Abstract
This study sought to determine the protective effect of dietary inclusion of sorghum leaf sheath dye on cisplatin-induced hepatotoxicity and oxidative stress in rats. Adult male rats were randomly divided into four groups with six animals in each group. Groups I and II were fed a basal diet, while groups III and IV were fed diets containing 0.5% and 1% sorghum leaf sheath dye, respectively, for 20 days before cisplatin administration. Hepatotoxicity was induced by a single dose of cisplatin (7 mg/kg body weight, i.p.), and the experiment was terminated at 3 days after cisplatin injection. The liver and plasma were studied for hepatotoxicity and antioxidant capacity. Cisplatin caused a significant (P<.05) alteration in plasma and liver enzymatic (catalase, glutathione-S-transferase [GST], and superoxide dismutase [SOD]) and nonenzymatic (glutathione [GSH] and vitamin C) antioxidant indices with a concomitant increase in the malondialdehyde (MDA) content; however, there was a significant (P<.05) restoration of the antioxidant status coupled with a significant (P<.05) decrease in the tissue MDA content, after consumption of diets containing sorghum leaf sheath dye. Furthermore, dietary inclusion of sorghum leaf sheath dye caused a marked reduction in the activities of alanine aminotransferase and aspartate aminotransferase after cisplatin administration. However, the ability of the dye to prevent significant cisplatin-induced alteration of both plasma and liver antioxidant indices suggests an antioxidant mechanism of action. Hence, this protective effect of Sorghum bicolor leaf sheath dye against cisplatin-induced hepatotoxicity in rats reflects its potential and beneficial role in the prevention of liver damage associated with cisplatin administration.
Key Words: : anthocyanin, antioxidants, cis-dichlorodiammineplatinum (II) (CDDP), liver damage, oxidative stress, red dye, Sorghum bicolor (L.) Moench
Introduction
Cisplatin [cis-dichlorodiammineplatinum (II), CDDP] is a platinum-based synthetic antineoplatic drug extensively used for the treatment of a variety of solid tumors.1,2 Although one of the mostly used antineoplatic drugs, cisplatin has a number of dose-dependent toxicities such as nephrotoxicity and hepatotoxicity that interfere with its therapeutic efficacy.3 Nephrotoxicity of cisplatin has been recognized as the most important dose-limiting factor in its treatment of some solid tumors, with findings also suggesting the occurrence of hepatotoxicity at higher doses.4 The precise mechanisms of cisplatin hepatotoxicity have not been categorically stated or fully elucidated, but suggestions have been made that cisplatin hepatotoxicity could be a result of the liver metabolizing cisplatin to more toxicity adducts/metabolites;5 this results in reduced liver function in cancer patients as indicated by elevated levels of liver enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP).6
The side effects caused by the administration of cisplatin in cancer patients needs to be holistically reduced due to a number of reasons. First, cisplatin causes oxidative stress via the depletion of endogenous antioxidants.6,7 The platinum (a transition metal) in cisplatin complexes with reduced glutathione (GSH) by binding to the thiol group of the protein8 results in the depletion of GSH stores, which is responsible for ameliorating the effects of free radicals entering into the system.9 The depletion of the GSH stores could result in decreased bioavailability of vitamin C and antioxidant enzymes such as glutathione-S-transferase (GST) and glutathione peroxidase (GPx), because GSH is necessary for the recycling of vitamin C and the antioxidant enzymes in vivo.2,10 Second, cisplatin causes oxidative stress via increased generation of reactive oxygen species, such as superoxide anion and hydroxyl radical,11 and also causes lipid peroxidation and DNA damage.10,12,13 It has been established that lipid peroxidation might also participate in hepatotoxicity found in cisplatin-treated animals despite the activation of antioxidant enzymes.14
The use of some synthetic drugs has been developed as one of the strategies proposed for the prevention/management of cisplatin-induced hepatotoxicity; however, these drugs have their own associated risks and side effects.15 Hence, there is a need to explore natural alternatives (plant and food materials) with little or no side effect. The use of plants with color or dye for the prevention and management of diseases has been employed in folklore since time immemorial. Sorghum [Sorghum bicolor (L.) Moench] is one of the numerous species of sorghum grasses raised mainly for grain, and many are also cultivated as fodder plants for pasture. The plant is native to tropical and subtropical regions,16 and it is one of the major grain crops cultivated for human food in Africa and other tropical regions of the world. The people of south-west Nigeria usually ferment the grains into gruels, which serve as weaning food for babies. Furthermore, sorghum leaf sheath (dried leafs and stems) and extracts have been employed as infusion, colorant, or dye in therapy for the management of anemia and sickle cell disease and they have also found use as an antimalarial, anthelminthic, and insecticide.17,18 The therapeutic roles of sorghum and its extracts have been linked to its phytoconstituents, such as anthocyanin.19 Anthocyanins have been reported to possess vasoprotective and anti-inflammatory properties,20 inhibit lipid peroxidation, and scavenge free radicals.21 Furthermore, the anti-cancer and chemoprotective,22 as well as anti-neoplastic properties23 of anthocyannins have been reported.
Although less toxic platinum compounds have been developed, cisplatin remains the drug of choice in platinum-based therapy regimens, and one of the most commonly used chemotherapeutic drugs.24 Thus, prevention/management of the side effects of cisplatin is one of the major issues in treating cancer patients.13 Therefore, this study sought to assess the antioxidant and hepatoprotective potentials of red dye extract from sorghum leaf sheath on cisplatin-induced hepatotoxicity in rats.
Materials and Methods
Materials
Sorghum (SRN 4841) leaf sheath (dried leafs and stems) was purchased at Oja Oba market in Akure metropolis, Nigeria. The sample was authenticated at the Department of Plant Science, Ekiti State University (Ado-Ekiti, Nigeria), where a voucher specimen (UHAE 2013/23) was deposited at the herbarium. The sample was oven dried and pulverized, and later stored in an air-tight container before dye/pigment extraction. Cisplatin was sourced from Korea United Pharm., Inc. (Sejong, Korea). All the kits used for the bioassay were sourced from RANDOX Laboratories Ltd., Crumlin, Co. (Antrim, United Kingdom). All chemicals were of analytical grade. Diet ingredients were purchased from VITAL Feeds, Jos, Nigeria Ltd (Jos, Nigeria). High-performance liquid chromatography (HPLC) with a Shimadzu Prominence Auto Sampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps, was connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector (DAD), and LC solution 1.22 SP1 software.
Animals
The handling and use of the animals were in accordance with NIH Guide for the care and use of laboratory animals. Male albino rats weighing about 165±10 g used for this experiment were purchased from a private animal colony within Akure metropolis. The rats were maintained at 25°C on a 12 h light/dark cycle with free access to food and water. They were acclimatized under these conditions for 2 weeks before the commencement of the experiments. The experimental study was approved by the Institutional Ethics Committee.
Extraction of sorghum leaf sheath dye
The extraction of the red dye from the sorghum leaf sheath was carried out using a slightly modified method of Adetuyi et al.25 Briefly, the dye was extracted by soaking 100 g of pulverized sample in 1.8 L of distilled water and kept overnight (12 h). Thereafter, the mixture was filtered and the filtrate was collected before the residue was rinsed with another 200 mL of distilled water. This again was filtered, and the filtrate was collected and added to the previous one. The filtrate was later lyophilized and kept in an air-tight container before analysis. This was designated as the dye used for this study.
Determination of total anthocyanin content
The total anthocyanin content of the sorghum sheath dye was determined according to the pH differential method reported by Fuleki and Francis.26 Briefly, 0.2 mL aliquots of the dye solution were diluted with 2.8 mL of buffer, pH 1.0 (consisting of 125 mL of 0.2 N KCl, and 385 mL of 0.2 N HCl) and another 0.2 mL of the dye solution was diluted with 2.8 mL of buffer, pH 4.5 (consisting of 400 mL of 1 N sodium acetate, 240 mL of 1 N HCl, and 360 mL distilled water) solution. Thereafter, the absorbance readings at 510 nm for the two solutions were taken. Total anthocyanin content was calculated using the value obtained from the difference between absorbance in pH 1.0 and 4.5 buffers. This was subsequently expressed as milligram cyanidin-3-glucoside equivalent/100 g of dye (cyanidin-3-glucoside, ɛ=26,900 M−1 cm−1).
Experimental design and induction of hepatotoxicity
The animals were randomly divided into four groups of six animals each. Groups I and II were fed a basal diet (50% skimmed milk, 36% corn starch, 10% groundnut oil, and 4% mineral and vitamin premix), while groups III and IV were fed a basal diet supplemented with 0.5% and 1% sorghum leaf sheath dye, respectively, for 20 days before the administration of cisplatin. The diets fed to the rats were prepared according to the modified method of Oboh et al.27 and were kept in air-tight containers, which were stored at 4°C until needed for use. On day 20, group I receive sterile water (1 mL/kg, i.p.), while hepatotoxicity was induced in groups II, III, and IV by intraperitoneal administration of a single dose of cisplatin (7 mg/kg body weight),28 and the experiment was terminated at 3 days after cisplatin administration. The animals were decapitated after an overnight fast by cervical dislocation, the blood was rapidly collected by direct heart puncture into an ethylenediaminetetraacetic acid (EDTA) bottle, and the liver was rapidly isolated, weighed, and kept on ice.
Analytical procedures
Plasma AST, ALT, albumin, triglyceride, total cholesterol, low-density lipoprotein (LDL)-cholesterol, and high-density lipoprotein (HDL)-cholesterol were determined using commercially available kits (Randox Laboratories, Northern Ireland, United Kingdom). Lipid peroxidation was determined by thiobarbituric acid (TBA) reaction29 and quantified as malondialdehyde (MDA) content. Furthermore, assays to determine the plasma activities of superoxide dismutase (SOD),30 catalase,31 and GST32 were carried out. However, reduced glutathione, vitamin C, and total protein contents were determined according to Ellman,33 Benderitter et al.34 and Lowry et al.,35 respectively.
Quantification of compounds by RP-HPLC-DAD
Reverse-phase (RP) chromatographic analyses were carried out under gradient conditions using C18 column (4.6×150 mm) packed with 5 μm-diameter particles; the mobile phase was water containing 2% acetic acid (A) and methanol (B), and the composition gradient was 5% of B until 2 min and was changed to obtain 25%, 40%, 50%, 60%, 70%, and 100% B at 10, 20, 30, 40, 50, and 80 min, respectively, following the method described by Laghari et al.36 with slight modifications. The red dye extract was analyzed at a concentration of 5 mg/mL. The flow rate was 0.6 mL/min, injection volume was 40 μL, and the wavelength ranged from 254 to 365 nm. The samples and mobile phase were filtered through a 0.45 μm membrane filter (Millipore, Darmstadt, Germany) and then degassed by an ultrasonic bath before use. Stock solutions of standard compounds were prepared in the HPLC mobile phase at a concentration range of 0.030–0.350 mg/mL for rutin and kaempferol; and 0.050–0.250 mg/mL for ferulic, caffeic, and gallic acids. The chromatography peaks were confirmed by comparing their retention time with those of reference standards and by DAD spectra (200–500 nm). All chromatography operations were carried out at ambient temperature and in triplicate. The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the responses and the slope using three independent analytical curves, as defined by ICH.37 LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve.
Data analysis
The results of replicate readings were pooled and expressed as mean±standard deviation. One-way analysis of variance was used to analyze the results, and Duncan multiple test was used for the post hoc.38 Statistical package for Social Science (SPSS, Chicago, IL, USA) 16.0 for Windows was used for the analysis. The significance level was taken at P<.05.
Results
The study revealed that the dye has 1.12 mg cyanidin-3-glucoside equivalent/100 g, total anthocyanin content. Furthermore, there was no significant (P>.05) difference in the average feed intake of animals in group I (normal rats fed basal diet), group II (control rats administered cisplatin and fed basal diet), and groups III and IV (cisplatin-administered rats fed diet supplemented with 0.5% and 1% of the red dye, respectively) (Table 1). However, there was a significant (P<.05) weight loss in the cisplatin-treated groups as compared with the normal rats. Nevertheless, diets containing either 0.5% or 1% of the dye were able to ameliorate the observed weight loss in cisplatin-treated groups (Table 1).
Table 1.
Effect of Diets Supplemented with Sorghum Leaf Sheath Red Dye on Some Biochemical Indices in Cisplatin (7 mg/kg i.p.) Administered Rats
| Treatment groups | ||||
|---|---|---|---|---|
| I | II | III | IV | |
| Average feed intake (g/rat/day) | 9.2±3.4a | 9.1±4.0a | 9.0±5.2a | 9.5±2.6a |
| Average weight gain/loss (%) | 5.9d | −2.8a | −1.6b | −1.2c |
| Plasma indices | ||||
| ALT (IU/L) | 61.1±3.6a | 70.3±11.0a | 57.6±10.6a | 53.6±7.9a |
| AST (IU/L) | 21.1±5.5b | 26.3±10.3b | 17.6±0.6a | 15.5±1.0a |
| Albumin (mg/dL) | 3.2±0.4b | 2.8±0.1a | 3.2±0.4b | 3.7±0.3c |
| Plasma enzymatic antioxidant indices | ||||
| Catalase activity (mmol H2O2 consumed/min/mg protein) | 180.0±20.0bc | 131.1±22.1a | 174.0±82.1b | 200.2±7.5c |
| GST activity (mmol CDNB conjugates formed/min/g protein) | 3.4±0.5d | 2.5±0.1a | 3.0±0.2c | 2.9±0.1b |
| SOD activity (Units/mg protein) | 90.2±0.5a | 61.1±0.5b | 140.4±1.3a | 200.3±1.2a |
| Plasma nonenzymatic antioxidant indices | ||||
| Vitamin C content (mmol/mg protein) | 9.4±0.4c | 4.6±0.3a | 6.0±0.1b | 5.7±0.2b |
| MDA content (mmol/100 g protein) | 1.1±0.6b | 8.2±1.9c | 0.9±0.2b | 0.6±0.2a |
| Plasma lipid profile (mg/dL) | ||||
| Triglycerides | 160.2±39.2a | 200.3±62.0c | 207.2±18.0c | 192.3±57.7b |
| Total cholesterol | 129.1±7.6a | 157.0±10.1c | 150.5±14.5c | 138.3±14.7b |
| LDL-cholesterol | 73.4±21.3a | 114.9±17.3b | 57.4±17.4a | 56.8±17.8a |
| HDL-cholesterol | 12.7±6.2b | 8.2±12.2a | 71.6±3.8d | 59.5±5.9c |
| Liver enzymatic antioxidant indices | ||||
| Catalase activity (mmol H2O2 consumed/min/mg protein) | 25.2±0.8b | 23.8±0.4a | 26.7±0.3b | 29.5±0.2c |
| GST activity (mmol CDNB conjugates formed/min/g protein) | 4.18±0.1c | 3.92±0.3a | 4.04±0.1b | 4.22±0.1c |
| SOD activity (Units/100 g protein) | 3.4±0.4b | 2.7±0.2a | 5.0±0.2c | 5.4±0.3c |
| Liver nonenzymatic antioxidant indices | ||||
| Vitamin C (mmol/mg protein) | 6.8±0.5a | 5.8±0.1a | 6.5±0.7a | 7.2±0.2a |
| MDA (mmol/mg protein) | 8.3±0.1a | 9.3±0.5b | 8.9±1.2b | 8.9±0.6b |
| GSH (mg/100 g protein) | 1.6±0.1b | 0.62±0.7a | 1.7±0.2b | 1.8±1.1b |
Values represent mean±standard deviation (n=6).
Values not sharing the same superscript letter on the same row are significantly (P<.05) different.
Adult male rats were divided into four groups of six animals each. Groups I (normal rats fed basal diet), group II (control rats administered cisplatin and fed basal diet), group III (cisplatin administered rats fed diet supplemented with 0.5% sorghum leaf sheath red dye), and group IV (cisplatin-administered rats fed diet supplemented with 1% sorghum leaf sheath red dye). The rats were maintained on these diets for 20 days before cisplatin administration. A single dose of cisplatin (7 mg/kg body weight, i.p.) was administered to the rats on the 20th day, and the experiment was terminated 3 days later. Plasma and liver biochemical indices were examined as evidence of cisplatin toxicity.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GSH, glutathione; GST, glutathione-S-transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDA, malondialdehyde; SOD, superoxide dismutase.
As observed also in Table 1, group II animals experienced a significant (P<.05) elevation in their plasma levels of liver damage marker enzymes such as AST and ALT compared with group I. However, consumption of sorghum leaf sheath dye fortified diets before cisplatin administration (groups III and IV) resulted in maintenance of the plasma levels of these marker enzymes at levels close to normal rats. Furthermore, consumption of the dye before cisplatin injection protected against alteration in plasma albumin levels.
Table 1 also revealed that cisplatin administration led to the reduction in the activities of plasma antioxidant enzymes (catalase, GST, and SOD), and dietary treatment with sorghum leaf sheath dye (groups III and IV) protected against this alteration (except GST activity) in a dose-dependent pattern. Likewise, cisplatin treatment caused a marked decrease in plasma vitamin C content with a concomitant increase in the MDA content of the treated rats. Furthermore, this trend was maintained at near normal in the rats fed diets containing either 0.5% or 1% of the red dye (Table 1). As shown in Table 1, administration of cisplatin caused a significant (P<.05) reduction in the activities of some liver antioxidant enzymes such as catalase, GST, and SOD; while the trend was maintained at near normal levels in either 0.5% or 1% red dye supplemented diet (groups III and IV). Furthermore, cisplatin administration also caused a marked reduction in both liver vitamin C and GSH contents, which was accompanied by an increase in the liver MDA content while these liver antioxidant indices were maintained at near normal levels in 0.5% or 1% red dye supplemented diet groups (Table 1).
In addition, the observed increase in the plasma atherogenic lipids (triglycerides and total cholesterol) and LDL-cholesterol, with a concomitant decrease in the plasma HDL-cholesterol in the cisplatin administered rats, was mitigated against in rats treated with diets containing 0.5% or 1% red dye supplementation with the effect most prominent in group IV animals (Table 1).
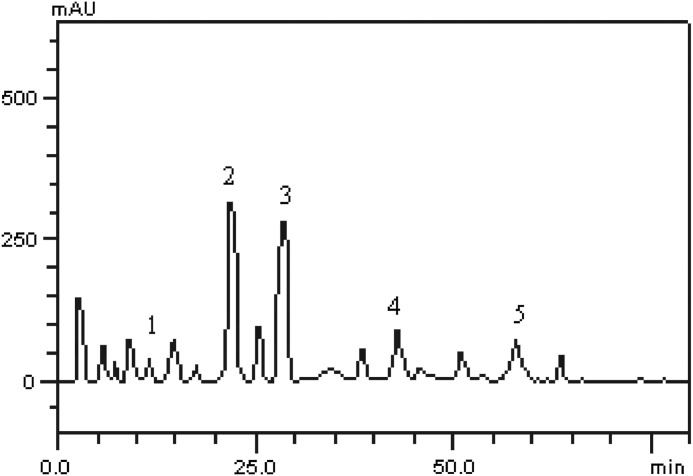
HPLC fingerprinting of the sorghum sheath red dye revealed the presence of the gallic acid (peak 1), caffeic acid (tR=24.97 min; peak 2), ferulic acid (tR=29.73 min; peak 3), rutin (tR=39.12 min; peak 4), and kaempferol (tR=56.98 min; peak 5) (Fig. 1 and Table 2).
FIG. 1.
Reverse phase–high performance liquid chromatography finger printing of the constituent phenolic compounds in the sorghum leaf sheath red dye. Gallic acid (peak 1), caffeic acid (peak 2), ferulic acid (peak 3), rutin (peak 4), and kaempferol (peak 5). Chromatographic conditions are described in the Materials and Methods section.
Table 2.
High-Performance Liquid Chromatography Fingerprinting of the Sorghum Leaf Sheath Red Dye Polyphenol Contents
| Polyphenol compounds | Composition mg/g | LOD μg/mL | LOQ μg/mL |
|---|---|---|---|
| Gallic acid | 5.83±0.01 | 0.04 | 0.12 |
| Caffeic acid | 70.95±0.01 | 0.01 | 0.04 |
| Ferulic acid | 61.39±0.02 | 0.02 | 0.08 |
| Rutin | 21.06±0.01 | 0.02 | 0.06 |
| Kaempferol | 24.52±0.03 | 0.01 | 0.05 |
Results are expressed as mean±SD of three determinations. Chromatographic conditions are as described in the Materials and Methods section.
LOD, limit of detection; LOQ, limit of quantification; SD, standard deviations.
Discussion
Cisplatin is an anti-neoplastic drug with known cytotoxicity at high-dose treatment, with hepatotoxicity being one of them.39 This study revealed that a single intraperitoneal administration of cisplatin (7 mg/kg body weight) to rats resulted in deterioration of hepatic function as indicated by elevated AST and ALT activities, and a concomitant reduction in the plasma albumin level. This finding is consistent with the study of Mansour et al.,40 where administration of cisplatin induced a significant increase in serum ALT and AST and a significant decrease in albumin and calcium levels. The ability of cisplatin to alter the activities of these enzymes may be a secondary event arising from cisplatin-induced liver damage and leakage of these enzymes from the hepatocytes into blood circulation.40
Liver is known to bio-accumulate significant amounts of cisplatin, after the kidney41; thus, hepatotoxicity may be associated with cisplatin treatment.42 Clinical evidence of cisplatin-induced hepatotoxicity is demonstrated by elevated activities of serum enzymes and bilirubin levels, and the development of jaundice.43 However, this study revealed that consumption of diet containing sorghum dye inclusion protected against the alteration in the plasma AST, ALT, and albumin levels, and maintained them close to normal values. This agreed with previous studies where various plant extracts and compounds protected the liver against cisplatin-induced hepatotoxicity.3,40 This hepatoprotective effect of the sorghum leaf sheath dye could be attributed to its constituent phytochemicals such as anthocyanins and other phenolic compounds (Table 2). Sorghum is a rich source of various phytochemicals such as anthocyanins, tannins, phenolic acids, phytosterols, and policosanols, which are known to significantly impact human health.19
Cisplatin administration also resulted in a significant alteration in the plasma and liver antioxidant enzymes (catalase, SOD, and GST) activity and nonenzymatic antioxidant indices (GSH, vitamin C, and MDA) with a significant reduction in the activities of these enzymes, GSH, and vitamin C contents. This is coupled to a significant increase in the plasma and liver MDA (a marker of lipid peroxidation) content. This finding is consistent with earlier studies that reported cisplatin-induced alteration in endogenous antioxidant status in rats.40,44 Oxidative stress has been implicated as one of the possible mechanisms of cisplatin-induced nephrotoxicity.45,46 Findings have shown that cisplatin could induce tissue lipid peroxidation in both in vivo and in vitro studies. It alters tissue thiol status with concomitant alterations in the activities of antioxidant enzymes.40 This might be due to increased free radical generation induced by cisplatin administration.47 These free radicals can inactivate antioxidant enzymes,48 and alter membrane lipids, leading to tissue injury or cell death.
Depletion in the tissue GSH content could affect the functional abilities of some glutathione-dependent antioxidant enzymes, thus rendering the tissue susceptible to oxidative stress. Furthermore, the increased liver MDA content (a biomarker of lipid peroxidation) could be associated with the elevation in the plasma AST and ALT activities, resulting from damaged hepatocytes. However, the protective effect rendered by previous consumption of diets fortified with the dye, as evidenced by the maintenance of the in vivo antioxidant indices (antioxidants and nonantioxidants) near normal values, suggests an antioxidant effect; and it could be attributed to the anthocyanin and phenolic constituents (Table 2). Phenolics are potent antioxidants that could help augment in vivo antioxidant status, by rendering a sparing effect to the GSH or directly scavenging the free radicals produced by cisplatin administration. Nevertheless, the observed ameliorative effect of the diets containing the sorghum dye gives credence to the fact that oxidative stress might be responsible for the cisplatin-induced liver damage.49,50 Oxidative stress is a pathogenic mechanism implicated in the initiation and progression of hepatic damage in a variety of liver diseases.
The observed increase in the plasma atherogenic lipids (triglycerides and total cholesterol) and LDL-cholesterol, coupled with a concomitant decrease in the plasma HDL-cholesterol in cisplatin administered rats, could be attributed to impaired hepatic function. The liver is responsible for lipid homeostasis, most especially, cholesterol metabolism.51 However, maintenance of the balance in lipid homeostasis that accompanied previous feeding with diets containing either 0.5% or 1% sorghum leaf sheath dye might be due to the protective/healing effect of its constituent phenolic compounds. Polyphenols are potent antioxidants and have been reported to be involved in the healing process of tissue injury mediated by oxidative stress.52 The presence of these bioactive polyphenolic compounds in significant amounts in the sorghum leaf sheath red dye highlights the promising and potential use of the dye (as natural food colourants) in the therapy and management of hepatotoxicity.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Tikoo K, Bhatt DK, Gaikawad AB, Sharma V, Kabra DG: Differential effects of tannic acid on cisplatin-induced nephrotoxicity in rats. FEBS Lett 2007;581:2027–2035 [DOI] [PubMed] [Google Scholar]
- 2.Ajith TA, Usha S, Nivitha V: Ascorbic acid and a-tocopherol protect against anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clin Chim Acta 2007;375:82–86 [DOI] [PubMed] [Google Scholar]
- 3.Ezz-Din D, Gabry MS, Farrag AH, Abdel-Moneim AE: Physiological and histological impact of Azadirachta indica (neem) leaves extract in a rat model of cisplatin-induced hepato and nephrotoxicity. J Med Plants Res 2011;5:5499–5506 [Google Scholar]
- 4.Zicca A, Cafaggi S, Mariggio MA, Vannozzi MO, Ottone M, Bocchini V, Caviglioli G, Viale M: Reduction of cisplatin hepatotoxicity by procainamide hydrochloride in rats. Eur J Pharmacol 2004;442:265–272 [DOI] [PubMed] [Google Scholar]
- 5.Mistry P, Lee C, McBrien DC: Intracellular metabolites of cisplatin in the rat kidney. Cancer Chemother Pharmacol 1989;24:73–79 [DOI] [PubMed] [Google Scholar]
- 6.Martins NM, Santos NA, Curt C, Bianchi ML, Santos AC: Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J Appl Toxicol 2008;28:337–344 [DOI] [PubMed] [Google Scholar]
- 7.Santos NA, Martins NM, Curti C, Bianchi ML, Santos AC: Dimethylthiourea protects against mitochondrial oxidative damage induced by cisplatin in liver of rats. Chem Biol Int 2007;170:177–186 [DOI] [PubMed] [Google Scholar]
- 8.Fuertes MA, Alonso C, Pérez JM: Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistence. Chem Rev 2002;103:645–662 [DOI] [PubMed] [Google Scholar]
- 9.Kadikoylu G, Bolaman Z, Demir S, Balkaya M, Akalin N, Enli Y: The effects of desferrioxamine on cisplatin-induced lipid peroxidation and the activities of antioxidant enzymes in rat kidneys. Hum Exp Toxicol 2004;23:29–34 [DOI] [PubMed] [Google Scholar]
- 10.Badary OA, Abdel-Maksoud S, Ahmed WA, Owieda GH: Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci 2005;76:2125–2135 [DOI] [PubMed] [Google Scholar]
- 11.Chirino YI, Pedraza-Chaverri J: Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol 2008;61:223–242 [DOI] [PubMed] [Google Scholar]
- 12.De Martinis BS, Bianchi M: Effect of vitamin C supplementation against cisplatin-induced toxicity and oxidative DNA damage in rats. Pharmacol Res 2001;44:317–320 [DOI] [PubMed] [Google Scholar]
- 13.Chang BJ, Nishikawa M, Sato E, Utsumi K, Inouea M: L-Carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch Biochem Biophys 2002;405:55–64 [DOI] [PubMed] [Google Scholar]
- 14.Christova TY, Gorneva GA, Taxirov SI, Duridanova DB, Setchenska MS: Effect of cisplatin and cobalt chloride on antioxidant enzymes in the livers of Lewis lung carcinoma-bearing mice: protective role of heme oxygenase. Toxicol Lett 2003;138:235–242 [DOI] [PubMed] [Google Scholar]
- 15.Skinner S: Strategies to prevent nephrotoxicity of anticancer drugs. Curr Opin Oncol 1995;7:310–315 [DOI] [PubMed] [Google Scholar]
- 16.Mutegi E, Sagnard F, Muraya M, Kanyenji B, Rono B, Mwongera C, Marangu C, Kamau J, Parzies H, Villiers S, Semagn K, Traore P, Labuschagne M: Ecogeographical distribution of wild, weedy and cultivated Sorghum bicolor (L.) Moench in Kenya: implications for conservation and crop-to-wild gene flow. Genet Resour Crop Evol 2010;57:243–253 [Google Scholar]
- 17.Ilori OO, Odukoya OA: Hibiscus sabdarifa and Sorghum bicolor as natural colorants. Electron J Environ Agric Food Chem 2005;4:858–862 [Google Scholar]
- 18.Okpuzor J, Adebesi O, Ogbunugafor H, Amadi I: The potential of medicinal plants in sickle cell disease control: a review. Int J Biomed Health Sci 2008;4:47–55 [Google Scholar]
- 19.Awika JM, Rooney LW: Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004;65:1199–1221 [DOI] [PubMed] [Google Scholar]
- 20.Lietti A, Cristoni A, Picci M: Studies of Vaccinium myrtillus anthocyanosides: vasoprotective and anti-inflammatory activity. Arzneim-Forsch 1976;26:829–832 [PubMed] [Google Scholar]
- 21.Tsuda T: The role of anthocyanins as an antioxidant under oxidative stress in rats. Biofactors 2000;13:133–139 [DOI] [PubMed] [Google Scholar]
- 22.Karaivanova M, Drenska D, Ovcharov R: A modification of the toxic effects of platinum complexes with anthocyanins. Eksp Med Morfol 1990;29:19–24 [PubMed] [Google Scholar]
- 23.Kamei H, Kojima T, Hasegawa M, Koide T, Umeda T, Yukawa T, Terabe K: Suppression of tumor cell growth by anthocyanins in-vitro. Cancer Invest 1995;13:590–594 [DOI] [PubMed] [Google Scholar]
- 24.Hanigen MH, Devarajan P: Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther 2003;1:47–61 [PMC free article] [PubMed] [Google Scholar]
- 25.Adetuyi AO, Akpambang VO, Oyetayo VO, Adetuyi FO: The nutritive value and antimicrobial property of Sorghum bicolor L. stem (Poporo) flour used as food colour additive and its infusion drink. Am J Food Technol 2007;2:19–86 [Google Scholar]
- 26.Fuleki T, Francis FJ: Quantitative methods for anthocyannins. 2. Determination of total anthocyanin and degradation index for cranberry juice. J Food Sci 1968;33:78–83 [Google Scholar]
- 27.Oboh G, Akomolafe TL, Adetuyi AO: Inhibition of cyclophosphamide induced oxidative stress in brain by dietary inclusion of red dye extracts from sorghum (Sorghum bicolor) stem. J Med Food 2010;13:1075–1080 [DOI] [PubMed] [Google Scholar]
- 28.Yüce A, Ateşşahin A, Ceribaşi AO, Aksakal M: Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol 2007;101:345–349 [DOI] [PubMed] [Google Scholar]
- 29.Ohkawa H, Ohishi N, Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–358 [DOI] [PubMed] [Google Scholar]
- 30.Alia M, Horcajo C, Bravo L, Goya L: Effect of grape antioxidant dietary fibre on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr Res 2003;23:1251–1267 [Google Scholar]
- 31.Sinha AK: Colorimetric assay of catalase. Anal Biochem 1972;47:389–394 [DOI] [PubMed] [Google Scholar]
- 32.Habig WH, Pabst ML, Jakpoly WB: Glutathione transferase: a first enzymatic step in mercapturic acid and formation. J Biol Chem 1974;249:7130–7139 [PubMed] [Google Scholar]
- 33.Ellman GL: Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70–77 [DOI] [PubMed] [Google Scholar]
- 34.Benderitter M, Maupoil V, Vergely C, Dalloz F, Briot F, Rochette L: Studies by electron paramagnetic resonance of the importance of iron in the hydroxyl scavenging properties of ascorbic acid in plasma: effects of iron chelators. Fundam Clin Pharmacol 1998;12:510–516 [DOI] [PubMed] [Google Scholar]
- 35.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the folinphenol reagent. J Biol Chem 1951;193:265–275 [PubMed] [Google Scholar]
- 36.Laghari AH, Memon S, Nelofar A, Khan KM, Yasmin A: Determination of free phenolic acids and antioxidant activity of methanolic extracts obtained from fruits and leaves of Chenopodium album. Food Chem 2011;126:1850–1855 [DOI] [PubMed] [Google Scholar]
- 37.ICH: Text on validation of analytical procedures: methodology: Q2 (R1). www.ich.org, 2005. (Accessed 24September2012)
- 38.Zar JH: Biostatistical Analysis. Prentice-Hall, Inc., Upper Saddle River, NJ, 1984, p. 620 [Google Scholar]
- 39.Pratibha R, Sameer R, Rataboli PV, Bhiwgade DA, Dhume CY: Enzymatic studies of cisplatin induced oxidative stress in hepatic tissue of rats. Eur J Pharmacol 2006;532:290–293 [DOI] [PubMed] [Google Scholar]
- 40.Mansour HH, Hafez HF, Fahmy NM: Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. J. Biochem. Mol. Biol 2006;39:656–661 [DOI] [PubMed] [Google Scholar]
- 41.El-Sayyad HI, Ismail MF, Shalaby FM, Abou-El-Magd RF, Gaur RL, Fernando A, Raj MHG, Ouhtit A: Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol Sci 2009;5:466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao Y, Lu X, Lu C, Li G, Jin Y, Tang H: Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol Res 2008;57:125–131 [DOI] [PubMed] [Google Scholar]
- 43.Moriya A, Hyodo I, Nishina T, Imaoka H, Imagawa A, Doi T, Endo H, Tanimizu M, Tajiri H: Extensive liver metastasis of gastric cancer effectively treated by hepatic arterial infusion of 5-fluorouracil and cisplatin. Gastric Cancer 2000;3:110–115 [DOI] [PubMed] [Google Scholar]
- 44.Avci A, Cetin R, Erguder IB, Devrim E, Kilicoglu B, Candir O, Ozturk HS, Durak I: Cisplatin causes oxidation in rat liver tissues: possible protective effects of antioxidant food supplementation. Turk J Med Sci 2008;38:117–120 [Google Scholar]
- 45.Yao X, Panichpisal K, Kurtzman N, Nugent K: Cisplatin nephrotoxicity: a review. Am J Med Sci 2007;334:115–124 [DOI] [PubMed] [Google Scholar]
- 46.El-Sayed EM, Abd-Ellah MF, Attia SM: Protective effect of captopril against cisplatin-induced nephrotoxicity in rats. Pak J Pharm Sci 2008;21:255–261 [PubMed] [Google Scholar]
- 47.Ulubas B, Cimen MY, Apa DD, Saritas E, Muslu N, Cimen OB: The protective effects of acetylsalicyclic acid on free radical production in cisplatin-induced nephrotoxicity: an experimental rat model. Drug Chem Toxicol 2003;26:259–270 [DOI] [PubMed] [Google Scholar]
- 48.Abheri DS, Anisur RM, Ghosh A: Free radicals and their role in different clinical condition. Int J Pharm Sci Res 2010;1:185–192 [Google Scholar]
- 49.Ramadan LA, El-Habit OH, Arafa H, Sayed-Ahmed MM: Effect of cremophor-el on cisplatin-induced organ toxicity in normal rat. J Egyptian Nat Cancer Inst 2011;13:139–145 [Google Scholar]
- 50.Yilmaz HR, Sogut S, Ozyurt B, Ozugurlu F, Sahin S, Isik B: The activities of liver adenosine deaminase, xanthine oxidase, catalase, superoxide dismutase enzymes and the levels of malondialdehyde and nitric oxide after cisplatin toxicity in rats: protective effect of caffeic acid phenethyl ester. Toxicol Ind Health 2005;21:67–73 [DOI] [PubMed] [Google Scholar]
- 51.Laura T, Marco S, Valentina P: Regulation and deregulation of cholesterol homeostasis: the liver as a metabolic “power station.” World J Hepatol 2012;4:184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haida R, Annie J: In-vitro effect of tea polyphenols on tea metabolism, oxidative stress and apoptosis in PC12 cells. Ann NY Acad Sci 2008;1138:328–365 [DOI] [PubMed] [Google Scholar]