Abstract
Accumulation of smooth muscle cells (SMC) results in neointima formation in injured vessels. Two graft models consisting of vein and artery grafts were created by anastomosing common carotid arteries to donor vessels. To identify the origin of the neointima cells from anastomosed arteries, we use Wnt1-Cre/reporter mice to label and track SMCs in the common carotid artery. The contribution of SMCs in the neighboring arteries to neointima formation was studied. On evaluating the artery grafts after 1 month, >90 % of the labeled neointima cells were found to have originated from the anastomosing host arteries. Most of the neointima cells were also smooth muscle α-actin positive (SMA-α+) and expressed the smooth muscle myosin heavy chain (SMMHC), the SMC terminal differentiation marker. In vein grafts, about 60 % SMA-α-positive cells were from anastomosing arteries. Bone marrow cells did not contribute to neointima SMCs in vein grafts, but did co-stain with markers of inflammatory cells. Wnt1 expression was not detected in the neointima cells in the vein or artery grafts, or the injured femoral arteries. Neointima SMCs showed the synthetic phenotype and were positively labeled with BrdU in vitro and in vivo. Treatment with the IGF-1 receptor inhibitor suppressed SMC proliferation and neointima formation in vein grafts. Our results indicate that SMCs from the neighboring artery are predominantly present in the neointima formed in both vein and artery grafts and that Wnt1-Cre mice can be used to explore the role of SMCs originating from neighboring vessels in vascular remodeling.
Keywords: VSMC, Wnt1, Neointima formation
Introduction
SMC accumulation and neointima formation are major contributors to vascular remodeling that can result in graft failure after angioplasty or coronary artery bypass surgery. In fact, formation of neointima is the main cause of failure in about 40 % of autologous vein grafts employed in coronary artery bypass surgery [11, 25]. Multiple types of cells have been implicated in neointima formation [3, 37], including the circulating fibrocytes, bone marrow-derived circulating progenitor cells [33, 34], and cells from endothelial–mesenchymal transition [1]. Besides distal source of cells, local cell types (including resident SMC progenitor cells, adventitial cells, or mature SMCs) can give rise to SMCs in the neointima [2, 13, 14, 16].
The problem with identifying the origin of cells that constitute a neointima is the absence of animal models that can specifically label these “SMC precursors”. Therefore, while transgenic mice overexpressing LacZ or GFP are used frequently to study the origin of neointima cells in vascular grafts, the lack of a tissue specific labeling strategy does not allow clear identification of the source of cells from the donor to that of the recipient.
To overcome this shortcoming, we studied Wnt1-Cre transgenic mice to label and track neural crest cells and their derivatives. It is well established that during development, SMCs of the cardiac outflow tract (including the common carotid artery) are derived from Wnt1-expressing neural crest cells [20] while those in other vessels including the vena cava and the descending aorta are not [20, 36]. In adult mice, most of the Wnt1 expression can be found in neurons, and not in neural crest-derived SMCs of the common carotid artery [24]. Applying these features of Wnt1 expression, we have bred Wnt1-Cre with the Floxed-reporter transgenic mice to generate offsprings in which the neural crest-derived SMCs in the common carotid artery (and cardiac outflow tract) are labeled permanently by the reporter genes, while SMCs in the other vessels remain unlabeled. We reasoned that after grafting an unlabeled vessel (vein or artery) onto the common carotid artery of Wnt1-Cre/reporter mice, the contribution of “local” neighboring SMCs to the neointima cells could be tracked and identified. SMCs of the common carotid arteries from the two transgenic mice, LacZ-Stopflox/Wnt1-Cre and RFPflox-GFP/Wnt1-Cre, expressing LacZ and GFP, respectively, were used for the current studies. Donor arteries or veins from wild-type (WT) mice were grafted onto the carotid artery of these reporter mice and the incorporation of labeled cells and BrdU proliferative cells in the neointima from the arterial or vein grafts were tracked and examined. We found that LacZ+ or GFP+ cells in the neointimas were co-stained with SMC specific markers, SMA-α and SMMHC, in the two graft models. Blocking SMC proliferation attenuates neointima formation in the vein graft. These results indicate that the migration and proliferation of SMCs from the neighboring arteries significantly contributes to neointima formation.
Materials and methods
Mice
WT mice, LacZ-Stopflox [B6.129S4-Gt(ROSA)26Sortm1Sor/J, stock number: 003474] and Wnt1-Cre transgenic mice [Tg(Wnt1-Cre)11Rth/MileJ, Stock number: 007807] were from Jackson Laboratory (Bar Harbor, Maine) [19]. RFPflox-GFP mice [B6.Cg-Tg(CAG-DsRed,-EGFP)5Gae/J, stock number: 008705] were used as described [26]. The RFPflox-GFP/Wnt1-Cre+ and LacZ-Stopflox/Wnt1-Cre+ mice were generated and handled in accordance with Baylor College of Medicine Institutional Animal Care and Use Committee guidelines. The RFP gene allele was deleted by Cre recombination, and GFP expression was observed in neural crest-derived cells and neurons in RFPflox-GFP/Wnt1-Cre+ mice. Similarly, expression of LacZ in these cells was observed in LacZ-Stopflox/Wnt1-Cre+ mice. The genotyping was performed according to the protocol provided by the Jackson Laboratory.
Antibodies and reagents
Antibodies against GFP (rabbit), SMA-α (rabbit), CD11b, SMMHC (rabbit) and Wnt1 were from Abcam (Cambridge, MA, USA); SMA-α-FITC (mouse), antibodies against β-actin, bromodeoxyuridine (BrdU), and picropodophyllotoxin (PPP), the IGF-1 receptor inhibitor were purchased from Sigma-Aldrich (St. Louis, MO, USA); the DsRed (rabbit) antibodies were from Rockland Inc (Danvers, MA, USA); antibodies against RFP (goat) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-GFP (mouse) antibodies were obtained from Invitrogen (Carlsbad, CA, USA). Anti-CD3 and neutrophil antibodies were purchased from eBioscience (San Diego, CA, USA). The anti-CD31 and -CD45 antibodies were from BD Pharmingen (San Jose, CA, USA). BrdU Labeling and Detection Kits were obtained from Roche (Indianapolis, IN, USA).
Vein graft procedure
All animal protocols were approved by IACUC. In brief, recipient mice were anesthetized with an intraperitoneal injection of xylazine and ketamine cocktail (8.8-mg/kg xylazine and 130-mg/kg ketamine). The right common carotid artery of a male mouse was mobilized and dissected. A cuff (∼1.0 mm × ∼0.5–0.8 mm, length × diameter) was placed on both ends of the artery. The ends of the artery were everted over the cuff and ligated with a 8.0 silk suture. Vena cava from donor mice was grafted between the two ends of the carotid artery by “sleeving” the ends of the vein over the cuff; they were secured with 8.0 silk sutures. After 4 weeks, mice were perfused with 4 % paraformaldehyde (PFA) via the left ventricle for 10 min, the vein grafts were collected, and cryo or paraffin sections were prepared based on proposed experiments. The vessel wall thickness was measured by the NIS-Elements BR 3.0 program as area of the vessel minus that of the lumen. In experiments designed to detect the effects of IGF-1 receptor inhibitor on neointima formation in vein grafts, mice were treated with PPP (20 mg/ kg) or drug-free solvent i.p. every 2 days for 3 weeks before the graft collection.
Artery graft procedure
The surgical procedure was similar to that for vein graft. The anesthetized donor mouse was perfused with saline, and the right common carotid artery was injured by wire (0.38 mm in diameter, Abbot Vascular Inc., Abbott Park, IL, USA) and placed in saline at room temperature. Recipient mice were anesthetized and the right common carotid artery of a male mouse was mobilized and dissected. A cuff was placed on both ends of the artery and the injured artery (∼8 mm) from donor mice was grafted between the two ends of the carotid artery. After 4 weeks, mice were perfused with 4 % PFA via the left ventricle for 10 min, the artery grafts were collected, and cryo or paraffin sections were prepared based on proposed experiments for X-Gal or immunostaining.
BrdU experiments
BrdU was administered to the mice (i.p., 1.5 mg/day) for 7 days before graft collection. BrdU immunofluorescent staining was performed according to the manufacturer's instructions (BrdU Labeling and Detection Kit, Roche, Indianapolis, IN, USA).
Mouse femoral artery injury model
The mice were anesthetized as above. The femoral artery was isolated, the vein and connective tissues around the artery were carefully removed with microsurgery forceps (Dumont S.A., Switzerland). The exposed muscular branch artery was dilated by topical application of one drop of 1 % lidocaine hydrochloride. The injury model of the femoral artery was performed as described [31] with some modifications. Briefly, the end-blunted 31-gauge needle (0.26 mm in diameter) was inserted into the profunda femoris artery, pushed forward for ∼5–10 mm toward the iliac artery and left in place for 1 min, to dilate the artery. The blood flow was reconstituted after ligation of the profunda femoris branch. After 4 weeks, mice were perfused with 4 % PFA via the left ventricle for 10 min. The femoral artery was carefully excised, fixed in 4 % PFA for 24 h, and embedded in paraffin.
X-Gal staining
5-μm sections of grafts were washed in PBS for 10 min, incubated in blocking buffer (1 mM MgCl2, 0.01 % Na-deoxycholate, 0.02 % IGEPAL-CA630, and 5 mM EGTA in PBS) for 20 min at room temperature, and incubated in the X-Gal mixture (1 mM MgCl2, 0.01 % Na-deoxycholate, 0.02 % IGEPAL-CA630, 5 mM EGTA, 5 mM K3-Fe(CN)6, 5 mM K4Fe(CN)6·3H2O, and 1 μg X-Gal; all from Sigma-Aldrich) for 16 h at 37 °C. Alternatively, cryo sections were incubated for 16 h with a commercially available β-Gal staining kit, specifically designed to minimize staining from endogenous β-Gal (Roche Diagnostics Corp).
Immunohistochemistry
For histological analysis, vein or artery grafts were perfused through the left ventricle as described [6]. Grafts were fixed with 4 % phosphate-buffered formaldehyde at 4 °C for 24 h and processed as described [5]. Sections were blocked with 10 % goat serum (Vector Laboratories, Burlingame, CA, USA) for 30 min and then incubated with primary antibodies. After washing in 0.5 % Tween 20 in PBS (PBST), these sections were incubated with a biotinylated secondary antibody (Vector lab) at room temperature. Following a repeat washing with PBST, tissue sections were incubated with an Elite® ABC reagent (Vector Laboratories) followed by a peroxidase substrate kit (Vector Laboratories) according to the manufacturer's protocol. The sections were counterstained by hematoxylin. For double immunofluorescent staining of samples, fluorescent secondary antibodies were applied to sections; DAPI was used as a counter stain. Pictures were recorded using a Nikon Eclipse 80i fluorescence microscope (Melville, NY, USA).
Bone marrow isolation and transplantation
Bone marrow (BM) transplantation was performed as described [6]. Briefly, mice were anesthetized with an intraperitoneal injection of xylazine and ketamine cocktail (8.8 mg/kg xylazine and 130 mg/kg ketamine). The BM donor mice were euthanized by cervical dislocation and cells were harvested by flushing their femurs and tibias. The transplant was created by injecting 5 × 106 bone marrow cells into the lateral tail vein of lethally irradiated (1,100 rads) mice recipients.
Statistical analysis
All data are presented as mean ± SEM. Comparison between groups was made using one-way ANOVA followed by pairwise comparisons with p value adjustment; p < 0.05 was considered statistically significant.
Results
Wnt1-Cre mediates a conditional expression of the reporter genes in SMCs of the common carotid artery
LacZ-Stopflox/Wnt1-Cre+ mice were generated to label Wnt1+ lineage cells. Staining for X-Gal was found to be positive only in the SMCs of the common carotid artery and was negative for cells of the endothelial layer (Fig. 1a). Likewise, there was no LacZ activity in SMCs in the vena cava (Fig. 1a). These observations were confirmed in RFPflox-GFP/Wnt1-Cre+ mice in which the expression of RFP was switched to GFP in SMCs of the common carotid artery (Fig. 1b). There was no expression of GFP in ECs as well as the SMCs of the vena cava or the descending aorta (because these SMCs were not of neural crest origin) in RFPflox-GFP/Wnt1-Cre+ mice (Fig. 1b). In Wnt1-Cre negative control mice, SMCs in carotid artery were RFP positive (Fig. 1b, left panel). Because neurons express Wnt1 [9], as expected, the vagus nerve was GFP+ in RFPflox-GFP/Wnt1-Cre+ mice (Fig. 1c). These results indicate that Wnt1-Cre reporter mice can be used to specifically label the SMCs in the common carotid artery, and leave SMCs in other vessels unlabeled.
Fig. 1.
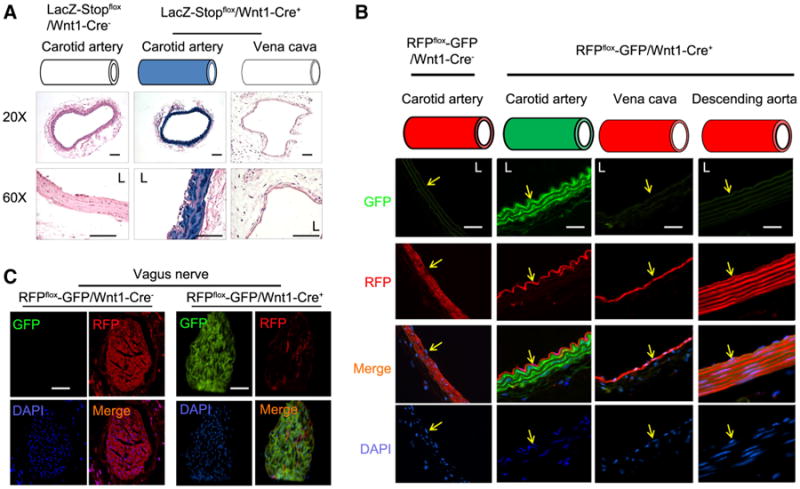
Characterization of Wnt1-Cre-mediated expression of reporter genes in vessels. a The common carotid artery and vena cava were collected from LacZ-Stopflox/Wnt1-Cre+ mice or control mice; X-Gal staining was performed. The blue color (X-Gal positive) was only found in the SMCs in the common carotid artery in LacZ-Stopflox/Wnt1-Cre+ mice (n = 5). b, c The cryo section of the common carotid artery, vena cava, and vagus nerve (c) was prepared from RFPflox/flox-GFP/Wnt1-Cre+ or control mice. The RFP and GFP fluorescence was photographed. DAPI was used to counter stain nuclei. Only the SMCs in carotid artery and vagus nerve were positive for GFP. Represented data were from five mice in each group (scales 50 μm, L, lumen; yellow arrows in b indicate the endothelium)
SMCs from a neighboring artery contribute to neointima formation in artery grafts
When artery grafts were created in WT mice, there was a significant increase in the accumulation of SMA-α-positive cells in the neointima after 1 month, thus leading to a smaller lumen when compared with that in control arteries (Fig. 2a, b). To identify the origin of these neointima cells, artery grafts were created in LacZ-Stopflox/Wnt1-Cre+ mice with the artery donor from LacZ-Stopflox/Wnt1-Cre− mouse. After 1 month, X-Gal staining in artery grafts revealed >90 % of neointima cells as being X-Gal positive, with none in the media of the artery grafts (Fig. 2c, e). These results suggest that X-Gal-positive cells found in the neointima were originated from the SMCs of the neighboring artery, because only SMCs in the host common carotid artery express LacZ. Conversely, when arteries from LacZ-Stopflox/Wnt1-Cre+ mice were grafted into LacZ-Stopflox/Wnt1-Cre− mice, most of the neointima cells did not exhibit X-Gal expression except for a few (Fig. 2d, e). Furthermore, since all SMCs in the media layer were X-Gal positive, this suggested that donor artery SMCs survived during the experiment, but did not contribute to neointima formation (Fig. 2d).
Fig. 2.
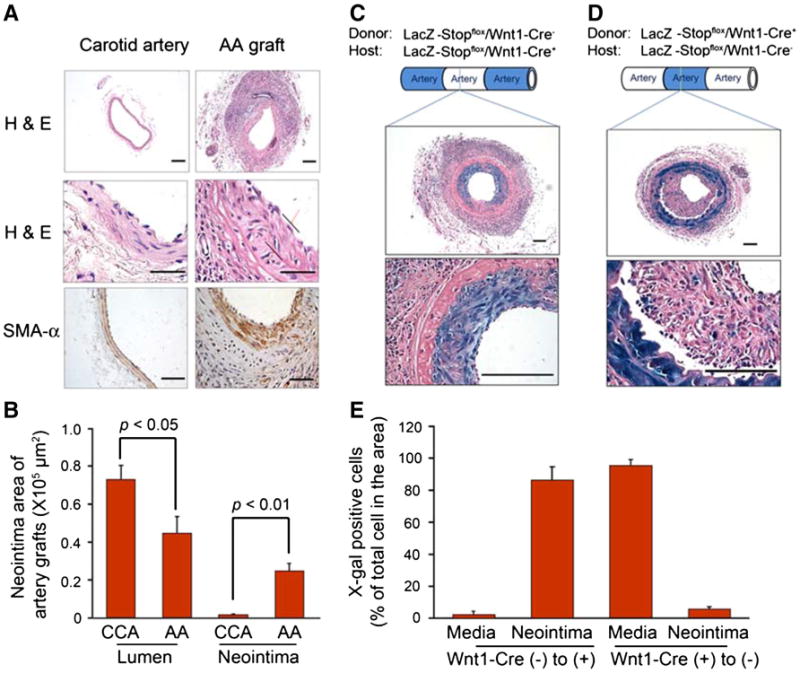
SMCs from neighboring artery contribute to neointima formation in artery grafts. a Artery grafts were created and collected after 1 month. The grafts were stained by H & E (upper panel) and SMA-α (lower panel). b The areas of lumen and neointima in the normal common carotid artery (CCA) and artery graft (AA graft) were measured (n = 5). c Artery grafts were created in LacZ-Stopflox/Wnt1-Cre+ mice with the donor common carotid artery from WT mice, and collected after 1 month. X-Gal staining was performed. The pictures with different magnification (×100 and ×600) are shown. d Artery grafts were created in WT mice with the donor common carotid artery from LacZ-Stopflox/Wnt1-Cre+ mice. X-Gal positive staining was performed. e The X-Gal-positive cells in the neointima found in c and d were counted and summarized (scales in panel a 50 μm; in panel c, d 100 μm; n = 5)
Arterial injury does not activate Wnt1 during neointima formation
We next tested whether Wnt1 begins to express during neointima formation, since such expression would stimulate Wnt1-Cre-mediated DNA recombination, which compromises the specificity to track the media SMCs from the neighboring artery. Immunostaining revealed no Wnt1-positive staining in artery grafts. However, the neointima cells expressed SMA-α and the SMMHC. The isotype IgG control did not show any specific staining for these markers (Fig. 3a). To further examine whether Wnt1 is activated during neointima formation, we performed a femoral arterial injury model in Wnt1-Cre reporter mice. This model was chosen because SMCs in the femoral artery are not neural crest-derived and not pre-labeled in Wnt1-Cre reporter mice (Fig. 3c, e). When the femoral artery was injured in RFPflox-GFP/Wnt1-Cre+ mice, the neointima formed after 1 month (Fig. 3b). The SMA-α-positive cells accumulated in the injured femoral artery but not in the uninjured control artery and there were no GFP-positive cells in the neointima (Fig. 3c, right panel). Only para-vessel nerves stained positively for GFP around the vessels (Fig. 3c). Conversely, there was no Wnt1 expression in the neointima in the injured femoral artery (Fig. 3d, left panel), while as a positive control, the femoral nerve was Wnt1 positive (Fig. 3d, right panel). In addition, the femoral arterial injury model was also performed in LacZ-Stopflox/Wnt1-Cre+ mice. Results obtained through X-Gal staining showed that only para-femoral arterial nerves were stained blue, as opposed to the smooth muscle layer (Fig. 3e, left panel). Likewise, we found that the femoral arterial injury in LacZ-Stopflox/Wnt1-Cre+ mice yielded no X-Gal-positive cells in the neointima, though the femoral nerve (Fig. 3e, right panel) and some para-vessel nerves of the injured artery were X-Gal positive (Fig. 3e, red arrows marked cells in middle panel). These results suggest that Wnt1 gene was not activated and expressed during arterial injury.
Fig. 3.
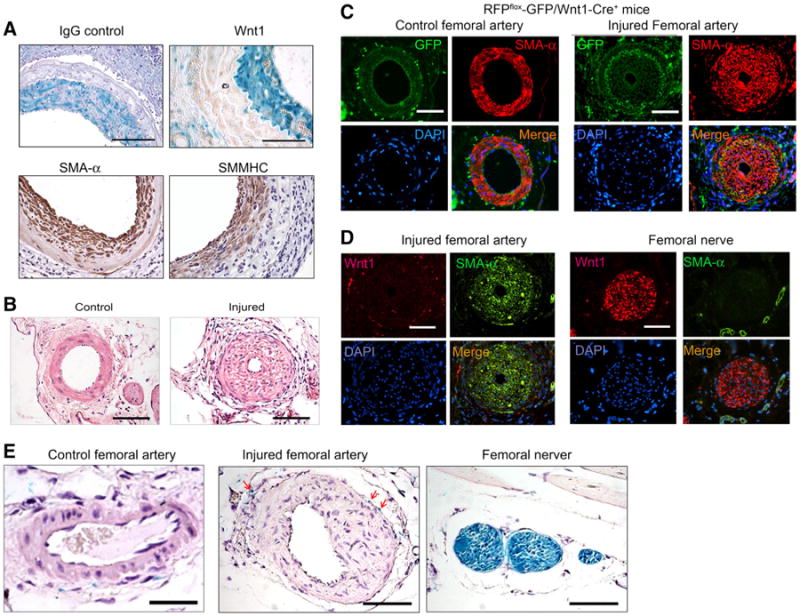
Wnt1 is not expressed in neointima cells in artery injury models. a X-Gal-positive cells did not express Wnt1 in neointima cells from artery grafts created in LacZ-Stopflox/Wnt1-Cre+ mice. The isotype IgG was used as control. Immunostaining of SMC markers, SMA-α and SMMHC, in artery grafts was performed and shown in the lower panel. b Wire injury of the femoral artery induced neointima formation after 1 month (n = 5). c Wnt1-lineage cells are not found in the neointima cells after femoral artery injury. Femoral artery injury was performed in RFPflox-GFP/Wnt1-Cre+ mice. Double staining of GFP and SMA-α was performed in control and injured femoral artery. The right panel shows the injured femoral artery with neointima formation. The non-injured contralateral femoral artery is shown in the left panel (n = 4). d Wnt1 is not expressed in neointima cells in the injured femoral artery. Wnt1 and SMA-α co-immunostaining were performed in the injured femoral artery and normal femoral nerve. Wnt1 expression was only found in femoral nerve. e Femoral artery injury was performed in LacZ-Stopflox/Wnt1-Cre+ mice. X-Gal staining was performed in control and injured femoral arteries (scales in panel a 100 μm; in panel b–e 50 μm; n = 4)
Neighboring arterial SMCs contribute to neointima formation in vein grafts
Vein grafts were created in RFPflox-GFP/Wnt1-Cre+ mice with donor vena cava from WT mice. After 1 month, multiple layers of neointima cells in the vein grafts were SMA-α positive (Fig. 4a, b). To detect whether Wnt1-Cre efficiently regulates GFP expression in the common carotid artery, the GFP was co-stained with SMMHC. As shown in Fig. 4c, all the SMCs were double positive for GFP/SMMHC and SMA-α/SMMHC in the common carotid artery in RFPflox-GFP/Wnt1-Cre+ mice, while the ECs were negatively stained (Fig. 4c, d), indicating that the GFP+ cells are SMCs. In vein grafts created in RFPflox-GFP/Wnt1-Cre+ mice, GFP-positive cells were found in the neointima (Fig. 4e). This suggests that SMCs from the common carotid artery (GFP+) were contributing to the formation of a neointima in the vein grafts. We also noted the presence of some RFP+/GFP− cells in the neointima (Fig. 4e). Double staining for GFP and SMA-α revealed that ∼44 % of GFP-positive cells were also positive for SMA-α (Fig. 4f, g) while, approximately, ∼33 % of GFP negative neointima cells were SMA-α positive (Fig. 4f, g). This suggested that these cells were not derived from the neighboring arterial SMCs and other sources (e.g., multipotent vascular stem cells, endothelial cells) could be involved in SMC accumulation in vein grafts. The presence of ∼10 % of GFP+/SMA-α− cells in the neointima (Fig. 4f, g) indicated that these cells were dedifferentiated SMCs that migrated from the neighboring artery.
Fig. 4.
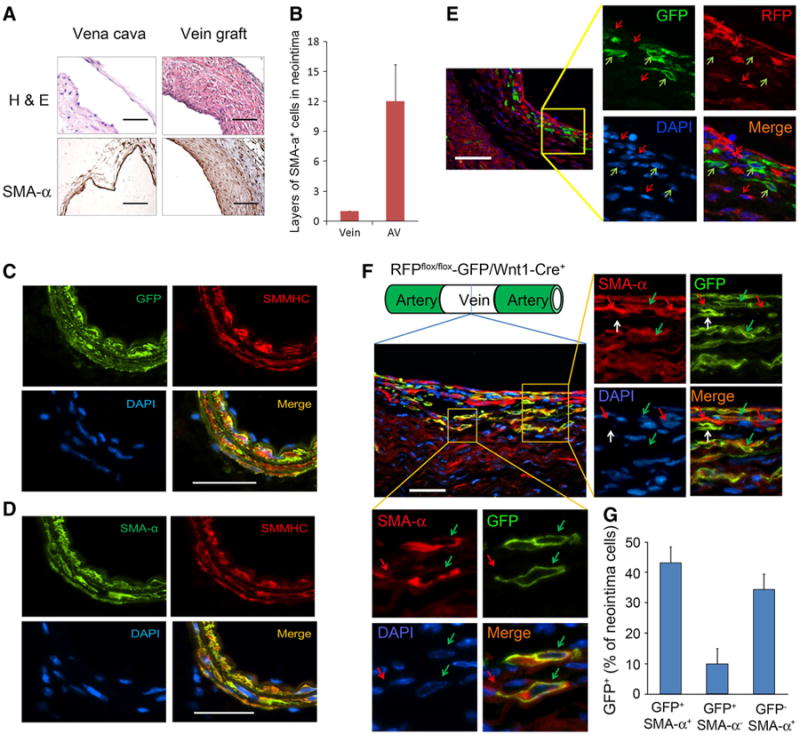
SMCs from the neighboring artery are involved in neointima formation in vein grafts. a, b Vein grafts were created in RFPflox-GFP/Wnt1-Cre+ mice with donor vein from WT mice. h, e SMA-α immunostaining was performed in 1-month vein graft and control vein. c, d Common carotid arteries from RFPflox-GFP/Wnt1-Cre+ mice were collected. Co-immunostaining of the SMC terminal differentiation marker SMMHC with GFP or SMA-α was performed. All the SMCs (SMMHC+ and SMA-α+) were GFP+. e Vein grafts were created in RFPflox-GFP/Wnt1-Cre+ mice with donor vein from WT mice. GFP and RFP were double immunostained. The GFP-positive cells were found in the neointima area, suggesting that the neighboring cells from the common carotid artery were involved in neointima formation. The green arrows indicate the GFP+ cells; red arrows for RFP+ cells. f Double staining of GFP and SMA-α in vein grafts. The framed areas were amplified. The green arrows indicate the GFP+/SMA-α+ cells; red arrows for GFP−/SMA-α+ cells; white arrows for GFP+/SMA-α− cells. g The percentage of labeled cells in SMA-α+ population (scales 50 μm; n = 5)
Wnt1 is not expressed in neointima cells in vein grafts
GFP-positive cells in vein grafts created in RFPflox-GFP/Wnt1-Cre+ mice could arise from two sources: (1) GFP-positive cells from the adjacent artery; or (2) neointima cells expressing Wnt1 which induced Wnt1-Cre activation and GFP expression. To distinguish between the two, Wnt1 expression was determined by immunostaining. Results showed that Wnt1 was only expressed in the vagus nerve, and was absent from the neointima cells (Fig. 5a, upper panel). On the other hand, GFP-positive cells were found in the neointima (Fig. 5a, middle panel), suggesting that these GFP+/Wnt1− cells in the neointima were derived from SMCs in neighboring arteries. Double staining for Wnt1 and GFP further revealed that GFP-positive cells of the neoin-tima did not express Wnt1 (Fig. 5b). As a positive control, there was a strong Wnt1 expression colocalizing with GFP in the vagus nerve near the vein graft (Fig. 5c, upper panel).
Fig. 5.
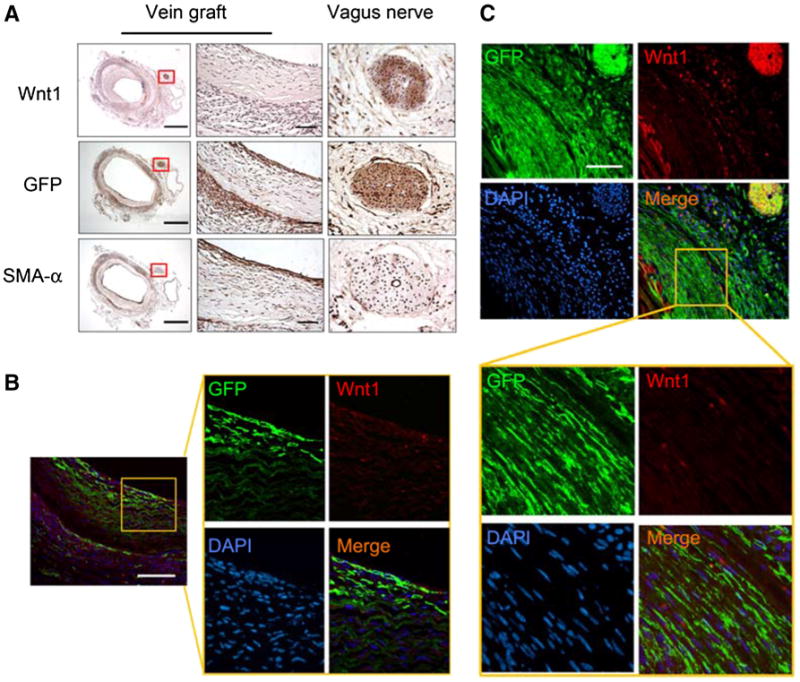
Wnt1 is not expressed in neointima cells in vein grafts. a Vein grafts created in RFPflox-GFP/Wnt1-Cre+ mice were collected and immunohistochemistry was carried out to analyze the expression of Wnt1, GFP, and SMA-α. The vagus nerve, marked with a red frame, positively stained with Wnt1 and GFP (n = 5). b, c Two areas in the vein grafts were selected to perform double staining for Wnt1 and GFP (scales a left panel 500 μm, right panel 50 μm; b 100 μm; c 50 μm; n = 5)
Bone marrow cells do not differentiate into SMCs in vein grafts
Because ∼50 % of neointima cells were not from neighboring arterial SMCs in the vein graft, they could be originating from the bone marrow. In order to explore the contribution of bone marrow cells, the vein grafts were placed in WT mice that had been transplanted with bone marrow cells isolated from RFP transgenic mice. After 1 month, we found bone marrow-derived RFP-positive cells in vein grafts, but these were not colocalized with SMA-α-positive cells (Fig. 6a). We also found that SMA-α-positive cells were terminally differentiated SMCs; they were stained positively by the SMMHC and that none of them co-stained with RFP+ (data not shown). Instead the bone marrow-derived RFP cells co-stained with inflammatory cell markers such as CD45, Mac2, and CD3 (Fig. 6b–f). These cells were mainly located between SMCs in the neointima and the adventitial area (Fig. 6b–e). Few bone marrow-derived cells localized within the endothelium (Fig. 6b–e).
Fig. 6.
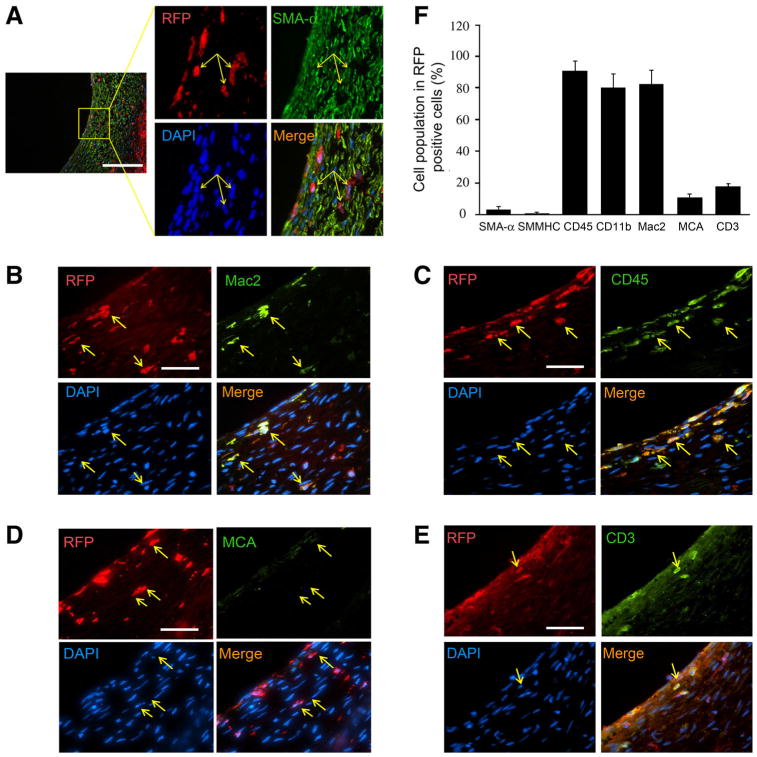
Bone marrow cells do not contribute to SMCs in neointima, but to inflammatory cells. Vein grafts were created in WT mice with transplanted-bone marrow cells from RFP transgenic mice (n = 4). The cryo sections of the 1-month vein grafts were stained with SMC markers: SMA-α (a); macrophage marker, Mac2 (b); monocyte markers, CD45 (c); and neutrophil marker, MCA (d); T-cell marker, CD3 (e). The RFP-positive cells were marked with yellow arrow. f The percentage of the SMCs or inflammatory cells in bone marrow-derived RFP-positive cells were counted and calculated (scales a 100 μm; b–e 50 μm)
IGF-1R inhibitor suppresses SMC proliferation and neointima formation
The above experiments show that SMC migration from anastomosed arteries contributes to neointima formation in both the artery and vein grafts. To explore whether these migrated cells also proliferate, we performed artery and vein grafts and used BrdU to label the proliferating cells. Significant amount of BrdU-positive cells was found in the artery grafts (Fig. 7a). Some BrdU-positive cells co-stained with SMA-α, indicating both migration- and proliferation-mediated SMCs accumulation in neointima in artery (Fig. 7a). Similar observations were confirmed in the vein grafts (Fig. 7b). To examine if blocking IGF-1R inhibits SMC proliferation, quiescent SMCs were treated with IGF-1 (1 ng/ml) in the presence or absence of PPP, the IGF-1R inhibitor. BrdU incorporation was measured. The number of BrdU-positive cells was found to be increased in IGF-1 treated cells compared with results from control. PPP pretreatment blocked IGF-1-induced BrdU incorporation in SMCs (IGF-1, 9.69 ± 1.03 % vs. IGF-1/PPP, 3.63 ± 0.38, p < 0.01, n = 3, Fig. 7c). To further detect the effect of PPP on neointima formation, it was administered (i.p.) in mice with vein grafts and found to suppress the accumulation of the SMCs in the neointima (Fig. 7d, f).
Fig. 7.
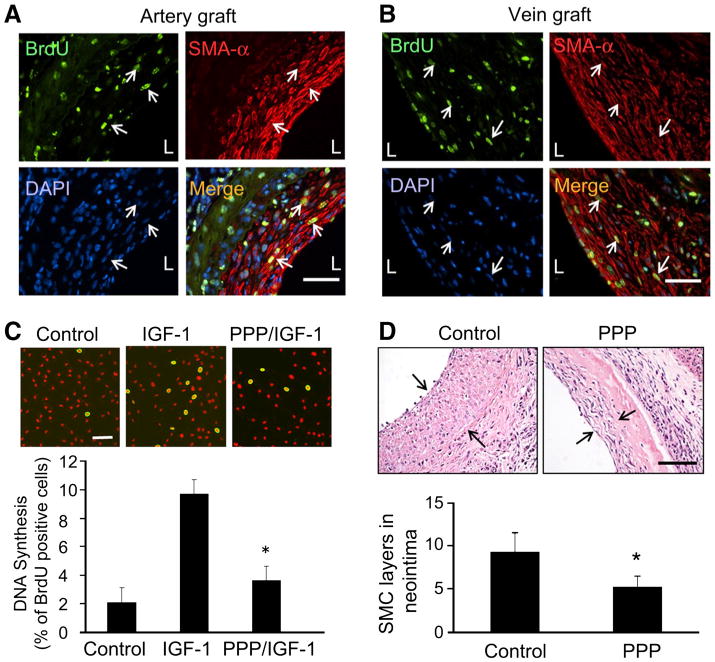
IGF-1R inhibitor inhibits neointima formation. a Artery grafts were performed in WT mice. BrdU was applied i.p. for 7 days before the grafts were collected. Double immunostaining was performed to detect BrdU (green) and SMA-α (red) (n = 3). b Vein grafts were performed in WT mice. BrdU incorporation in SMCs in neointima was detected by immunostaining (n = 3). c SMCs were seeded onto a glass coverslip and kept in serum-free media for 24 h, cells were treated with IGF-1 (10 ng/ml) in the presence or absence of PPP (2 μM) for 24 h. The SMCs were incubated with BrdU for 3 h before fixation. BrdU-positive cells are shown in green; nuclei were counterstained with propidium iodide (red). Cell proliferation is expressed as the percentage of cells that were BrdU-positive as determined in five view fields. d, f Vein grafts were performed in WT mice. After 3 weeks of the surgery, mice were treated with PPP or solvent for 3 weeks. The vein grafts (1 month) were collected and stained with SMA-α. The layers of the SMCs were calculated (n = 4)
Discussion
Restenosis following neointima hyperplasia limits the success of bypass grafts or balloon angioplasty. We examined the source of cells in the neointima in models of artery or vein grafts. The artery graft is a model of events occurring after angioplasty. The mouse vein graft model is used to examine the pathogenesis of atherosclerosis in the vein as might occur in the aorta-coronary saphenous vein graft in human patients [17], since it shares features similar to the vein graft in patients [11, 40].
To identify the source of SMA-α-positive cells of the neointima in artery and vein grafts, we developed a strategy based on lessons from developmental biology. The transgenic mice that express Wnt1-Cre to label local SMCs arising from the common carotid artery were used in our experiments. In the artery–artery graft, >90 % of SMCs in the neointima were traced to the adjacent artery, the donor artery made a small contribution to neointima formation. In the vein graft model, ∼44 % of SMA-α-positive cells in the neointima originated from the neighboring arteries. This is the first report indicating that SMCs from neighboring vessels directly contribute to neointima formation in vein or artery grafts using Wnt1-Cre reporter mice.
The Wnt1 promoter efficiently targets the neural crest-derived SMCs of the cardiac outflow tract composing the common carotid arteries. SMCs from other vasculature (e.g., veins) are not neural crest derived. Therefore, Wnt1-Cre/reporter transgenic mice were used to distinguish the source of SMCs of the cardiac outflow tract from other vasculatures. Several groups have used Wnt1-Cre/reporter mice to study the function of neural crest cells in the development of the cardiac outflow tract [19, 32]. In the current study, we used both LacZ-Stopflox/Wnt1-Cre+ and RFPflox-GFP/Wnt1-Cre+ mice to specifically label SMCs of the common carotid artery. These pre-labeled cells were found in both artery and vein grafts. Thus, the Wnt1-Cre/ reporter mice could be a tool to label and track SMCs in the aortic outflow during vascular remodeling.
The origin of the neointima cells in vein graft models is controversial. Some reports conclude that neointima cells are exclusively composed of donor-originated cells in a venous graft [7]. Others report that bone marrow or “graft-extrinsic” cells are predominant in the neointima [39]. In our experiments, we found that about 54 % of SMA-α-positive cells in the neointima originated from the neighboring artery. Bone marrow cells did not contribute to the SMCs of the neointima in vein grafts as they only co-stained with the inflammatory markers, and were negative for the SMC markers, SMA-α or SMMHC. About 10 % of the neighboring artery-derived GFP+ cells in the neointima cells did not express the SMC markers (SMA-α) (Fig. 4e, f). This could represent dedifferentiated SMCs, since it has been reported that the SMC cell phenotype is lost when contractile SMCs are dedifferentiated into synthetic SMCs [10]. Interestingly, the GFP−/SMA-α+ cells in the vein graft were not from the neighboring artery (Fig. 4e), indicating that there are other sources, in the host or recipient vessels which can be differentiated into SMCs [18]. We speculate that SMCs present in the neointima of the vein graft might be derived from SMC progenitors [8, 38], endothelial cells [4, 8], or adventitial stem cells [18].
In arterial remodeling, a long-standing concept in the field has been that the majority of neointima SMCs are derived from pre-existing media SMCs [21]. Different with this standing, we demonstrated that >90 % of SMCs in the neointima are from neighboring SMCs instead of the injured artery. Our results are consistent with recent findings, which showed that only flanking recipient SMCs contribute to vascular remodeling [12]. This observation is different with reports from several other groups who suggest that adventitial cells are the source of neointima cells in models of artery injury or vein grafts [27–30]. In their report, the authors had used BrdU to label proliferating adventitial fibroblasts. Because BrdU labels all proliferating cells, it could not distinguish the cell origin of fibro-blasts from medial SMCs in the neointima [29, 30].
A recent report by Tang et al. [35] demonstrated that the multipotent vascular stem cells (MVSC) from the arterial wall contribute to neointima formation in the artery injury model. MVSC isolated from arteries and veins can be differentiated into several types of cells, including SMCs. Interestingly, MVSCs were also labeled in the common carotid artery in the Wnt1-Cre reporter mice [35], suggesting that MVSCs could be involved in neointima formation in the vein or artery grafts. Because MVSCs are SMMHC negative [22, 35], while we found that the GFP-positive cells in common carotid artery in Wnt1-Cre+ transgenic mice were SMMHC positive (Fig. 4c), suggest that those GFP/SMMHC positive SMCs are precursors that contribute to neointima cells. We conclude that de-differentiation of SMCs in the anastomosed arteries are the major contributor for neointima formation. To specify the contribution of MVSCs to neotima formation, other strategies will be required to delineate the role of MVSCs. For example, a tamoxifen-inducible SMC-Cre reporter and/or SOX10-Cre reporter mice would be useful tools.
SMC proliferation plays an essential role in neointima formation. Our results also found that BrdU was incorporated in SMCs in the neointima. No BrdU-positive cells were found in the normal artery (data not shown). Thus, from Wnt1-Cre tracking and BrdU-labeling experiments, we conclude that both SMC migration and proliferation are involved in neointima formation. Among many factors that regulate SMC proliferation, growth factors (PDGF, TGF-β1) play an important role in promoting SMC hyperplasia and neointima formation [15, 23]. Earlier, we have shown the suppression of neointima formation in the vein grafts of the knockout IGF-1R mice [5]. Consistent with this report, application of IGF-1R inhibitor, PPP, in mice significantly blocked SMC proliferation and neointima formation.
To summarize, we demonstrate that SMCs from the neighboring artery play an essential role in the composition of neointima cells in both the vein and artery grafts. These findings will guide the development of intervention methods, based on the migration and proliferation of SMCs in vascular remodeling, after angioplasty or coronary artery bypass surgery.
Acknowledgments
We acknowledge Dr. William E Mitch for his constructive suggestions. We also thank Dr. Abha Sharma for critically reading and modifying the manuscript. This work was supported by grants from RO1 DK095867, the American Heart Association Grant 10SDG2780009 (to J.C.), the National Institutes of Health grants R37 and DK37175, and a generous grant from Dr. and Mrs. Harold Selzman.
Abbreviations
- VSMC
Vascular smooth muscle cells
- NC
Neural crest
- Wnt1
Wingless-type MMTV integration site 1 family
Contributor Information
Ming Liang, Department of Nephrology, Guangzhou First People's Hospital, Guangzhou Medical University, Guangzhou, China; Division of Nephrology, Department of Medicine, Baylor College of Medicine, 77030 Houston, TX, USA.
Anlin Liang, Division of Nephrology, Department of Medicine, Baylor College of Medicine, 77030 Houston, TX, USA.
Yun Wang, Division of Nephrology, Department of Medicine, Baylor College of Medicine, 77030 Houston, TX, USA.
Jun Jiang, Division of Nephrology, Department of Medicine, Baylor College of Medicine, 77030 Houston, TX, USA.
Jizhong Cheng, Email: jizhongc@bcm.edu, Division of Nephrology, Department of Medicine, Baylor College of Medicine, 77030 Houston, TX, USA.
References
- 1.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 2.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2696–2702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 3.Caplice NM, Wang S, Tracz M, Croatt AJ, Grande JP, Katusic ZS, Nath KA. Neoangiogenesis and the presence of progenitor cells in the venous limb of an arteriovenous fistula in the rat. Am J Physiol Ren Physiol. 2007;293:F470–F475. doi: 10.1152/ajprenal.00067.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012;2:1684–1696. doi: 10.1016/j.celrep.2012.10.021. doi:10.1016/j. celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol. 2007;27:1744–1751. doi: 10.1161/ATVBAHA.107.147371. [DOI] [PubMed] [Google Scholar]
- 6.Cheng J, Wang Y, Liang A, Jia L, Du J. FSP-1 silencing in bone marrow cells suppresses neointima formation in Vein Graft. Circ Res. 2012;110:230–240. doi: 10.1161/circresaha.111.246025. [DOI] [PubMed] [Google Scholar]
- 7.Cooley BC. Murine model of neointimal formation and AQ stenosis in vein grafts. 2004:1180–1185. doi: 10.1161/01.ATV.0000129330.19057.9f. [DOI] [PubMed] [Google Scholar]
- 8.Diez M, Musri MM, Ferrer E, Barbera JA, Peinado VI. Endothelial progenitor cells undergo an endothelial-to-mesenchymal transition-like process mediated by TGFbetaRI. Cardiovasc Res. 2010;88:502–511. doi: 10.1093/cvr/cvq236. [DOI] [PubMed] [Google Scholar]
- 9.Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- 10.Engelse MA, Lardenoye JHP, Neele JM, Grimbergen JM, de Vries MR, Lamfers MLM, Pannekoek H, Quax PHA, de Vries CJM. Adenoviral activin a expression prevents intimal hyperplasia in human and murine blood vessels by maintaining the contractile smooth muscle cell phenotype. 2002:1128–1134. doi: 10.1161/01.res.0000021044.53156.f5. [DOI] [PubMed] [Google Scholar]
- 11.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 12.Hagensen MK, Shim J, Falk E, Bentzon JF. Flanking recipient vasculature, not circulating progenitor cells, contributes to endothelium and smooth muscle in murine allograft vasculopathy. Arterioscler Thromb Vasc Biol. 2011;31:808–813. doi: 10.1161/ATVBAHA.110.221184. doi:10. 1161/atvbaha.110.221184. [DOI] [PubMed] [Google Scholar]
- 13.Hoglund VJ, Dong XR, Majesky MW. Neointima Formation. Arterioscler Thromb Vasc Biol. 2010;30:1877–1879. doi: 10.1161/ATVBAHA.110.211433. doi:10. 1161/atvbaha.110.211433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK. Origin of neointimal smooth muscle: we've come full circle. Arterioscler Thromb Vasc Biol. 2006;26:2579–2581. doi: 10.1161/01.ATV.0000249623.79871.bc. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Bock G, Wick G, Xu Q. Activation of PDGF receptor alpha in vascular smooth muscle cells by mechanical stress. Faseb J. 1998;12:1135–1142. doi: 10.1096/fasebj.12.12.1135. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Davison F, Ludewig B, Erdel M, Mayr M, Url M, Dietrich H, Xu Q. Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells. Circulation. 2002;106:1834–1839. doi: 10.1161/01.cir.0000031333.86845.dd. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Xu Q. New Mouse Model of Vein Bypass Graft Atherosclerosis. Heart Lung Circ. 2002;11:182–188. doi: 10.1046/j.1444-2892.2002.00138.x. doi:10.1046/j. 1444-2892.2002.00138.x. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Investig. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Cheng L, Li J, Chen M, Zhou D, Lu MM, Proweller A, Epstein JA, Parmacek MS. Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest. 2008;118:515–525. doi: 10.1172/JCI33304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–110. doi: 10.1016/j.semcdb.2006.12.004. doi:10. 1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13:279–284. doi: 10.1097/01.mnh.0000126791.93346.4a. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy E, Hakimjavadi R, Greene C, Mooney CJ, Fitzpatrick E, Collins LE, Loscher CE, Guha S, Morrow D, Redmond EM, Cahill PA. Embryonic rat vascular smooth muscle cells revisited—a model for neonatal, neointimal SMC or differentiated vascular stem cells? Vascular Cell. 2014;6:6. doi: 10.1186/2045-824X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi K, Yokote K, Fujimoto M, Yamashita K, Sakamoto A, Kitahara M, Kawamura H, Maezawa Y, Asaumi S, Tokuhisa T, Mori S, Saito Y. Targeted disruption of TGF-{beta}-Smad3 signaling leads to enhanced neointimal hyperplasia with diminished matrix deposition in response to vascular injury. Circ Res. 2005;96:904–912. doi: 10.1161/01.res.0000163980.55495.44. [DOI] [PubMed] [Google Scholar]
- 24.Mead TJ, Yutzey KE. Notch pathway regulation of neural crest cell development in vivo. Dev Dyn. 2012;241:376–389. doi: 10.1002/dvdy.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.CIR.97.9.916. [DOI] [PubMed] [Google Scholar]
- 26.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 27.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.CIR.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.CIR.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 29.Shi Y, O'Brien JE, Jr, Mannion JD, Morrison RC, Chung W, Fard A, Zalewski A. Remodeling of autologous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation. 1997;95:2684–2693. doi: 10.1161/01.CIR.95.12.2684. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Pieniek M, Fard A, O'Brien J, Mannion JD, Zalewski A. Adventitial remodeling after coronary arterial injury. Circulation. 1996;93:340–348. doi: 10.1161/01.CIR.93.2.340. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu T, De Wispelaere A, Winkler M, D'Souza T, Caylor J, Chen L, Dastvan F, Deou J, Cho A, Larena-Avellaneda A, Reidy M, Daum G. Sphingosine-1-Phosphate Receptor 3 Promotes Neointimal Hyperplasia in Mouse Iliac-Femoral Arteries. Arterioscler Thromb Vasc Biol. 2012;32:955–961. doi: 10.1161/atvbaha.111.241034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh N, Trivedi CM, Lu M, Mullican SE, Lazar MA, Epstein JA. Histone Deacetylase 3 Regulates Smooth Muscle Differentiation in Neural Crest Cells and Development of the Cardiac Outflow Tract/Novelty and Significance. Circ Res. 2011;109:1240–1249. doi: 10.1161/circresaha.111.255067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol. 2009;86:1111–1118. doi: 10.1189/jlb.0309132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka K, Sata M, Natori T, Kim-Kaneyama JR, Nose K, Shibanuma M, Hirata Y, Nagai R. Circulating progenitor cells contribute to neointimal formation in nonirradiated chimeric mice. Faseb J. 2008;22:428–436. doi: 10.1096/fj.06-6884com. [DOI] [PubMed] [Google Scholar]
- 35.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldo KL, Kumiski D, Kirby ML. Cardiac neural crest is essential for the persistence rather than the formation of an arch artery. Dev Dyn. 1996;205:281–292. doi: 10.1002/(SICI)1097-0177(199603. [DOI] [PubMed] [Google Scholar]
- 37.Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol. 2006;26:257–266. doi: 10.1161/01.ATV.0000198239.41189.5d. [DOI] [PubMed] [Google Scholar]
- 38.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C. SDF-1α/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.res.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Freedman NJ, Brian L, Peppel K. Graft-Extrinsic Cells Predominate in Vein Graft Arterialization. Athro Throm Vascul Biol. 2004;24:470–476. doi: 10.1161/01.atv.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]
- 40.Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse Model of Venous Bypass Graft Arteriosclerosis. Am J Pathol. 1998;153:1301–1310. doi: 10.1152/ajplung.00378.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


