Abstract
Macrophages have a critical role in inflammatory and immune responses through their ability to recognize and engulf apoptotic cells1. Here we show that macrophages initiate a cell-death programme in target cells by activating the canonical WNT pathway. We show in mice that macrophage WNT7b is a short-range paracrine signal required for WNT-pathway responses and programmed cell death in the vascular endothelial cells of the temporary hyaloid vessels of the developing eye. These findings indicate that macrophages can use WNT ligands to influence cell-fate decisions—including cell death—in adjacent cells, and raise the possibility that they do so in many different cellular contexts.
In most systems, it has largely been assumed that macrophage involvement in programmed cell death comes after the apoptotic event, and is a response to the presence of membrane-tethered or soluble ‘eat-me’ signals from dead and dying cells. However, in some circumstances phagocytes actively induce programmed cell death. In mice, macrophages are required for the programmed regression of temporary capillary networks within the developing eye2,3. In the worm Caenorhabditis elegans, phagocyte recognition pathways can act as a backup stimulus for the induction of programmed cell death when the autonomous caspase-driven pathway is deficient4,5. These data suggest that there are evolutionarily conserved pathways for phagocyte-induced programmed cell death.
Using ablation and repletion experiments2,3, it has been shown that resident macrophages are necessary and sufficient for the programmed cell death that drives the regression of the temporary ocular capillary networks. To determine whether mice deficient in the lymphomyeloid transcription factor PU.1, which lack macrophages6, are useful for studying programmed vascular regression, we confirmed that resident ocular macrophages were absent in these animals (Fig. 1a–d) and assessed ocular vessel network regression postnatally, when the hyaloid vessels would normally regress7. As expected2,3, the pupillary membrane (on the anterior surface of the lens, Supplementary Fig. 1) and the tunica vasculosa lentis (on the posterior surface of the lens, data not shown), as well as the hyaloid vessels (between the lens and retina, Fig. 1e–h), appeared to persist postnatally in PU.1-deficient mice. Counts of vessel numbers at postnatal day 8 (P8) (Fig. 1i) confirmed this, and also revealed that PU.1 heterozygotes had a partial regression phenotype. TdT-mediated dUTP nick end-labelling (TUNEL) analysis showed that PU.1-null mice have a defect in apoptotic cell clearance (data not shown) that precluded the meaningful quantification of apoptotic vascular endothelial cells (VECs). These data establish that PU.1-mutant mice are a useful model for studying macrophage function in programmed vascular regression.
Figure 1. Regression of the hyaloid vessels is macrophage-dependent.
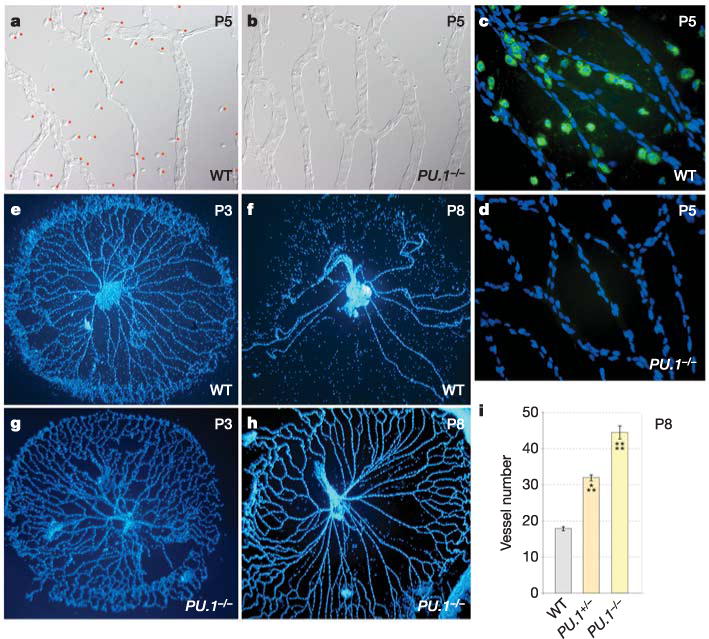
a–d, Hyaloid vessel preparations from wild-type (a, c) and PU.1−/− (b, d) mice at P5. a, b, Differential interference contrast illumination indicating the presence of macrophages in wild-type mice (a, with red dots adjacent) and the absence of macrophages in PU.1−/− mice (b). c, d, Fluorescent immunostaining for macrophages (F4/80, a macrophage-specific marker, green) and nuclei (blue). e–h, Hyaloid vessels from wild-type (e, f) and PU.1−/− (g, h) animals of the indicated ages stained with Hoechst 33258. i, Hyaloid vessel number in wild-type, PU.1−/− and PU.1−/− mice at P8. All error bars are standard errors. Significance levels: three asterisks, 0.0001 < P < 0.001; four asterisks, P < 0.0001. Original magnification: ×50 (e–h); ×400 (a–d). WT, wild type.
The WNT signalling pathway has a crucial function in developmental cell fate decisions8–11 and, when aberrantly activated, in the development of cancer12. In vertebrates, the canonical WNTresponse requires a receptor complex comprising the coreceptor low-density lipoprotein receptor-related protein (LRP)5 or LRP6 (ref. 13) and a multiple-pass transmembrane receptor of the Frizzled (FZD) family14. When activated by a WNT ligand, this complex initiates a cascade of events that culminates in the stabilization of β-catenin, its association with transcription factors of the Lef/Tcf family15 and the regulation of target genes including some that stimulate cell cycle entry8. Notably, as indicated by the phenotype of the homozygousnull Lrp5lacZ/lacZ mice16,17 (Fig. 2a–d, g), the coreceptor gene Lrp5 is required for the regression of the hyaloid vessels as a consequence of reduced VEC apoptosis17 (Fig. 2h). As LRP5 may have activities outside the canonical WNT pathway9, we assessed the consequences of mutating the Lef1 gene18 and again found persistent hyaloid vessels (Fig. 2e–g) and reduced levels of cell death (Fig. 2h). As the WNT pathway is often a stimulus for proliferation, we also performed 5-bromodeoxyuridine (BrdU) labelling and showed that in capillary cells of both the Lrp5 and Lef1 mutants, the rate of cell cycle entry is reduced (Fig. 2i). Taken together, these data indicate that canonical WNT signalling is required for VEC cell cycle entry, apoptosis and programmed capillary regression.
Figure 2. The WNT pathway response in VECs is required for hyaloid vessel regression.
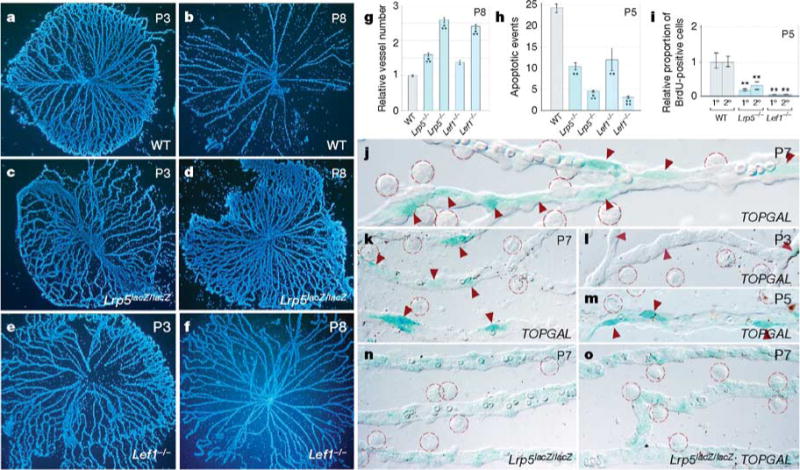
a–f, Hoechst-33258-labelled hyaloid vessel preparations from mice of the indicated ages and genotypes. g–i, Vessel number at P8 (g), number apoptotic events at P5 (h) and BrdU labelling index in primary (1°) and secondary (2°) capillary branches at P5 (i) in the hyaloid vessels of mice with the indicated genotypes. Lrp5−/− is an abbreviated reference to the Lrp5lacZ/lacZ allele. j–o, X-gal staining (blue) in hyaloid vessels from mice of the indicated ages and genotypes. Intensely stained TOPGAL-expressing cells (red arrowheads) and resident macrophages (red dashed circles) are apparent. All error bars are standard errors. Significance levels: two asterisks, 0.001 < P < 0.01; three asterisks, 0.0001 < P < 0.001; four asterisks, P < 0.0001. Original magnification: ×50 (a–f); ×630 (k–o); ×1,000 (j). WT, wild type.
To determine which cell type was WNT-responsive, we took advantage of canonical WNT pathway reporter mice (TOPGAL) that carry a transgene with Lef/Tcf binding sites and a minimal promoter upstream of an open reading frame encoding β-galactosidase (β-gal)19. Staining of hyaloid vessels from TOPGAL hemizygous mice with 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal) revealed β-gal-positive VECs adjacent to the vessel lumen (Fig. 2j). The intensity of X-gal staining in VECs increased from P3 (Fig. 2l) to P5 and P7 (Fig. 2m, k). Macrophages could be identified morphologically (Fig. 2j–m, dashed circles) and did not stain. The X-gal staining pattern in P7 hyaloid vessels from TOPGAL and Lrp5lacZ/lacZ mice were quite distinct, with TOPGAL mice showing sporadic intensely stained VECs (Fig. 2j, k) and the Lrp5lacZ/lacZ mice showing weak general staining (Fig. 2n). In compound Lrp5lacZ/lacZ;TOPGAL mice, the intense sporadic VEC labelling was lost (Fig. 2o), consistent with the expectation that LRP5 is required for WNT signalling. When combined with the reduced cell death and regression failure of the Lrp5- and Lef1-mutant mice, these data indicate that the WNT response of VECs is critical for scheduled vascular regression.
One way to explain the requirement for macrophages in scheduled vascular regression would be if they were a source of WNT ligands. To investigate this possibility, we determined whether the macrophagedeficient PU.1-null mice showed defects that were characteristic of perturbations in the WNT pathway. BrdU labelling showed that, like the Lrp5 and Lef1 mutants (Fig. 2i), PU.1-null mice show a reduced rate of S-phase labelling in cells of primary and secondary hyaloid capillary branches (Supplementary Fig. 2a). Crosses between PU.1 and TOPGAL mice produced macrophage-deficient TOPGAL animals that showed a 70% reduction in the number of X-gal-stained cells in the hyaloid vessels (Supplementary Fig. 2b). These data suggest that macrophages can stimulate WNT pathway activation in hyaloid VECs.
To determine whether macrophages might express a set of Wnt genes associated with vascular development20, we performed reverse transcription–polymerase chain reaction (RT–PCR) analysis on purified populations of hyaloid macrophages gathered from wildtype P5 hyaloid preparations using the laser-capture microdissection technique (Supplementary Fig. 2). This revealed that of a small number of Wnt genes expressed in macrophages, Wnt7b seemed to be specific for hyaloid macrophages (Supplementary Fig. 2). To investigate further the expression of Wnt7b, we performed X-gal staining on hyaloid vessel preparations from Wnt7b+/lacZ mice21 (Fig. 3a–c), which confirmed that Wnt7blacZ expression was restricted to macrophages. Furthermore, the level of X-gal staining increased dramatically from P1 to P5 (Fig. 3a–c) and correlated with increasing TOPGAL expression in VECs over the same time interval (Fig. 2k–m). These data suggested that macrophages are required for scheduled vascular regression because they are a source of WNT7b.
Figure 3. WNT7b is required for hyaloid vessel regression and is expressed in macrophages.
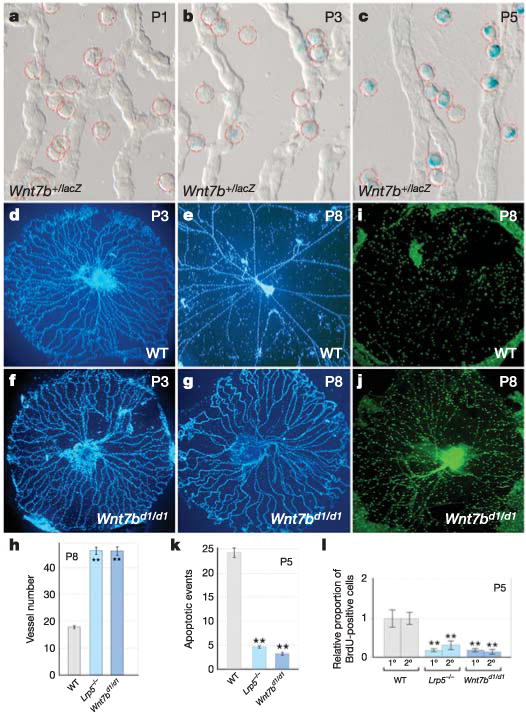
a–c, X-gal staining (blue) of hyaloid vessels from Wnt7b+/lacZ mice of the indicated ages. Macrophages are indicated by dashed red circles. d–g,i,j, Hyaloid vessels from wild-type (d,e,i) and Wnt7bd1/d1 (f,g,j) animals at the indicated ages, stained with Hoechst 33258 (d–g) or anti-F4/80 (i, j). h, k, l, Quantification, in hyaloid vessels from mice with the indicated genotypes, of vessel number at P8 (h), apoptotic events at P5 (k) and BrdU labelling index in primary (1°) and secondary (2°) capillary branches at P5 (l). All error bars are standard errors. Significance levels: two asterisks, 0.001 < P < 0.01. Original magnification: ×50 (d–g, i, j); ×630 (a–c). WT, wild type.
To determine whether this was the case, we generated a hypomorphic Wnt7bd1 gene-targeted allele (Supplementary Fig. 3). It was necessary to generate this allele for analysis as previously generated Wnt7b mutations, including the Wnt7blacZ allele used above21, result in lethality too early to permit analysis of hyaloid vessel regression. Interestingly, Wnt7bd1/d1 mice show persistence of the hyaloid vessels (Fig. 3d–h). Immunolabelling of wild-type and Wnt7bd1/d1 hyaloid vessels for the macrophage marker F4/80 showed that normal numbers of macrophages were present in mutant mice (Fig. 3i, j), eliminating the possibility that hyaloid vessel persistence was a consequence of a lack of macrophages at these sites. It should be noted that Wnt7bd1/d1 mice also showed levels of apoptosis (Fig. 3k) and BrdU labelling (Fig. 3l) that were much reduced from wild-type levels but comparable to the levels observed in Lrp5-mutant mice. As Wnt7b expression is restricted to macrophages, these data suggest that WNT7b is a macrophage product critical for inducing cell death in hyaloid VECs.
To determine which FZD receptor might mediate WNT7b signalling, we assessed the activity of FZD3–FZD8 using the SuperTOPFLASH WNT reporter cell line11 and a luciferase reporter assay (Supplementary Fig. 4). Although we could not exclude the participation of multiple FZDs, the expression of Fzd4 in hyaloid capillaries, its activity in mediating WNT7b signalling (Supplementary Fig. 4a, b) and the persistence of hyaloid vessels in Fzd4-mutant mice11 all implicated FZD4. For this reason, we also examined the specificity of the interaction using a mixed-cell assay in which we tested the ability of FZD4 ligand-binding domain mutants (see Methods) to mediate paracrine signalling by WNT7b (Supplementary Fig. 4c). This showed that subtle mutations in FZD4 prevent paracrine WNT7b signalling, and further support a model in which WNT7b produced by macrophages signals through FZD4 expressed on endothelial cells.
If macrophage WNT7b was signalling to hyaloid VECs and eliciting a WNT pathway response, we would predict that in TOPGAL;Wnt7bd1/d1 compound mice, the TOPGAL response would be reduced. To assess this, we generated X-gal-stained P8 hyaloid vessel preparations from control TOPGAL and TOP-GAL;Wnt7bd1/d1 mice (Fig. 4a, b) and counted the numbers of stained cells. The data were normalized to take into account the greater number of vessels in the Wnt7bd1/d1 mutant; this showed that the Wnt7b mutation reduced the TOPGAL response to 29% of the control value (Fig. 4c). This corresponds very closely to the reduced TOPGAL response observed when macrophages are absent (in PU.1-mutant mice, Supplementary Fig. 2b) and further argues that macrophage WNT7b elicits a WNT pathway response in hyaloid VECs.
Figure 4. Macrophages are a critical paracrine source of WNT7b required for hyaloid vessel regression.
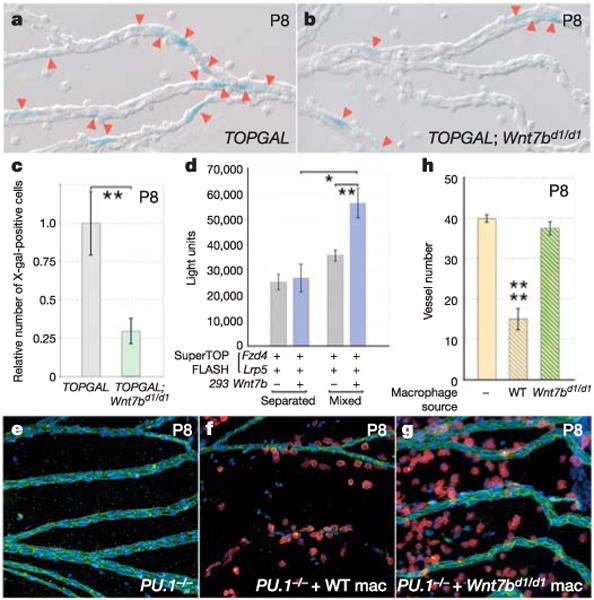
a, b, X-gal staining (blue) of P8 hyaloid vessel preparations in TOPGAL (a) and TOPGAL; Wnt7bd1/d1 (b) mice. Red arrowheads indicate stained cells. c, Relative number of X-gal-stained cells in TOPGAL (grey bar) and TOPGAL;Wnt7bd1/d1 (green bar) hyaloid preparations at P8. The data are normalized for the increased vessel number of the Wnt7bd1/d1 mutants. d, Quantification of WNT responsiveness in SuperTOPFLASH cells transfected with Fzd4 and Lrp5 and either mixed with WNT7b producer cells or separated from them by restricting SuperTOPFLASH responder cells and WNT7b producer cells to each half of a culture dish. e–g, Hyaloid vessel preparations at P8 from uninjected PU.1−/− mice (e), PU.1−/− mice injected with wild-type macrophages (f) and PU.1−/− mice injected with Wnt7bd1/d1 macrophages (g). Preparations are labelled for the F4/80 marker (red), vascular endothelial cell cadherin (green) and nuclei (blue). Original magnification: ×200. h, Vessel number at P8 in uninjected PU.1−/− mice (yellow bar), PU.1−/− mice injected with wild-type macrophages (yellow/grey bar) and PU.1−/− mice injected with Wnt7bd1/d1 macrophages (yellow/green bar). All error bars are standard errors. Significance levels: one asterisk, 0.01 < P < 0.05; two asterisks, 0.001 < P < 0.01; four asterisks, P < 0.0001. WT, wild type; Mac, macrophages.
WNT ligands can be lipid-modified and are therefore highly insoluble22. This suggests that macrophage WNT7b might elicit VEC responses only through cell–cell contact. Therefore, we used a technique described previously11 to assess the range of action of WNT7b using populations of producer and responder cells that were either closely apposed or widely separated in the culture dish. The SuperTOPFLASH cell line11 transfected with Fzd4 and Lrp5 expression plasmids was used to assess WNT pathway responses. Transfected 293 cells, containing a Wnt7b expression plasmid, were used as a producer cell line. Significant activity was detected only when WNT7b-producer cells were mixed with FZD4-expressing responder cells (Fig. 4d), suggesting that WNT7b had a short range of action.
Despite the reduced TOPGAL responsiveness in TOP-GAL;Wnt7bd1/d1 mice, it remained possible that another source of WNT7b in addition to the macrophage might be critical to initiate the hyaloid VEC death programme. To investigate this possibility, we took advantage of PU.1-mutant mice and asked whether injected macrophages could rescue the hyaloid vessel regression failure. Bone-marrow-derived macrophages were differentiated in the presence of CSF-1 (colony-stimulating factor 1) as described previously3 and were allowed to mature to 14 ± 3 days before injection. Intravitreal injection into the right eye was performed on the day of birth; the contralateral eye was used as an uninjected control. As expected, no macrophages were observed in uninjected PU.1-mutant eyes and the hyaloid vessels were persistent (Fig. 4e). Injected wild-type macrophages were localized to the hyaloid capillaries (Fig. 4f), and by P8 had rescued regression completely (Fig. 4f, h) according to the number of hyaloid vessels remaining at P8 in wild-type mice (Fig. 3h). Notably, when bone marrow macrophages from Wnt7bd1/d1 mice were injected, they also localized to hyaloid vessels (Fig. 4g) but completely failed to mediate hyaloid vessel regression (Fig. 4g, h). When combined with the range-of-action experiments and the results from the TOPGAL;Wnt7bd1/d1 compound mice, these data indicate that the critical, paracrine source of WNT7b required for scheduled vascular regression is the macrophage.
Here we have established that macrophages actively regulate life-and-death decisions in adjacent cells through the WNT pathway. In the context of the hyaloid vessel system, the critical mediator of cell death is WNT7b. Although it is possible that a more complex signalling pattern exists, the data support a model in which macrophage WNT7b activates the WNT pathway in adjacent vascular endothelial cells, most likely through cell–cell contact. As the WNT pathway can stimulate cell cycle entry in this and other systems8, there is a possibility that programmed cell death is dependent on cell cycle entry. Coupling of cell death and cell cycle entry has been noted in other systems23.
The current study defines a role for the WNT pathway in regulating vascular regression. Given the broad distribution of macrophages, they may be more generally involved in regulating vascularity through the activation of the WNT pathway in vascular structures10,11. In addition, tumour-associated macrophages are known to express WNT genes24 and can extrinsically regulate the growth of the tumour vasculature, and also perhaps of the tumour cells themselves25. Thus, the finding that macrophages can produce WNT ligands has important implications given the many circumstances in which the WNT pathway has a critical function. The short-range action of macrophage WNT7b is a mechanism for controlling the activity of this potent mediator.
Beyond vascular development, the major question that arises from this analysis is whether the ability of macrophages to actively signal cell death is a unique adaptation in the eye or a more general phenomenon. Although these alternatives need to be tested rigorously, there is some evidence to suggest the latter. For example, in mammalian systems, macrophage-related microglia can promote cell death in the retina through the production of nerve growth factor26. Recently, ablation experiments in a model of liver injury have shown that inflammatory macrophages promote myofibroblast proliferation and apoptosis as a mechanism of resolution and repair27. It has also been shown that phagocytes can promote programmed cell death outside of mammalian systems. In C. elegans, the machinery required for the recognition and engulfment of dead cells can promote apoptosis when CED3 (caspase) activity is compromised, suggesting that phagocytes provide a backup mechanism for disposing of superfluous cells4,5. All this would suggest that phagocytes may have a conserved function in signalling cell death in a variety of contexts.
METHODS
Mouse breeding and genotyping
Genotyping of Lrp5lacZ/lacZ (ref. 17), Lef1−/− (ref. 18), PU.1−/− (ref. 6) and TOPGAL (ref. 19) mice was performed as previously described. When Lef1-mutant mice were produced as C57BL/6 × 129Sv F1 hybrids they showed enhanced survival that allowed the analysis of hyaloid vessel regression postnatally.
Dissections, immunostaining and imaging
Hyaloid vessel preparations were generated as previously described17 with the exception that 5% (w/v) gelatin was used at 56 °C and allowed to set on ice before completion of the dissection. X-gal staining was done according to established protocols21. Indirect immunofluorescent staining and BrdU labelling were performed as previously described28. Primary antibodies used were anti-vascular-endothelial-cell-cadherin (Santa Cruz), anti-BrdU (Dako) and anti-F4/80 (Caltag laboratories), all at a 1:100 dilution. Secondary antibodies labelled with Alexa fluorochromes (Molecular Probes) were used at a 1:500 dilution. TUNEL labelling of apoptotic cells was performed using an in situ cell death detection kit (Roche).
Plasmids
All Fzd, Wnt and Norrin complementary DNAs used were mouse. The plasmid encoding the Alkaline phosphatase (AP)–Norrin fusion protein has been described elsewhere11. WNT7b was expressed from pCDNA3.1 as a V5- and His-epitope-tagged fusion protein. In addition, we generated Fzd4m1 and Fzd4m2 in pCDNA3.1 using a PCR-based mutagenesis strategy. In Fzd4m1, the sequence encoding the cysteine-rich domain (residues 54-QNLGYNV-60) was modified to encode AALAYAA. In Fzd4m2, the sequence encoding residues 105-MCT-107 was modified to encode ACA. These alanine mutations in the equivalent regions of murine FZD8 were shown to eliminate binding of Xenopus WNT8–AP (ref. 29). These expression plasmids were used in the paracrine and range-of-action signalling assays (Supplementary Fig. 4).
Laser-capture microdissection
To purify hyaloid macrophages, we performed laser-capture microdissection from whole-mount hyaloid vessel preparations using the PixCell II laser-capture microdissection system (Arcturus). The macrophages shown in Supplementary Fig. 2c encircled in red are shown after they have been lifted from the preparation (Supplementary Fig. 2d) on the lasermelted polymer membrane.
Reverse transcription–polymerase chain reaction
RNA from isolated macrophages was purified using PicoPure RNA isolation kit (Arcturus) and subsequent RT–PCR performed using the OneStep RT–PCR kit (Qiagen). Nested primers were used for a second round of PCR-amplification using Takara LA Taq (Takara). Primer nucleotide sequences used were: Gapdh, forward (F) 5′ -ACT CCACTCACGGCAAATTC-3′, reverse (R) 5′ -CACATTGGGGGTAGGAACAC-3′; Wnt2, F 5′ -CGGCCTTTGTTTACGCCATC-3′, R 5′ -TGAATACAGTAGTCTG GAGAA-3′; Wnt7b, F 5′ -AAGAACTCCGAGTAGGGAGTCG-3′, R 5′ -TGCG TTGTACTTCTCCTTGAGC-3′; Wnt7b, second round F 5′ -CCGAGTAGGG AGTCGAGAGG-3′, R 5′ -CACACCGTGACACTTACATTCC-3′; Wnt10b, F 5′ -GTGGTAACGGAAAACCTGAAGC-3′, R 5′ -CTCATCACACAGCACATAA CAGC-3′; Wnt10b, second round F 5′ -GCCAATTCAAGACCTGTTGG-3′, R 5′ -CATAACAGCACCAGTGGAAACG-3′; Fzd4, F 5′ -GCTACAACGTGACCAA GATGC-3′, R 5′ -CAAACCCAAATTCTCTCAGGAC-3′; Fzd4, second round F 5′ -AACTTAGTGGGACACGAGCTG-3′, R5′ -CAGCGTCTCTTGACTGAAAGG-3′; Norrin, F 5′ -GAGAAATCATGTACTAGCTGCATCC-3′, R 5′ -TGTACCGG TAAGTGGCAGTAAG-3′; Norrin, second round F 5′ -GGCCATAATGGGAGATA CAGAC-3′, R 5′ -GAGGACAGTGCTGAAGGACAC-3′; Lef1, F 5′ -CTACAGCG ACGAGCACTTTTC-3′, R 5′ -AGGATCTGGTTGATAGCTGCAC-3′.
Macrophage rescue
Mouse bone-marrow-derived macrophages were isolated as previously described3. Approximately 4,000 macrophages from wild-type or Wnt7bd1/d1 animals were injected into the vitreous at P1 in a volume of 200 nl using a modification of the trans-corneal technique described previously3. Animals were killed at P8 and hyaloid vessels dissected.
Luciferase and range-of-action assays
For the Frizzled receptor activity screen, SuperTOPFLASH cells11 (which carry a reporter plasmid with Lef/Tcf binding sites and a minimal promoter upstream of an open reading frame encoding luciferase) were plated into 60-mm dishes and transfected after 24 h with combinations of 1 μg Lrp5–Flag, 1μg Wnt7b–V5His and 0.1μg Fzd plasmid made up to 4μg with pIRES–GFP using Fugene 6 (Roche). Forty-eight hours after transfection, cells were washed with PBS and luciferase activity measured with a luciferase assay kit (Promega). For the mixed-cell paracrine signalling assay, 293 cells were transfected with WNT pathway ligands and overlaid on SuperTOPFLASH responder cells that were separately transfected with plasmids encoding LRP5 and a FZD receptor. Luciferase activity was measured as indicated above. WNT7b range-of-action experiments were carried out as described11.
Statistical analysis
Vessel number was quantified using established methods7. At least four hyaloid vessel preparations were quantified for each experiment. All data are presented with standard error bars. Student’s t-test and one-way analysis of variance were used to assess statistical significance.
Supplementary Material
Acknowledgments
P. Speeg for technical assistance; E. Fuchs, L. Niswander and C. Dean for the TOPGAL mice; S. McKercher and R. Maki for the PU.1-null mice; L. Chan for the Lrp5-null mice; and, R. Grosschedl for the Lef1-null mice. We are indebted to Q. Xu and J. Nathans for providing the SuperTOPFLASH cell line and for the Fzd and Norrin expression plasmids. This work was supported by NIH grants to E.E.M., A.P.M., G.K. and R.A.L. G.K. was also supported by funds from the March of Dimes, and R.A.L. by funds from the Abrahamson Pediatric Eye Institute Endowment at the Children’s Hospital Medical Center of Cincinnati.
Footnotes
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
The authors declare no competing financial interests.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nature Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 2.Lang RA, Bishop MJ. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- 3.Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997;124:3633–3638. doi: 10.1242/dev.124.18.3633. [DOI] [PubMed] [Google Scholar]
- 4.Hoeppner DJ, Hengartner MO, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature. 2001;412:202–206. doi: 10.1038/35084103. [DOI] [PubMed] [Google Scholar]
- 5.Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in C. elegans. Nature. 2001;412:198–202. doi: 10.1038/35084096. [DOI] [PubMed] [Google Scholar]
- 6.McKercher SR, et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 7.Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol (Berl) 1999;200:403–411. doi: 10.1007/s004290050289. [DOI] [PubMed] [Google Scholar]
- 8.Nusse R. WNT targets. Repression and activation. Trends Genet. 1999;15:1–3. doi: 10.1016/s0168-9525(98)01634-5. [DOI] [PubMed] [Google Scholar]
- 9.Huelsken J, Birchmeier W. New aspects of Wnt signalling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa T, et al. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- 11.Xu Q, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand–receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 12.Bienz M, Clevers H. Linking colorectal cancer to Wnt signalling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 13.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signalling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 14.Perrimon N. Serpentine proteins slither into the wingless and hedgehog fields. Cell. 1996;86:513–516. doi: 10.1016/s0092-8674(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 15.Behrens J, et al. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 16.Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 17.Kato M, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Genderen C, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 19.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin AM, D’Amore PA. Wnt signalling in the vasculature. Angiogenesis. 2002;5:1–9. doi: 10.1023/a:1021563510866. [DOI] [PubMed] [Google Scholar]
- 21.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- 22.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 23.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 24.Smith K, et al. Up-regulation of macrophage Wnt gene expression in adenoma–carcinoma progression of human colorectal cancer. Br J Cancer. 1999;81:496–502. doi: 10.1038/sj.bjc.6690721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 26.Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 27.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diez-Roux G, Argilla M, Makarenkova H, Ko K, Lang RA. Macrophages kill capillary cells in G1 phase of the cell cycle during programmed vascular regression. Development. 1999;126:2141–2147. doi: 10.1242/dev.126.10.2141. [DOI] [PubMed] [Google Scholar]
- 29.Dann CE, et al. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


