Summary
Purpose
Neurobehavioral comorbidities are common in pediatric epilepsy with enduring adverse effects on functioning, but their neuroanatomical underpinning is unclear. Striatal and thalamic abnormalities have been associated with childhood-onset epilepsies, suggesting that epilepsy-related changes in the subcortical circuit might be associated with the combordities of children with epilepsy. We aimed to compare subcortical volumes and their relationship with age in children with complex partial seizures (CPS), childhood absence epilepsy (CAE), and healthy controls (HC). We examined the shared versus unique structural-functional relationships of these volumes with behavior problems, intelligence, language, peer interaction, and epilepsy variables in these two epilepsy syndromes.
Methods
We investigated volumetric differences of caudate, putamen, pallidum, and thalamus in children with CPS (N= 21), CAE (N=20), and HC (N=27). Study subjects underwent structural MRI, intelligence, and language testing. Parent-completed Child Behavior Checklists provided behavior problem and peer interaction scores. We examined the association of age, IQ, language, behavioral problems, and epilepsy variables with subcortical volumes that were significantly different between the children with epilepsy and HC.
Results
Both children with CPS and CAE exhibited significantly smaller left thalamic volume compared to HC. In terms of developmental trajectory, greater thalamic volume was significantly correlated with increasing age in children with CPS and CAE but not in HC. With regard to the comorbidities, reduced left thalamic volumes were related to more social problems in children with CPS and CAE. Smaller left thalamic volumes in children with CPS were also associated with poor attention, lower IQ and language scores, and impaired peer interaction.
Significance
Our study is the first to directly compare and detect shared thalamic structural abnormalities in children with CPS and CAE. These findings highlight the vulnerability of the thalamus and provide important new insights on its possible role in the neurobehavioral comorbidities of childhood-onset epilepsy.
Introduction
Behavior problems, as well as impaired cognition, language, and social skills are common comorbidities of pediatric epilepsy with enduring adverse effects on functioning (see review in Lin et al.).1 Population-based epidemiological investigations have uniformly documented higher rates of Attention Deficit Hyperactive Disorder (ADHD), anxiety disorders and depression, as well as lower IQ and poor social skills in children with epilepsy compared to children with other medical problems,2 healthy controls,3 and siblings without epilepsy.4 These behavioral, cognitive, linguistic, and social comorbidities can collectively exert a negative impact on daily activities, such as relationships within the family, peer interactions, school performance, and extracurricular activities.5
There is now substantial evidence indicating that childhood-onset epilepsy is associated with altered brain development6–9 and these structural abnormalities are linked to neurobehavioral comorbidities.9–14 In healthy growing children, cortical gray matter volume and thickness declines with increasing age.15 In addition to age, the dynamic process of change in cortical thickness is associated with the maturation of intellectual abilities.16 Against this background of expected developmental trajectory, Tosun and colleagues, in a cross-sectional study, showed that pruning of the frontal and temporal regions is altered in children with complex partial seizures (CPS). Specifically, children with CPS with average IQ demonstrated greater cortical thickness than controls whereas those with below average IQ scores show more thinning with age compared to typically developing children.17 Behavior problems and linguistic deficits are also associated with abnormal cortical development in pediatric epilepsy. Smaller inferior frontal gyrus white matter volumes are related to the presence of a psychiatric diagnosis, mainly ADHD in children with chronic CPS.13 Further, frontal lobe volumes are increased in children with new-onset epilepsy and comorbid ADHD relative to both children with epilepsy but without comorbid ADHD and to controls.18 Structural abnormalities involving language-related cortex are associated with impaired language skill in children with epilepsy with average intelligence.19
Similar to the cortex, subcortical regions undergo age-related developmental changes with increasing volumes in childhood followed by decreasing volumes in adolescence.20–22 Although the link between age and subcortical volumes has not been extensively examined in childhood epilepsy, several lines of evidence suggest that children with epilepsy might have aberrant subcortical developmental trajectory compared to typically developing peers. First, volumetric reductions in the striatum and thalamus have been found in children with localization-related epilepsy23 and idiopathic generalized epilepsy8, 10, 24, 25 compared to their healthy peers. Second, thalamic and striatal volumetric abnormalities are evident in children with new-onset epilepsy, thus implying a neurodevelopmental impact of childhood epilepsy on subcortical maturation prior to the onset of seizures.8, 9 Third, subcortical development is closely linked to cortical development in healthy children.21 Given our previous findings of abnormal age-related cortical development in childhood epilepsy,26 we hypothesized that subcortical development might also deviate from normal.
Subcortical structural volumes are related to cognitive performance in pediatric epilepsy. In children with new-onset juvenile myoclonic epilepsy, baseline frontothalamic volume is associated with deficits in executive function,10 and striatal enlargement predicts better executive function performances in children with new-onset benign epilepsy with centrotemporal spikes.9 These subcortical structures that are vulnerable to childhood-onset epilepsy are also implicated in pediatric psychiatric disorders. Specifically, structural and functional abnormalities in the thalamus and basal ganglia are found in children with ADHD,27 depression,28 internalizing symptoms,29 and anxiety disorder.30
Therefore, in the current study, we focused on subcortical regions in the corticostriatal circuit that have been implicated in both childhood-onset epilepsy and neuropsychiatric disorders, including the caudate, putamen, pallidum, and thalamus. Among these subcortical regions, we targeted structures that would differentiate children with localization related epilepsy (LRE) and idiopathic generalized epilepsy (IGE) from healthy controls (HC). Cryptogenic epilepsy with CPS and childhood absence epilepsy (CAE) were chosen as epilepsy syndromes that represent the two respective broad band epilepsy diagnoses, namely LRE and IGE. We predicted that children with CPS and CAE would have significantly smaller volume of the striatum and thalamus compared to the HC group. We also hypothesized that we would identify a different age relationship with striatal and thalamic volumes in the epilepsy and HC groups. We then examined the shared versus unique structural-functional relationships with behavior problems, intelligence, language, peer interaction, and epilepsy variables in these two epilepsy syndromes. In terms of behavioral problems, we focused on attention problems (e.g. day dreaming, difficulties with concentration, social problems (e.g. poor interpersonal skills and/or school performances, few hobbies), and thought problems (e.g. impaired or illogical reasoning, disorganized communication skills) because a recent meta-analyzes (N=2434) showed that these problems were more common in childhood epilepsy compared to other pediatric chronic diseases.31 Several studies have also shown that children with epilepsy have impaired social competence and peer interaction (See review in Hamiwka et al.).32 Our previous study also linked linguistic skills with frontal lobe white matter volumes.19 Given the integral connections among thalamus, basal ganglia, and prefrontal cortex33 and evidence for the role of the thalamus in language (See review in Llano et al.),34 we hypothesized that subcortical volumes will be related to linguistic skills. Therefore, among the children with epilepsy, we posited that smaller striatal and thalamic volumes will be related to more attention, social, and thought problems; lower IQ; impaired language skill; poor peer interaction; and severity of epilepsy. Together with previously described cortical findings, these predicted findings might provide a neuroimaging signature of the neurobehavioral comorbidities of pediatric epilepsy.
Methods
Participants
This study was approved by the Institutional Review Boards of both the University of California, Los Angeles and the University of California, Irvine. Study participants included 20 children with CPS, 21 children with CAE, and 27 HC, aged 6–16 years. The primary study inclusion criterion for each epilepsy subject was that he/she had a diagnosis of epilepsy (CPS or CAE) and at least one seizure in the year prior to participation in the study.
Participants were recruited from tertiary centers (UCLA and USC) (N= 16, 40%) as well as community clinics (Los Angeles and Anaheim Kaiser Permanente) and the San Diego Chapter of Epilepsy Foundation (N= 24, 60%). A board certified pediatric neurologist made the diagnosis of epilepsy (CPS or CAE), according to criteria of the International classification of epilepsy and all children with epilepsy experienced at least one seizure in the past year.35 The diagnosis of CPS was made based on clinical criteria of having focal seizures with alteration of consciousness of unknown cause and normal findings on clinical magnetic resonance imaging scans (MRIs). The diagnosis of CAE was based on clinical evidence of absence seizures induced by hyperventilation and EEGs showing the typical 3-Hz generalized spike and wave discharges. We excluded children with other types of epilepsy (i.e. mixed seizure disorder, juvenile myoclonic epilepsy), prior epilepsy surgery, neurological illness other than epilepsy, metabolic disorders, hearing impairments, intellectual disability, and bilingual speakers of American English who did not attend English speaking schools or speak English at home.
Socioeconomic status was based on the parental occupational and educational status, using the Hollingshead two factor index.36 Hollingshead levels I and II were classified as high and levels III-V were classified as low. Table 1 presents the demographic characteristics of the study participants. The three study groups did not differ significantly on age, gender, ethnicity, and socioeconomic status.
Table 1.
Demographic, comorbidity, and epilepsy variables of study groups
| Variables | HC (n=27) |
CPS (n=20) |
CAE (n=21) |
|---|---|---|---|
| Chronological age (yr) (SD) | 10.9 (2.6) | 10.9 (2.7) | 9.6 (2.1) |
| Sex (%) | |||
| Male | 52 | 45 | 29 |
| Female | 48 | 55 | 71 |
| Ethnicity (%) | |||
| Caucasian | 37 | 53 | 43 |
| Non-Caucasian | 63 | 47 | 57 |
| Socioeconomic status (%) | |||
| High | 26 | 20 | 24 |
| Low | 74 | 80 | 76 |
| Age of seizure onset (yr) (SD) | 6.9(3.7) | 7.0 (2.1) | |
| Duration of illness (yr) (SD) | 4.2 (2.4) | 2.6 (2.5) | |
| Seizure frequency per year (log) | 2.9 (2.7) | 5.2 (3.8) | |
| Prolonged seizures (%) | 25 | 9.5 | |
| Febrile seizures (%) | 10 | 4.7 | |
| Antiepileptic drugs (%) | |||
| None | 0 | 9.5 | |
| Monotherapy | 70.0 | 61.9 | |
| Polytherapy | 30.0 | 28.6 | |
| Full Scale IQa | 109.8(12.9) | 94.5 (12.9) | 105.6 (15.9) |
| SLQb | 103.8(16.3) | 93.2 (14.7) | 98.9 (17.2) |
| CBCL Scores | |||
| Social Problemsc | 51.0 (2.9) | 58.6 (8.3) | 55.7 (7.9) |
| Thought Problemsd | 51.7 (3.9) | 58.8 (6.7) | 59.8 (7.9) |
| Attention Problemse | 51.9 (2.6) | 59.9 (7.8) | 61.7 (10.6) |
| Peer Interactionf | 48.5 (5.4) | 39.6 (9.8) | 44.4 (7.3) |
CPS significantly lower than both CAE (t(65) = 2.62, p = .01) and HC (t(65) = 3.75, p = .0004)
Speech language quotient for CPS significantly lower than for HC (t(65) = 2.21, p = .03)
Both CPS (t(65) = 3.76, p = .0004) and CAE (t(65) = 2.44, p = .02) significantly higher than HC
Both CPS (t(65) = 3.67, p = .0005) and CAE (t(65) = 4.35, p = .0001) significantly higher than HC
Both CPS (t(65) = 3.49, p = .001) and CAE (t(65) = 4.90, p = .0001) significantly higher than HC
CPS significantly lower than both CAE (t(65) = 1.96, p = .05) and HC (t(65) = 3.81, p = .0003)
The parents’ reports and children’s medical records provided information on seizure frequency, antiepileptic drugs (AEDs), age of onset, illness duration, as well as the number of febrile convulsions and prolonged seizures (i.e., > 5 minutes) (Table 1). Of the 20 children with CPS, 7 had no focal epileptic activity on EEGs conducted at the time of the initial epilepsy diagnosis; 3 had left, 3 had right, 7 had bilateral epileptic activity; and 12 had epileptic activity in the fronto-temporal region. Seven children with CPS had background slowing, and one had secondary generalization. Of the 21 children with CAE, 2 had generalized tonic-clonic convulsions, and 1 had background slowing.
To include control subjects from ethnic and socioeconomic status backgrounds similar to the CAE group, we recruited the normal subjects from four public and two private Los Angeles schools. The study coordinator screened potential participants for neurological, psychiatric, language, and hearing disorders through a telephone conversation with a parent. In children without epilepsy, we also excluded children with ADHD, depression, and anxiety disorder in the past or who met criteria for these disorders in a structured psychiatric interview once enrolled in the study.
Procedures
Image acquisition
MRI data were obtained at UCLA with a 1.5-Tesla GE Signa MR scanner. T1-weighted sagittal spoiled gradient (SPGR) images were collected using an imaging acquisition matrix of 256 × 256 × 124 and slice thickness of 1.2 mm. Imaging parameters were as follows: repetition time = 24 ms, echo time = 9 ms, flip = 22°, field of view = 240 mm × 240 mm, number of excitations = 2.
Image processing
Image processing and analysis were performed using tools from the Oxford Center for Functional MRI of the Brain (FMRIB, FSL version 4.1.5, http://www.fmrib.ox.ac.uk/fsl). T1-weighted images were initially skull-stripped using the Brain Extraction Tool (BET)37 and each image was visually inspected for proper brain extraction. Brain extracted images were then processed using FMRIB’s Automated Segmentation Tool (FAST)38 to correct for spatial intensity variations and segment images into gray matter, white matter, and CSF. Total intracranial volume was calculated by summing the volumes of gray matter, white matter, and CSF, as generated by FAST.
Subcortical gray matter structures, including caudate, putamen, pallidum and thalamus, were automatically segmented using FMRIB’s Integrated Registration and Segmentation Tool (FIRST).39 FIRST is a segmentation tool that is based on a template originating from manually segmented images, with subcortical labels parameterized as surface meshes. This process first registered the T1 images to the template via 12 degrees of freedom affine transformation, and then 12 degrees of freedom registration to a standard MNI 152 template subcortical mask. This subcortical mask was then used to label and segment subcortical structures based on T1 image intensity. Image registration and subcortical segmentations were visually inspected to confirm accuracy of the results. Subcortical volumes obtained from automated segmentation have been shown to be highly comparable to manual labeling techniques.39
Behavior, cognition, language, and peer interaction
Child Behavior Checklist (CBCL): Parents completed the 118 behavioral problem and 20 social competence items of the CBCL40 which includes two major scales: a behavioral problem scale and a social competence scale. Although the CBCL generates broad band (i.e., externalizing, internalizing) and narrow band scales, in this study we targeted behavior problems that were more common in childhood epilepsy compared to other chronic pediatric illnesses: 1) attention problems, 2) social problems, and, 3) thought problems.31 Individual item intraclass correlations (ICC) are greater than 0.90. Stability of ICCs over a 3-month period is 0.84 for behavior problems and 0.97 for social competencies. Test/retest reliability is 0.89. Tests of criterion-related validity for referred/non-referred children support the validity of the instrument and normative data are extensive.40 The cut point for borderline/clinically significant pathology in this study was based on the age and gender normed T score of 67 for the narrow band scales.40
The three social subscales measure competencies in the following domains: activities (e.g., sports, hobbies); social (e.g., friendships, interpersonal skills); and school (e.g., performance, ability, school problems). In this paper we focus on the peer friendship and interaction component, which has a cut point of 30. Scores below 30 are in the clinical range.
IQ: The Wechsler Intelligence Scale for Children-3rd edition (WISC-III),41 administered to the study participants, generated Full Scale, Verbal, and Performance IQ scores.
The Test of Language Development (TOLD):42
The TOLD has 3 forms: the TOLD-2 Primary for children aged 4–8 years; the TOLD-2 Intermediate for children aged 8–12 years; and the TOAL for adolescents 12–18 years. Each form of the TOLD-2 consists of a series of subtests through which it assesses both vocabulary and grammar. We administered the TOLD Primary to 26.5%, the TOLD Intermediate to 57.3%, and the TOAL to 16.2% of the children.42 Spoken language quotient (SLQ) derived from each of these tests was used as an independent variable.
Statistical analysis
We first compared volumes of caudate, putamen, pallidum, and thalamus among the three groups of children, CPS, CAE and HC of comparable age, using mixed models with repeated measures, with group (CPS, CAE, HC) as the inter-subject and hemisphere (left, right) as the intra-subject variable. Four mixed models were estimated, one for each subcortical volume, and age, gender and total intracranial volumes were used as covariates. For those volumes that were significantly different between the groups, secondary analyses were conducted to determine which hemisphere was driving the findings. Then, to reduce the number of comparisons, only those specific subcortical volumes where children with epilepsy were significantly different from controls were further analyzed. Within each group, we examined the association of these volumes with age, IQ, SLQ, CBCL behavior problem scores and peer interaction scores, as well as epilepsy-related variables using Spearman correlation coefficients. The associations with the CBCL scores and epilepsy related variables were studied only within the two epilepsy groups, due to the limited range of CBCL scores within the HC group. To accommodate non-linear relationships between variables, we used rank-based Spearman correlations to study the associations. For the plots depicting the significant associations, we employed a loess procedure (as implemented in PROC LOESS of SAS v9.3) to determine the best fit. The loess procedure fits weighted quadratic or linear least squares regression to localized subsets of the data. This is done at each point at which the regression line is to be estimated, thus building a best fit point by point. The epilepsy-related variables that were studied included age of seizure onset, duration of illness (time from age of onset to participation in study), log seizure frequency in the year prior to recruitment to the study, and the number of AEDs subdivided into no AEDs, monotherapy, and polytherapy. All tests were two-tailed and an alpha level of 0.05 was adopted for all inferences.
Results
Subcortical volumes: Group comparisons
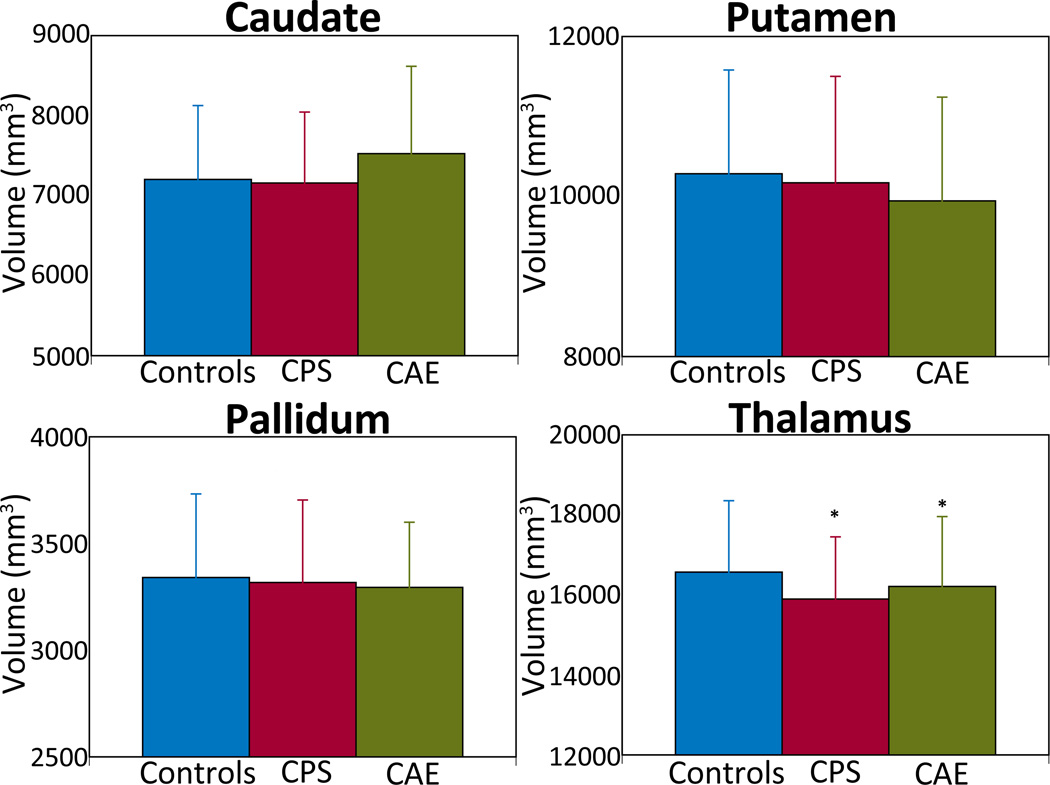
A significant group difference was found for thalamus volumes, controlling for the effects of gender, age, and total intracranial volume (F(2,62) = 3.37, p=0.04). The interaction between hemisphere and group was significant (F(2,64) = 3.75, p=0.03), and post-hoc pair-wise analyses showed left thalamic volumes were reduced in CPS (8036.9±802.6; t(64) = −2.84, p=0.01) and CAE (8146.2±899.7; t(64) = −2.02, p=0.05) compared to HC (8419.6+911.2). There were no significant group differences in the other subcortical regions.
Thalamus volume asymmetry: Group comparisons
Given that the thalamic volumes exhibited significant group differences, we then examined volume asymmetries (left - right) among the three study groups. A significant group difference for thalamic asymmetry was found, after controlling for age and gender (F(2, 62)=3.93; p=0.02). Post-hoc pair-wise analyses revealed that the CAE group (89.6±195.2 mm3) had significantly less leftward asymmetry compared to controls (294.8±228.5 mm3); t(62) = −2.78, p=0.007), while the difference between the CPS group (176.5±341.6 mm3) and controls approached statistical significance (t(62) = −1.67, p=0.10).
Cognitive, linguistic, behavior and social profiles
Table 1 presents the cognitive, linguistic, behavior and social profiles of the three groups. While there were no significant between-group differences in the demographic variables, the CPS group was significantly more impaired than the other groups in cognition and peer interaction, and both epilepsy groups exhibited significantly more behavior problems than the HC. In addition, the CPS group was more impaired than the HC on the language measure.
Association of volumes with comorbidities and epilepsy variables
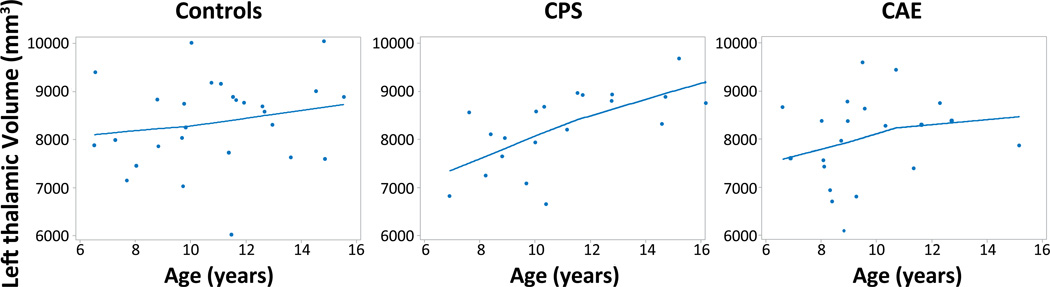
As described in the statistical analysis section, since the left thalamus volumes were significantly different between groups, we investigated the association of only left thalamus volumes with age, IQ, SLQ, behavior and social profiles. The left thalamus volume was positively associated with age in the CPS group (rs=0.72, p=0.0008) and CAE group (rs=0.49, p=0.03), while controlling for duration of illness (Figure 2). Age was not related to thalamic volume in the HC group (rs=0.22, p=0.3). Post-hoc analyses revealed that age was significantly correlated with duration of illness in the CAE group (CAE: rs=0.64, p=0.002) but not in the CPS group (rs=0.08, p=0.75). However, no significant relationship was found between thalamic volume and age of epilepsy onset, seizure frequency, and number of AEDs.
Figure 2.
Plot of left thalamus volumes versus age for controls, CPS, and CAE. The lines shown were the best fit lines determined by a loess (local regression) procedure.
In the CPS group, the left thalamic volume was significantly linked to Full Scale IQ (rs=0.52, p=0.03) and SLQ (rs=0.58, p=0.01) scores while controlling for age and gender, but these relationships did not reach significance in the CAE (Full Scale IQ: rs=0.39, p=0.10; SLQ: rs=0.30, p=0.20) or HC groups (Full Scale IQ: rs=0.30, p=0.14; SLQ: rs=0.30, p=0.16). In CPS and CAE, the left thalamic volume was significantly correlated with CBCL social problem scores (CPS, r=−0.52, p=0.04; CAE, r=−0.46, p=0.05). In CPS, left thalamus volume was also associated with CBCL attention problem (r=−0.54, p=0.04) and peer interaction scores (r = .53, p = .04).
Discussion
Four primary findings were derived from examining the subcortical structural integrity of children with CPS and CAE: 1) Among subcortical regions measured, both children with CPS and CAE exhibited significantly smaller left thalamic volume compared to HC, 2) In terms of developmental trajectory, greater thalamic volume was correlated with increasing age in children with CPS and CAE but not in HC, 3) With regard to the comorbidities, reduced left thalamic volumes were related to more social problems in children with CAE and CPS, and, 4) Smaller left thalamic volumes in children with CPS were also associated with poor attention, lower IQ and SLQ scores, as well as impaired peer interaction. These findings highlight the age-related vulnerability of the thalamus in children with epilepsy and its association with unique versus shared functional-structural relationships in CPS and CAE.
Reduced thalamic volume
Our study is the first to directly compare and detect shared thalamic structural abnormalities in children with CPS and CAE with similar age of seizure onset, duration of illness, mean chronological age, and demographic features, as well as MRI scanner and protocol. Several lines of evidence suggest that the thalamus is critical for seizure generation and propagation in both CAE and CPS. Animal models of CAE showed that the signature 3- to 4-Hz spike-and-wave discharges likely reflect aberrant thalamocortical oscillations,43 and AEDs that specifically target T-type calcium channels in the thalamus effectively block this rhythm.44 Volumetric reductions in the thalamus have been documented in children with CAE.24, 25 Functional neuroimaging and electrophysiology studies45, 46 also implicate the thalamus in seizure generation and propagation. Thalamic volumes are also reduced in children6, 23 and adults47, 48 with CPS and the degree of volume loss has been correlated with seizure duration.49 Furthermore, a number of studies showing microstructure50 and metabolic abnormalities51 also support thalamic involvement in CPS.
In the current study, we showed that thalamic volumes were selectively reduced in children with epilepsy, whereas basal ganglia volumes were preserved relative to healthy controls. Consistent with existing literature, thalamus abnormalities have been frequently found in pediatric epilepsy8, 10, 23–25, 52 but the involvement of basal ganglia structures has been less common. In contrast to our findings, Luo et al. showed that basal ganglia as well as thalamic volumes were reduced in children with CAE, but age differences among the participants of these two studies may have contributed to the discrepant findings. The average age of the study participants in Luo and coworker’s study (12.1±5.1 years old)25 was older than our study cohort (9.6±2.1 years old). Interestingly, neuroimaging studies in adults have consistently demonstrated basal ganglia structural abnormalities in LRE53–57 and IGE.58, 59 The cross-sectional design of our study precludes examination of the origin, development, and trajectory of subcortical brain structural abnormalities. We, nevertheless, speculate that left thalamic abnormality may precede the onset of epilepsy or occur early in the course of the illness, while basal ganglia abnormalities might emerge with longer duration of epilepsy (i.e., in the older youth in Luo et al. and in adults with epilepsy).
It is unclear why the thalamic atrophy was found on the left rather than the right. The left hemisphere might be more vulnerable to developmental insults due to a more protracted maturation process. Supporting this explanation, gene transcription analyses of the embryonic human brain reveal less expression of genes that are critical for cortical development in the left hemisphere, and suggest that left hemisphere development lags behind that of the right hemisphere.60 In addition, lower cerebral blood volume and oxygenation in the left compared to the right hemisphere of newborns implies that a delayed maturation in the left hemisphere may enhance its plasticity to environmental stimuli as well as its vulnerability to insults.61 Finally, patients with temporal lobe epilepsy (TLE) arising from the left side exhibit greater anatomical abnormalities than patients with right-sided TLE.62
Thalamic asymmetry
The significantly decreased leftward asymmetry in the CAE and trend in the CPS group might reflect the selective left thalamic volume reduction in our study. This finding further emphasizes that the left thalamus might be more susceptible than the right thalamus to the neurodevelopmental impact of childhood-onset epilepsy. Although previous studies have demonstrated mixed findings in terms of thalamic volumetric asymmetries in healthy children and adults, other than rightward asymmetry in Xie et al.,63 the general trend corroborates our leftward asymmetry findings.22, 48, 64 A recent diffusion tensor imaging study also revealed that thalamic-prefrontal connectivity was greater on the left side compared to the right in healthy children between the ages of 8 and 17 years old,65 the age range of the children in our study. Although the developmental origin of leftward thalamic asymmetry is unknown, several authors have speculated that hemispheric lateralization of language and cognition might underlie this finding.66 In children with epilepsy, we showed that the normal leftward asymmetry is disrupted, with the CAE group showing significantly reduced asymmetry and the CPS group a trend for reduction.
Thalamic volume and age
Although cross-sectional, our study showed different age-related trajectories in the CPS, CAE, and HC groups. In contrast to normal subcortical developmental patterns of an inverted “U” curve, with volumetric increases in childhood followed by decreases in adolescence,20–22 we did not find an association between left thalamic volume and age in our healthy controls. The narrower age range and modest sample size of our healthy cohort might have obscured important normal developmental trajectories. Specifically, our mean age of 10.9 years (standard deviation = 2.6) in the HC group likely fell on the plateau of the inverted “U” curve, evident in larger normative studies. Yet, despite the similar age range in the two epilepsy and control groups, the statistically significant positive correlation between left thalamic volume and age in the epilepsy groups suggest a different developmental trajectory compared to typical developing children.
Relationships between thalamic volume and comorbidities
This is the first study to demonstrate involvement of the thalamus in behavior problems, lower IQ, impaired language, and poor peer interaction in children with epilepsy. Two of the three problem behaviors thought to occur more frequently in childhood epilepsy than other chronic pediatric illnesses,31 social problems and attention problems, were associated with reduced left thalamic volumes. The role of the orbital frontal cortex in regulation of emotions and social decision-making, 67 together with the connection between the thalamus and this brain region68 probably underlies our findings. In addition, the shared association between social problem scores and left thalamic volume in the CPS and CAE groups might also reflect the significantly smaller orbital frontal gyrus volumes in both children with CPS13 and CAE19 compared to age and gender matched healthy controls. However, it is important to emphasize that the thalamus is composed of several nuclei with distinct thalamocortical connections33 serving multiple different functions, including sensory integration, language, memory, attention, and executive function.34, 69, 70 Furthermore, previous studies suggest different patterns of thalamic involvement in IGE71, 72 versus LRE.73 Even though the overall thalamic volumes were linked to social problems and attention problems in both epilepsy groups, it is possible that specific nuclei may exert unique influences on these neurobehavioral comorbidities. Therefore, additional studies are warranted to determine the relationship between volume of specific thalamic nuclei and the comorbidities in children with epilepsy.
The relationship of IQ, SLQ, and peer interaction scores with left thalamic volume only in the CPS group probably reflects the significant differences between the CPS and CAE groups in these scores (Table 1). Despite similar social and attention problems scores in both epilepsy groups (Table 1), smaller left thalamic volumes were associated with higher social problem scores in CPS and CAE but with higher attention problem scores only in CPS. These findings imply different underlying subcortical mechanisms for the comorbidities in children with CPS and CAE. Similar findings are evident for the association between the comorbidities and cortical volumes.13, 19, 74 These subcortical and cortical findings emphasize the need for prospective studies to determine how CPS and CAE differentially impair cortical and subcortical development while manifesting similar behavioral, cognitive, linguistic, and social deficits.
Limitations and Conclusions
Study limitations include the relatively small sample size, cross-sectional study design, and multiple statistical comparisons. Thus, the findings of our study will require confirmation in a larger sample of children with epilepsy. However, using a conservative statistical approach for the analyses, we confirmed the study’s hypotheses regarding smaller volume of the thalamus in both CPS and CAE, abnormal relationship of thalamic volumes with age in CPS, and the role of the thalamus in a wide range of CPS comorbidities but limited to social problems in CAE.
Figure 1.
Subcortical structural volumetric differences among children with complex partial seizures (CPS, red bar), childhood absence epilepsy (CAE, green bar), and healthy controls (blue bar). CPS and CAE group demonstrated significantly reduced thalamic volumes compared to controls. There were no significant volumetric differences in the caudate, putamen, and pallidum. Volumes are expressed in mm3, with error bars representing standard deviations. * denote p ≤ 0.05, controls vs. CPS and controls vs. CAE
Acknowledgements
Supported by grants from the National Institutes of Health: K23 NS060993 (JJL) and NS32070 and MH 6718 (RC).
Footnotes
Disclosure
The authors have no conflicts of interests. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380:1180–1192. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Developmental medicine and child neurology. 2003;45:292–295. doi: 10.1017/s0012162203000550. [DOI] [PubMed] [Google Scholar]
- 3.Hoie B, Sommerfelt K, Waaler PE, et al. Psychosocial problems and seizure-related factors in children with epilepsy. Developmental medicine and child neurology. 2006;48:213–219. doi: 10.1017/S0012162206000454. [DOI] [PubMed] [Google Scholar]
- 4.Berg AT, Vickrey BG, Testa FM, et al. Behavior and social competency in idiopathic and cryptogenic childhood epilepsy. Developmental medicine and child neurology. 2007;49:487–492. doi: 10.1111/j.1469-8749.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 5.Baca CB, Vickrey BG, Caplan R, et al. Psychiatric and medical comorbidity and quality of life outcomes in childhood-onset epilepsy. Pediatrics. 2011;128:e1532–e1543. doi: 10.1542/peds.2011-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tosun D, Dabbs K, Caplan R, et al. Deformation-based morphometry of prospective neurodevelopmental changes in new onset paediatric epilepsy. Brain. 2011;134:1003–1014. doi: 10.1093/brain/awr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson E, Pulsipher D, Dabbs K, et al. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy research. 2010;88:208–214. doi: 10.1016/j.eplepsyres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulsipher DT, Dabbs K, Tuchsherer V, et al. Thalamofrontal neurodevelopment in new-onset pediatric idiopathic generalized epilepsy. Neurology. 2011;76:28–33. doi: 10.1212/WNL.0b013e318203e8f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JJ, Riley JD, Hsu DA, et al. Striatal hypertrophy and its cognitive effects in new-onset benign epilepsy with centrotemporal spikes. Epilepsia. 2012;53:677–685. doi: 10.1111/j.1528-1167.2012.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulsipher DT, Seidenberg M, Guidotti L, et al. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009;50:1210–1219. doi: 10.1111/j.1528-1167.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Muircheartaigh J, Vollmar C, Barker GJ, et al. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology. 2011;76:34–40. doi: 10.1212/WNL.0b013e318203e93d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vollmar C, O'Muircheartaigh J, Barker GJ, et al. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain. 2011;134:1710–1719. doi: 10.1093/brain/awr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daley M, Levitt J, Siddarth P, et al. Frontal and temporal volumes in children with epilepsy. Epilepsy Behav. 2007;10:470–476. doi: 10.1016/j.yebeh.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Caplan R, Levitt J, Siddarth P, et al. Thought disorder and frontotemporal volumes in pediatric epilepsy. Epilepsy Behav. 2008;13:593–599. doi: 10.1016/j.yebeh.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 17.Tosun D, Caplan R, Siddarth P, et al. Intelligence and cortical thickness in children with complex partial seizures. Neuroimage. 2011;57:337–345. doi: 10.1016/j.neuroimage.2011.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermann B, Jones J, Dabbs K, et al. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain. 2007;130:3135–3148. doi: 10.1093/brain/awm227. [DOI] [PubMed] [Google Scholar]
- 19.Caplan R, Siddarth P, Vona P, et al. Language in pediatric epilepsy. Epilepsia. 2009;50:2397–2407. doi: 10.1111/j.1528-1167.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 20.Sowell ER, Trauner DA, Gamst A, et al. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental medicine and child neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 21.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brain Development Cooperative G. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cormack F, Gadian DG, Vargha-Khadem F, et al. Extra-hippocampal grey matter density abnormalities in paediatric mesial temporal sclerosis. Neuroimage. 2005;27:635–643. doi: 10.1016/j.neuroimage.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Chan CH, Briellmann RS, Pell GS, et al. Thalamic atrophy in childhood absence epilepsy. Epilepsia. 2006;47:399–405. doi: 10.1111/j.1528-1167.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 25.Luo C, Xia Y, Li Q, et al. Diffusion and volumetry abnormalities in subcortical nuclei of patients with absence seizures. Epilepsia. 2011;52:1092–1099. doi: 10.1111/j.1528-1167.2011.03045.x. [DOI] [PubMed] [Google Scholar]
- 26.Tosun D, Siddarth P, Toga AW, et al. Effects of childhood absence epilepsy on associations between regional cortical morphometry and aging and cognitive abilities. Hum Brain Mapp. 2011;32:580–591. doi: 10.1002/hbm.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia S, Li X, Kimball AE, et al. Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2012;204:161–167. doi: 10.1016/j.pscychresns.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo K, Rosenberg DR, Easter PC, et al. Striatal volume abnormalities in treatment-naive patients diagnosed with pediatric major depressive disorder. Journal of child and adolescent psychopharmacology. 2008;18:121–131. doi: 10.1089/cap.2007.0026. [DOI] [PubMed] [Google Scholar]
- 29.Herba CM, Roza SJ, Govaert P, et al. Infant brain development and vulnerability to later internalizing difficulties: the Generation R study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1053–1063. doi: 10.1016/j.jaac.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Guyer AE, Choate VR, Detloff A, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. The American journal of psychiatry. 2012;169:205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodenburg R, Stams GJ, Meijer AM, et al. Psychopathology in children with epilepsy: a meta-analysis. Journal of pediatric psychology. 2005;30:453–468. doi: 10.1093/jpepsy/jsi071. [DOI] [PubMed] [Google Scholar]
- 32.Hamiwka L, Jones JE, Salpekar J, et al. Child psychiatry. Epilepsy Behav. 2011;22:38–46. doi: 10.1016/j.yebeh.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Draganski B, Kherif F, Kloppel S, et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llano DA. Functional imaging of the thalamus in language. Brain Lang. 2013;126:62–72. doi: 10.1016/j.bandl.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engel J., Jr International League Against E. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 36.Hollingshead AB. Medical sociology: a brief review. The Milbank Memorial Fund quarterly Health and society. 1973;51:531–542. [PubMed] [Google Scholar]
- 37.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 39.Patenaude B, Smith SM, Kennedy DN, et al. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Achenbach T. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Vermont: Department of Psychiatry, University of Vermont; 1991. [Google Scholar]
- 41.Wechsler D. Weschsler Intelligence Scale for Children, (WISC-III) 3rd Edition. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- 42.Newcomer PL, Hammil DD. Test of Language Development-2 Primary. Austin: Pro-ed; 1988. [Google Scholar]
- 43.Hughes JR. Absence seizures: a review of recent reports with new concepts. Epilepsy Behav. 2009;15:404–412. doi: 10.1016/j.yebeh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Huguenard JR, Prince DA. A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992;12:3804–3817. doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masterton RA, Carney PW, Jackson GD. Cortical and thalamic resting-state functional connectivity is altered in childhood absence epilepsy. Epilepsy Res. 2012;99:327–334. doi: 10.1016/j.eplepsyres.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Berman R, Negishi M, Vestal M, et al. Simultaneous EEG, fMRI, and behavior in typical childhood absence seizures. Epilepsia. 2010;51:2011–2022. doi: 10.1111/j.1528-1167.2010.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seidenberg M, Hermann B, Pulsipher D, et al. Thalamic atrophy and cognition in unilateral temporal lobe epilepsy. J Int Neuropsychol Soc. 2008;14:384–393. doi: 10.1017/S1355617708080399. [DOI] [PubMed] [Google Scholar]
- 48.McDonald CR, Hagler DJ, Jr, Ahmadi ME, et al. Subcortical and cerebellar atrophy in mesial temporal lobe epilepsy revealed by automatic segmentation. Epilepsy research. 2008;79:130–138. doi: 10.1016/j.eplepsyres.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keller SS, Schoene-Bake JC, Gerdes JS, et al. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross-sectional evidence for progressive neurologic injury. PLoS One. 2012;7:e46791. doi: 10.1371/journal.pone.0046791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Widjaja E, Kis A, Go C, et al. Abnormal white matter on diffusion tensor imaging in children with new-onset seizures. Epilepsy Res. 2013;104:105–111. doi: 10.1016/j.eplepsyres.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Henry TR, Mazziotta JC, Engel J., Jr Interictal metabolic anatomy of mesial temporal lobe epilepsy. Arch Neurol. 1993;50:582–589. doi: 10.1001/archneur.1993.00540060022011. [DOI] [PubMed] [Google Scholar]
- 52.Carney PW, Masterton RA, Harvey AS, et al. The core network in absence epilepsy. Differences in cortical and thalamic BOLD response. Neurology. 2010;75:904–911. doi: 10.1212/WNL.0b013e3181f11c06. [DOI] [PubMed] [Google Scholar]
- 53.Riley JD, Moore S, Cramer SC, et al. Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 2011;21:80–87. doi: 10.1016/j.yebeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dreifuss S, Vingerhoets FJ, Lazeyras F, et al. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57:1636–1641. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- 55.Bouilleret V, Semah F, Chassoux F, et al. Basal ganglia involvement in temporal lobe epilepsy: a functional and morphologic study. Neurology. 2008;70:177–184. doi: 10.1212/01.wnl.0000297514.47695.48. [DOI] [PubMed] [Google Scholar]
- 56.Pulsipher DT, Seidenberg M, Morton JJ, et al. MRI volume loss of subcortical structures in unilateral temporal lobe epilepsy. Epilepsy Behav. 2007;11:442–449. doi: 10.1016/j.yebeh.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeCarli C, Hatta J, Fazilat S, et al. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol. 1998;43:41–45. doi: 10.1002/ana.410430110. [DOI] [PubMed] [Google Scholar]
- 58.Keller SS, Ahrens T, Mohammadi S, et al. Microstructural and volumetric abnormalities of the putamen in juvenile myoclonic epilepsy. Epilepsia. 2011;52:1715–1724. doi: 10.1111/j.1528-1167.2011.03117.x. [DOI] [PubMed] [Google Scholar]
- 59.Seeck M, Dreifuss S, Lantz G, et al. Subcortical nuclei volumetry in idiopathic generalized epilepsy. Epilepsia. 2005;46:1642–1645. doi: 10.1111/j.1528-1167.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 60.Sun T, Patoine C, Abu-Khalil A, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin PY, Roche-Labarbe N, Dehaes M, et al. Regional and hemispheric asymmetries of cerebral hemodynamic and oxygen metabolism in newborns. Cereb Cortex. 2013;23:339–348. doi: 10.1093/cercor/bhs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kemmotsu N, Girard HM, Bernhardt BC, et al. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia. 2011;52:2257–2266. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie Y, Chen YA, De Bellis MD. The relationship of age, gender, and IQ with the brainstem and thalamus in healthy children and adolescents: a magnetic resonance imaging volumetric study. J Child Neurol. 2012;27:325–331. doi: 10.1177/0883073811419260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahsan RL, Allom R, Gousias IS, et al. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. Neuroimage. 2007;38:261–270. doi: 10.1016/j.neuroimage.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Alkonyi B, Juhasz C, Muzik O, et al. Thalamocortical connectivity in healthy children: asymmetries and robust developmental changes between ages 8 and 17 years. Ajnr. 2011;32:962–969. doi: 10.3174/ajnr.A2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toga AW, Thompson PM. Mapping brain asymmetry. Nature reviews. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 67.Bachevalier J, Machado CJ, Kazama A. Behavioral outcomes of late-onset or early-onset orbital frontal cortex (areas 11/13) lesions in rhesus monkeys. Ann N Y Acad Sci. 2011;1239:71–86. doi: 10.1111/j.1749-6632.2011.06211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Price DJ, Kennedy H, Dehay C, et al. The development of cortical connections. Eur J Neurosci. 2006;23:910–920. doi: 10.1111/j.1460-9568.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- 69.Jakab A, Blanc R, Berenyi EL. Mapping changes of in vivo connectivity patterns in the human mediodorsal thalamus: correlations with higher cognitive and executive functions. Brain imaging and behavior. 2012;6:472–483. doi: 10.1007/s11682-012-9172-5. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe Y, Funahashi S. Thalamic mediodorsal nucleus and working memory. Neuroscience and biobehavioral reviews. 2012;36:134–142. doi: 10.1016/j.neubiorev.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Zhang Z, Jiao Q, et al. Impairments of thalamic nuclei in idiopathic generalized epilepsy revealed by a study combining morphological and functional connectivity MRI. PLoS One. 2012;7:e39701. doi: 10.1371/journal.pone.0039701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyvaert L, Chassagnon S, Sadikot A, et al. Thalamic nuclei activity in idiopathic generalized epilepsy: an EEG-fMRI study. Neurology. 2009;73:2018–2022. doi: 10.1212/WNL.0b013e3181c55d02. [DOI] [PubMed] [Google Scholar]
- 73.Langlois M, Polack PO, Bernard H, et al. Involvement of the thalamic parafascicular nucleus in mesial temporal lobe epilepsy. J Neurosci. 2010;30:16523–16535. doi: 10.1523/JNEUROSCI.1109-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caplan R, Levitt J, Siddarth P, et al. Language and brain volumes in children with epilepsy. Epilepsy Behav. 2010;17:402–407. doi: 10.1016/j.yebeh.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]