM. Opt. Lett. 2007;32:3525. doi: 10.1364/ol.32.003525. [DOI] [PubMed] [Google Scholar]