Non-alcoholic Fatty Liver Disease (NAFLD) is now the most common liver disease in the United States and it has quickly become a global problem paralleling the rise in obesity prevalence. In the hunt for a dietary trigger for NAFLD, fructose has been identified as a tempting candidate, particularly because of its tendency to promote visceral adiposity and hypertriglyceridemia [1]. Fructose is a six-carbon ketone sugar that is common in the diet and makes up ~10% of calories of a typical American [2]. The primary sources for fructose are added sugars, such as high fructose corn syrup and sucrose, that are found in beverages and processed foods [2]. Growing clinical evidence links fructose consumption and NAFLD [3,4]. The article by Spruss, et al. examines one possible mechanism for fructose-induced hepatic steatosis in mice, namely the interaction(s) between Toll-like receptor 4 (TLR4) and gut-derived endotoxin (LPS).
Fructose metabolism is distinct from glucose metabolism. Dietary fructose is absorbed in the intestine via a saturable, facilitative transporter, GLUT5, and healthy persons have the ability to absorb up to 25 gms, with malabosorption occurring at higher doses which can lead to increased fructose fermentation by gut bacteria [5]. After transport across the basolateral membrane, fructose is taken up by the liver with a high rate of extraction compared to glucose. In the hepatocyte, fructose is rapidly phosphorylated to form fructose-1-phosphate in a reaction catalyzed by fructokinase. The next steps in fructose metabolism result in the production of glyceraldehyde, dihydroxyacetone phosphate, and glyceraldehyde-3-phosphate. This is the point where glucose metabolism and fructose metabolism “merge”; however fructose metabolites reached this stage without passing through the rate limiting step of phosphofructokinase, effectively avoiding the regulating action of insulin. It is this lack of regulation that has been often hypothesized to contribute to the differential effects of fructose feeding compared to glucose[1].
Research has clearly shown that fructose feeding of animals (reviewed in [1]) and humans results in increased de novo lipogenesis, elevated plasma triglyceride levels, increased hepatic lipids and visceral adiposity [5–7]. Oxidative stress has been shown in animals, and more recently several papers demonstrate links between oxidative stress and fructose in humans as well [6,8]. However, the mechanisms for these effects are not known. In this paper by Spruss et al., the authors use a fructose fed animal model to examine possible mechanisms for the development of steatosis, specifically looking at the role of innate immunity, bacterial overgrowth and endotoxin using a TLR4 mutant mouse model. The authors seek to demonstrate an independent effect of intestinal translocation of bacterial endotoxin as a partial or additional source of the increased hepatic steatosis seen in the model, rather than simply the effects assumed from rapid and relatively unregulated hepatic fructose metabolism. They demonstrate that fructose fed mice have endotoxemia, hepatic steatosis with increased ALT, indicators of oxidative stress, and increased MyD88 andTNF mRNA production, all of which, except endotoxemia, were attenuated/blocked in TLR-4 mutant mice.
The approach taken by Spruss et al. is similar to that used in animal models of alcoholic liver disease. It is well documented that alcohol increases gut permeability through multiple mechanisms including alterations in signaling molecules such nitric oxide, effects of alcohol/acetaldehyde on gut barrier proteins, and zinc deficiency induced by chronic alcohol intake [9]. Moreover, alcohol fed animals and humans have alterations in gut flora [9,10]. These gut alterations are associated with endotoxemia, activation of TLR4 signaling, increased TNF production, and liver injury. Moreover, when C3H/HeJ TLR4 mutant mice are fed alcohol, fatty liver is attenuated and liver injury is blocked [11]. Multiple research groups used similar strategies in diet-induced NAFLD produced by feeding a high fat diet. Mice consuming a high fat diet exhibit significantly increased plasma LPS that has been postulated to play a major role in complications of the metabolic syndrome, and this has been termed “metabolic endotoxemia” [reviewed-12]. High fat fed rodents also have an altered gut microbiota profile and increased gut permeability. Mice fed a high fat diet not only develop NAFLD, but other complications of the metabolic syndrome such as vascular inflammation and insulin resistance that are markedly attenuated in animals lacking TLR4 receptor [reviewed-12]. Elegant work from Cani and co-workers demonstrated that mice chronically infused with a very low dose of LPS, (to simulate blood levels induced by a high fat diet) mimic the “high fat phenotype” of obesity, steatosis, hepatic insulin resistance, etc [13]. Lastly, translational research showed that high fat feeding in humans caused low grade post-prandial metabolic endotoxemia [reviewed-12]., The endotoxin absorption following high fat feeding has recently been postulated to not only occur through increased gut permeability but also through binding to chylomicrons formed following high fat consumption [14]. Thus, both chronic alcohol and high fat intake mimic the gut permeability, endotoxemia, increased hepatic TNF production and hepatic steatosis observed by Spruss et al. with high fructose feeding, and the liver/metabolic abnormalities in all three models are blocked/attenuated with loss of TLR-4 function.
Toll-like receptors are highly conserved, pattern recognition receptors that function as pathogen sensors and play a critical role in the innate immune system, and endotoxin is the best studied TLR4 ligand. In liver disease, the role of gut derived endotoxin has been recognized for over a half century. Pioneering studies by Broitman et al. showed that neomycin attenuated liver injury and blocked cirrhosis in choline deficient rats. When endotoxin was added to the diet in neomycin treated animals, liver injury and cirrhosis again developed [15]. Of great importance, it is increasingly clear that there are other agents that activate TLR4 in addition to endotoxin, with certain saturated fatty acids, such as palmitate, representing one example of potential relevance to NAFLD [16]. LPS stimulates an inflammatory response and causes an elevation in levels of free fatty acids as well as impairing insulin sensitivity. Endotoxin increases lipolysis in adipose tissue, elevates circulating free fatty acids levels, and induces insulin resistance in rodents. The lipolytic action of endotoxin is mediated by its lipid A moiety and is blocked by anti-endotoxin peptides [17]. Fatty acids such as palmitate have been shown to activate macrophages to produce TNF and other cytokines such as IL-6 [16]. In in vivo animal models of obesity/NAFLD it is unclear if it is a direct effect of endotoxin that is inducing TLR4 activation or whether other mediators such as fatty acids are mediating this activation. In this study by Spruss, et al., fatty acid levels were not determined in the liver or serum of these animals. To complicate the TLR-4 story further, there are other non-endotoxin ligands for TLR4 that could play a role in NAFLD; with the alarmin, or danger signal high mobility group box-1 (HMGB-1), being an excellent example. This nuclear protein can be released with cell necrosis or by activation of monocytes/Kupffer cells, and is increasingly being appreciated as a mediator of several forms of liver injury [18].
Certain saturated fatty acids also have been shown to cause activation of TLR4 signaling and inflammation in the hypothalamus, which has major implications for obesity [19]. Indeed, both the TLR4 mutation leading to loss of function as well as immunological inhibition of TLR4 protected mice from diet induced obesity. It is important to note in the manuscript by Spruss, et al. that the TLR4 mutant C3H/HeJ mice weighed less than the wild type mice fed fructose. Thus, the lower weight gain may also have attenuated the fatty liver observed in the fructose fed TLR4 mutant mice.
While there are many similarities between diet/obesity and alcohol-induced hepatic steatosis and liver injury, there also are important differences. For example, TLR4 signaling through the MyD88 pathway appears to be important for diet/obesity induced hepatic steatosis and liver injury, and that appears to be the case in the fructose model. On the other hand, the MyD88 independent pathway appears critical for alcohol induced liver injury [20]. The reasons for this, and which cell types are most critical (e.g., Kupffer cells vs. hepatocytes) are unclear at this time. Immunohistochemical studies by Spruss et al. indicated the MyD88 is upregulated in Kupffer cells in their fructose fed mice. Further defining the interactions of the microbiome, gut permeability, the importance of various TLR4 ligands, and differences in TLR4 signaling pathways will be critical for our understanding of alcoholic, high fat and fructose-induced fatty liver disease, and for defining targets for therapeutic interventions.
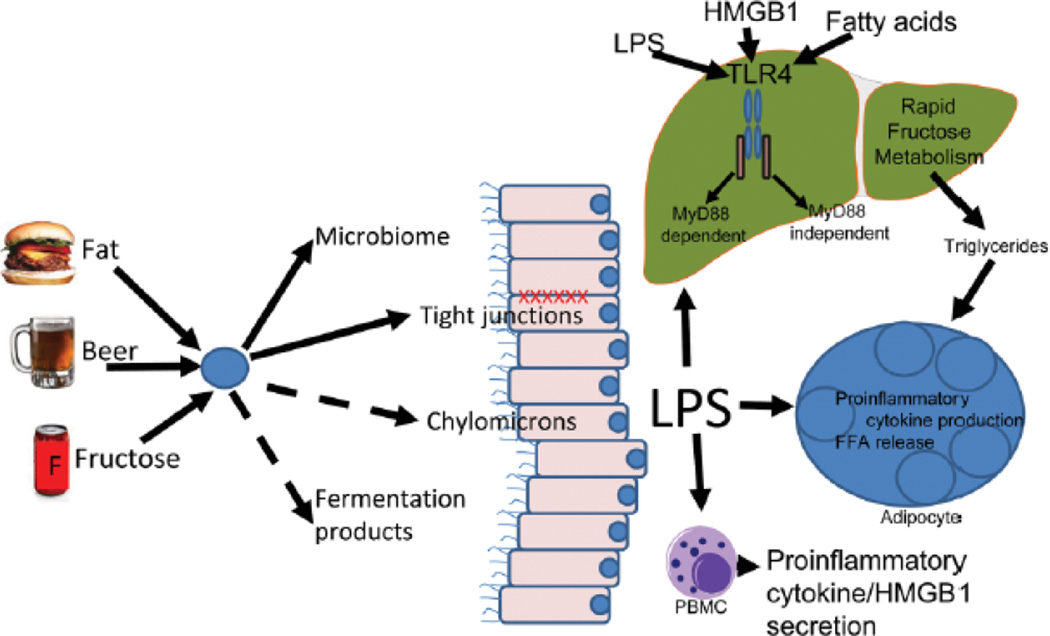
Fig. 1.
A high fructose diet induces changes similar to those seen in models of chronic alcohol intake and high fat diets, including increase gut permeability, endotoxemia, increased hepatic TNF production and hepatic steatosis. As shown in the figure, changes in the microbiome and altered tight junctions (solid arrows) result in increased LPS reaching the liver; LPS transfer via chylomicrons and fermentation products may also play a role (broken arrows). LPS has multiple effects including stimulation of TLR4 through both the MyD88 dependent (fructose induced liver injury) and independent pathways (alcohol-induced liver injury) as well as through stimulation of an inflammatory response that results in elevation in levels of FFA. These inflammatory products including FFA and HMGB1 (released by cell necrosis or by activation of Kupffer cells) are also known to stimulate TLR4. Loss of TLR4 function results in blockage or attenuation of the metabolic derangements in all 3 models, suggesting TLR4 signaling pathways play an important role in each of these forms of fatty liver. Abbreviations: FFA, free fatty acid; HMGB1, high mobility group box 1; LPS, lipopolysaccharide; MyD88, myeloid differentiation protein 88; PBMC, peripheral blood mononuclear cell; TLR4, Toll-like receptor 4.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- TLR4
Toll-like receptor 4
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor
- HMGB-1
high mobility group box-1
Contributor Information
Miriam B. Vos, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta, mvos@emory.edu.
Craig J. McClain, Departments of Medicine and Pharmacology & Toxicology, Distinguished University Scholar, Associate Vice President for Translational Research, Director, Clinical and Translational Sciences Institute, University of Louisville, Louisville VA Medical Center.
References
- 1.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 2.Vos MB, et al. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10(7):160. [PMC free article] [PubMed] [Google Scholar]
- 3.Thuy S, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138(8):1452–1455. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang X, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48(6):993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao SS, et al. Ability of the normal human small intestine to absorb fructose: evaluation by breath testing. Clin Gastroenterol Hepatol. 2007;5(8):959–963. doi: 10.1016/j.cgh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanhope KL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le KA, et al. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr. 2006;84(6):1374–1379. doi: 10.1093/ajcn/84.6.1374. [DOI] [PubMed] [Google Scholar]
- 8.Vos MB, et al. Fructose and oxidized low-density lipoprotein in pediatric nonalcoholic fatty liver disease: a pilot study. Arch Pediatr Adolesc Med. 2009;163(7):674–675. doi: 10.1001/archpediatrics.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008 Aug;42(5):349–361. doi: 10.1016/j.alcohol.2008.03.131. PMID:18504085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008 Dec;42(8):675–682. doi: 10.1016/j.alcohol.2008.08.006. PMID:19038698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001 Jul;34(1):101–108. doi: 10.1053/jhep.2001.25350. PMID:11431739. [DOI] [PubMed] [Google Scholar]
- 12.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15(13):1546–1558. doi: 10.2174/138161209788168164. PMID:19442172. [DOI] [PubMed] [Google Scholar]
- 13.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007 Jul;56(7):1761–1772. doi: 10.2337/db06-1491. Epub 2007 Apr 24. PMID:17456850. [DOI] [PubMed] [Google Scholar]
- 14.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009 Jan;50(1):90–97. doi: 10.1194/jlr.M800156-JLR200. Epub 2008 Sep 24. PMID: 18815435. [DOI] [PubMed] [Google Scholar]
- 15.Broitman SA, Gottlieb LS, Zamcheck N. Influence of Neomycin And Ingested Endotoxin In The Pathogenesis Of Choline Deficiency Cirrhosis In The Adult Rat. J Exp Med. 1964 Apr 1;119:633–642. doi: 10.1084/jem.119.4.633. PMID:14151103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin Invest. 2006 Nov;116(11):3015–3025. doi: 10.1172/JCI28898. Epub 2006 Oct 19. PMID:17053832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zu L, He J, Jiang H, Xu C, Pu S, Xu G. Bacterial endotoxin stimulates adipose lipolysis via toll-like receptor 4 and extracellular signal-regulated kinase pathway. J Biol Chem. 2009 Feb 27;284(9):5915–5926. doi: 10.1074/jbc.M807852200. Epub 2009 Jan 3. [DOI] [PubMed] [Google Scholar]
- 18.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008 Jul-Aug;14(7–8):476–484. doi: 10.2119/2008-00034.Klune. PMID:18431461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009 Jan 14;29(2):359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. PMID:19144836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X-J, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 Binding to the TNF Promoter Results in Macrophase TNF Dysregulation and Steatosis Induced by Chronic Ethanol. J Immunol. 2008 Sep 1;181(5):3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]