Abstract
In this study, two recombinant plasmids containing the ORF2 gene of porcine circovirus type 2 (PCV2) with or without porcine interleukin-18 (IL-18) were constructed and evaluated for their ability to protect piglets against PCV2 challenge. Transient expression of the plasmids in PK-15 cells could be detected using Western blot. Piglets were given two intramuscular immunizations 3 weeks apart and were challenged with a virulent Wuzhi strain of PCV2 at 42 days after the initial immunization. All animals vaccinated with pBudCE4.1-ORF2 or with pBudCE4.1-ORF2/IL18 developed PCV2-specific antibody and T-lymphocyte proliferative responses. The levels of T-lymphocyte proliferation in piglets immunized with pBudCE4.1-ORF2/IL18 were significantly higher than in those immunized with pBudCE4.1-ORF2, and pBudCE4.1-ORF2/IL18 stimulated a significantly increased production of IFN-γ and IL-2. Furthermore, PCV2 challenge experiments showed that the DNA vaccine-immunized groups can partially prevent PCV2 viremia and significantly reduce the amount of PCV2 virus in the lymphoid tissues, and the piglets immunized by pBudCE4.1-ORF2/IL18 exhibit a marked inhibition of PCV2 replication compared to the pBudCE4.1-ORF2 group. These data demonstrate that the plasmid pBudCE4.1-ORF2/IL18 may be an effective approach for increasing PCV2 DNA vaccine immunogenicity.
Introduction
Porcine circovirus type 2 (PCV2), classified as a member of the Circoviridae family, is an etiologic agent that is associated with postweaning multisystemic wasting syndrome (PMWS), resulting in great economic losses in many swine-producing countries (2,29). PCV2 is a small nonenveloped single-stranded circular DNA virus with a 1,767 nucleotide (nt) or 1,768 nt ambisense genome that contains at least two major open reading frames (ORF1 and ORF2) (16). ORF1 encodes the replication proteins (Rep and Rep′) involved in rolling circle PCV2 DNA replication, and ORF2 encodes the major structural Cap protein (20). Studies of candidate antigens involved in protective immunity against PCV2 have focused mainly on the Cap protein. Neutralizing monoclonal antibodies to PCV2 react with the Cap protein (18), and neutralizing sera from pigs have also been shown to recognize this protein (28).
Immunization against PCV2 has been studied intensely and found to be the most effective strategy for protecting pigs to date (11,13,21). However, the current vaccines do have some disadvantages. Viral titers of the commercially inactivated whole virus, expressed as 50% tissue culture infectious dose (TCID50) per milliliter, obtained from PK-15 cell cultures are usually low. The subunit vaccines are the PCV2 capsid-based subunit vaccines expressed in a Baculovirus vector with high cost. Currently, the commercially inactivated whole virus versus subunit vaccines cause low antibody levels, and the duration of immunity available ranges from 4 to 6 months (12,21,30). Multi-immunization is therefore required to achieve a lasting and efficient immunity response, which increases the cost for farms (9,26). Thus, development of new-generation vaccines is necessary to control PCV2 infection. Direct injection of plasmid DNA has been used as a promising approach to protect animals and humans against pathogens (34,35). DNA vaccines against PCV2 have been investigated, which often have limited efficacy and need two or three immunizations to achieve a good level of immunity (1,3,8). DNA vaccines often have limited efficacy. Therefore, methods to augment the immunogenicity of PCV2 vaccines are desirable.
Interleukin-18 (IL-18), also known as interferon-gamma (IFN-γ)–inducing factor due to its ability to stimulate T-helper 1 (Th1) cells to secrete IFN-γ, has been widely used as an adjuvant to enhance immune responses of many vaccine antigens. IL-18 is a pleiotropic cytokine that plays an important role in both innate and acquired immunity (23). Similar to IL-12, IL-18 facilitates Th1 immune responses, and depending on the cytokine constellation, IL-18 may also promote Th2 type responses. As a vaccine adjuvant (15,19) and an immunomodulatory molecule, IL-18 modulates the immune response toward a Th1 type and enhances the immune responses to DNA vaccines (19,33).
In this study, two recombinant plasmids—pBudCE4.1-ORF2/IL18 and pBudCE4.1-ORF2—containing the ORF2 gene of PCV2 with or without porcine IL-18 were constructed. The immunogenicity of the two recombinant plasmids was investigated using a piglet model. Furthermore, the protective effects of pBudCE4.1-ORF2/IL18 vaccination were compared with those of pBudCE4.1-ORF2 vaccination against PCV2.
Materials and Methods
Cell, virus, and experimental animals
The PK-15 cell line was purchased from China Institute of Veterinary Drug Control, Beijing, China, and maintained in minimal essential medium (GIBCO BRL, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (FBS; GIBCO BRL). PK-15 cells were free of porcine circovirus type 1 (PCV1) and PCV2 according to polymerase chain reaction (PCR) analyses, and were selected through a serial screening for their high PCV2 yield. The Wuzhi strain of PCV2 was originally isolated from the lymph nodes of an 8-week-old pig with naturally occurring PMWS and serially passaged 25 times in PK-15 cells. The virulent PCV2 Wuzhi isolate belonged to the PCV2b genotype according to phylogenetic analysis, and was propagated in a PK-15 subclone cell line. The genome sequence of PCV2 strain Wuzhi has been deposited in GenBank under accession no. HQ650833.
The 3-week-old crossbred piglets, which were negative for PCV2 infections according to PCR analyses, were purchased from the Laboratory Animal Center, Zhengzhou University, Zhengzhou, China, and raised in automatic extrusion-independent venting isolation cages (Fengshi Laboratory Animal Equipment Co. Ltd., Jiangsu, China). The selected animals were provided commercial diets and water ad libitum, and allowed to acclimatize for 7 days before the PCV2 vaccination. All animal procedures were in accordance with the Guidelines for the Care and Use of Animals at Henan Agricultural University (license number SCXK (Henan) 2011-0001), and were reviewed and approved by the Henan Agriculture University Animal Care and Use Committee.
Construction of recombinant eukaryotic expression plasmids
The eukaryotic co-expression vector pBudCE4.1 (Invitrogen, Carlsbad, CA) contains the human cytomegalovirus (CMV) immediate-early promoter and the human elongation factor-1alpha subunit (EF-1α) promoter for high-level, constitutive, independent expression of two recombinant proteins. The ORF2 gene was amplified by PCR from the virulent PCV2 Wuzhi strain using specific primers: ORF2fs and ORF2rs (Table 1). The PCR reaction mixture consisted of 3 μL template DNA, 12 μL rTaq (Takara Bio, Inc., Shiga, Japan), 0.5 μL of each primer (25 μM), and ddH2O to a total volume of 25 μL. The reaction was performed by preheating for 5 min at 95°C, followed by 35 cycles at 94°C for 30 sec, at 58°C for 50 sec, and at 72°C for 1 min, with a final extension for 10 min at 72°C. The ORF2 gene was digested with Sal I and Sca I, and then cloned into the Sal I and Sca I sites of the vector pBudCE4.1 under the control of the CMV promoter to generate the plasmid pBudCE4.1-ORF2. Another pair of specific primers—pIL18fs and pIL18rs—for amplifying the porcine IL-18 gene was designed as shown in Table 1. Porcine IL-18 gene was amplified by PCR from previously cloned cDNA constructs (GenBank accession No. DQ499825) using the porcine IL-18–specific primers, and the PCR reaction mixture was as described above. The reaction was performed by preheating for 5 min at 95°C, followed by 35 cycles at 94°C for 30 sec, at 60°C for 50 sec, and at 72°C for 1 min, with a final extension for 10 min at 72°C. The PCR amplification was digested with Not I and Xho I and then inserted into the Not I and Xho I sites of the EF-1α promoter in the pBudCE4.1-ORF2 construct. The resulting plasmids—pBudCE4.1-ORF2 and pBudCE4.1-ORF2/IL18 (Fig. 1)—were used to transform into Escherichia coli DH5α and sequenced to ensure correct insertions.
Table 1.
Primers Used for PCR Amplification of Target Genes in This Study
| Target gene | Primer | Sequencea(5′-3′) | Restriction site | Expected product (bp) |
|---|---|---|---|---|
| PCV2 ORF2 | ORF2fs | CTT AGT CGA CAT GAC GTA TCC AAG GAG | Sal I | 722 |
| ORF2rs | CGG GAG TAC TAT TCA TTA AGG GTT AAG | Sca I | ||
| Porcine IL-18 | pIL18fs | TAA GCG GCC GCA TGT ATA AGA TGC AGC T | Not I | 599 |
| pIL18rs | CGT CTC GAG TCA AGT CAG TGT TG | Xho I | ||
| PCV2 ORF1 | ORF1fs | TGG GTG TGG CAA AAG CAA ATG | 191 | |
| ORF1rs | TAG TCT CAA CAG TCA AAG GAT |
The restriction enzyme sites used for the construction are underlined.
PCR, polymerase chain reaction; PCV2, porcine circovirus type 2.
FIG. 1.
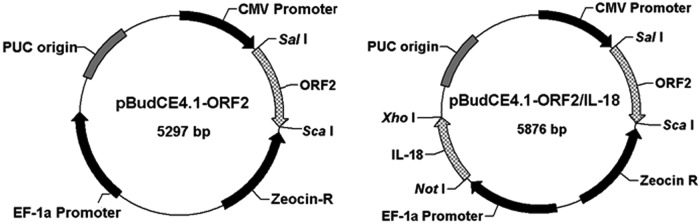
Map of pBudCE4.1-ORF2 and pBudCE4.1-ORF2/IL18. pBudCE4.1-ORF2 was constructed by cloning the PCV2 ORF2 gene into the Sal I and Sca I sites of CMV MCS of pBudCE4.1. To generate pBudCE4.1-ORF2/IL18, the porcine IL-18 DNA fragment was inserted into the Not I and Xho I sites of the constructed pBudCE4.1-ORF2 plasmid in the frame with the PCV2 ORF2 gene.
Preparation of DNA plasmids and transient expression in PK-15 cells
Functional antigen expression from the constructed DNA plasmids was confirmed by Western blot. The eukaryotic expression plasmids pBudCE4.1-ORF2, pBudCE4.1-ORF2/IL18, and pBudCE4.1 were purified using PureYield™ Plasmid Midi-prep System (Promega, Madison, WI) as specified by the manufacturer, and dissolved in endotoxin-free phosphate-buffered saline (PBS, pH 7.2).
PK-15 cells were grown to approximately 80% confluence prior to transfection of the purified plasmids using Lipofectamine™ 2000 Reagent according to the manufacturer's instructions (Invitrogen). Three days after transfection, the total cellular lysates were collected and electrophoresed through an SDS-12% polyacrylamide gel. Afterwards, proteins were then transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA) as described previously (7). The blots were probed with mouse anti-PCV2 mAb (Rural Technologies, Inc., Brookings, SD) or mouse anti-porcine IL-18 mAb (produced by our laboratory, unpublished data), washed, and exposed to horseradish peroxidase (HRP)–labeled anti-mouse IgG antibody (Southern Biotechnology Associates, Inc., Birmingham, AL). The blots were then developed by adding the substrate 3,3′,5,5′-tetramethylbenzidine (Promega).
Experimental design and detection of PCV2-specific antibodies
For vaccination, 20 four-week-old piglets were randomly divided into four groups of five piglets each. Two groups of five piglets were immunized with pBudCE4.1-ORF2/IL18 or pBudCE4.1-ORF2 in a total volume of 1 mL in PBS pH 7.2 (300 μg per piglet).
Other groups included piglets administered with 300 μg of empty vector pBudCE4.1 (group 3), and piglets injected with 1 mL PBS only (group 4). All groups were vaccinated intramuscularly on one side of the neck at 4 weeks and boosted on the same side with an equivalent dose at 3 weeks after the initial inoculation.
At 0, 1, 2, 3, 4, 5, and 6 weeks after the initial immunization, blood samples from all piglets were collected via the vena cava. Total serum immunoglobulin G (IgG) specific for PCV2 was measured by enzyme-linked immunosorbent assay (ELISA) according to a previous described method (31). Briefly, ELISA plates were coated overnight at 4°C with PCV2 lysates as an antigen, and then blocked with 5% skim milk. Serum samples were tested at 1:20 dilution, and IgG against PCV2 was detected with HRP-labeled goat-anti-swine conjugate. The substrate 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma-Aldrich, Shanghai, China) was used to visualize the reaction. The optical density at 450 nm was measured in an ELISA microplate reader. Sera were run in duplicate. Negative and positive control sera were included in each assay. Total serum IgG specific for PCV2 are represented by the optical density.
Peripheral blood lymphocyte proliferation assay
Peripheral blood lymphocyte proliferation assay was performed according to a previous described method (6). Briefly, blood samples from all piglets were collected at 21 days after the boost immunization. Peripheral blood mononuclear cells (PBMCs) were isolated from each blood sample by Ficoll-Paque density gradient centrifugation, and seeded in a 96-well plate in triplicate. Cells were stimulated for 60 h at 37°C in 5% CO2 with 5 μg/mL concanavalin A (Con A; positive control), 5 μg/mL purified Cap antigen (specific antigen) from PCV2, 5 μg/mL bovine serum albumin (BSA; irrelevant antigen), or medium alone (negative control). A 20 μL aliquot of CellTiter 96 Aqueous One Solution Reagent (Promega) was added into each well according to the protocol provided by the manufacturer. The absorbance at 490 nm was measured after incubation for 4 h at 37°C.
Cytokine release assays
The effect of pBudCE4.1-ORF2 and pBudCE4.1-ORF2/IL18 on the generation of Th1 and Th2 phenotypes was investigated. PBMCs as isolated above were resuspended at 5×106/mL and seeded (3×106 cells/well) in a 24-well plate in triplicate. Cells were incubated for 24 h at 37°C with 500 μL PCV2 (106.8TCID50/mL) purified by sucrose density gradient centrifugation or 500 μL complete RPMI medium alone (negative control). Culture supernatants were harvested, and IFN-γ, IL-2, and IL-4 were detected by using commercially available swine IFN-γ, IL-2, and IL-4 sandwich ELISA kits (Biosource, Camarillo, CA) according to the manufacturer's instructions. The concentrations of swine IFN-γ, IL-2, and IL-4 in the samples were determined from appropriate standard curves.
Virus challenge experiment
At 42 days after the initial immunization, all piglets from each group were challenged with 5 mL (2.5 mL intranasally and 2.5 mL intramuscularly) of the virulent PCV2 Wuzhi strain (106.8 TCID50/mL; GenBank accession no. HQ650833). Blood samples were collected at the time of challenge and on a weekly basis thereafter, and sera were stored at −80°C. All piglets in each group were euthanized with an intravenous overdose of sodium pentobarbital on day 28 after the virus challenge. Necropsies were performed immediately postmortem, and heart, liver, spleen, lung, and lymph node were collected to measure the amounts of PCV2 antigens.
SYBR green I real-time PCR for evaluation of viremia
Viral DNA was extracted from the serum samples after the virus challenge using a commercial test kit (QIAamp DNA Mini Kit; Qiagen, Hilden, Germany) according to the manufacturer's instructions, and used to quantify the PCV2 genomic DNA copy numbers by SYBR green I real-time PCR. Potential primer sequences were identified by determination of sequence regions conserved across all available PCV2 genome sequences and not found in PCV1 genomes, and required to be closely matched in predicted annealing temperature with show little potential for dimerization and self-interaction. The primers (Table 1) were selected using the Primer Express v2.0 software, and were based on a highly conserved sequence within the ORF1 region of PCV2 genome.
The SYBR green I real-time PCR assay was performed in a total volume of 25 μL. Each reaction mixture contained 2 μL DNA, 12.5 μL 2×SYBR Green PreMix, and 0.5 μL of 20 μM each primer and 9.5 μL ddH2O. All reactions were conducted in triplicate on an ABI7500 (Applied Biosystems. Foster City, CA).The reaction condition was 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 15 sec, annealing at 55°C for 10 sec, and extension at 72°C for 15 sec. For a standard curve, serial dilutions of plasmid pORF1 (the ORF1 gene cloned into the pGEM-T Easy Vector) were used to quantify the virus genomic copy number. The numbers of virus copies for each sample were presented as the mean value of triplicate reactions.
Immunohistochemistry
The PCV2-specific antigens were detected by using immunohistochemistry (IHC) from the heart, liver, spleen, lung, and lymph node collected during the necropsy on day post-challenge (DPC) 28. A mouse anti-PCV2 mAb was used for IHC following procedures described previously (9). The amount of PCV2 antigen distributed in these tissues was scored in a blinded fashion by assigning a score ranging from 0 for no signal to 3 for a strong positive signal. The mean score was determined for each tissue and compared between groups.
Statistical analysis
As to the analysis of the data, normality in the repeated measures was tested with the Shapiro–Wilk test, while homogeneity of variance was tested using Levene's test. Differences between groups were analyzed by one-way analysis of variance (ANOVA) using the SPSS for Windows v12.0 (SPSS, Inc., Chicago, IL) and Statistical Analysis System (SAS) for Windows v6.12 (SAS Institute, Inc., Cary, NC). p-Values of<0.05 were regarded as significant, and those <0.01 were regarded as highly significant.
Results
Confirmation of functional antigen expression
Prior to vaccination, the Cap and porcine IL-18 proteins secreted by the transfected PK-15 cells were assessed by Western blot analysis using anti-PCV2 mAb and anti-porcine IL-18 mAb. One band with a molecular weight of 27.9 kDa (Cap protein) was detected in transfected cells with both pBudCE4.1-ORF2 and pBudCE4.1-ORF2 /IL18, and one band (porcine IL-18, 22.9 kDa) was detected in transfected cells with pBudCE4.1-ORF2 /IL18, but not in cells transfected with pBudCE4.1 (data not shown). These data demonstrate that the ORF2 and IL-18 genes were expressed in the PK-15 cells.
Antibody responses to PCV2 in piglets vaccinated with recombinant plasmids
Antibody responses in sera were determined by ELISA using PCV2 lysates as a coating antigen. PCV2-specific antibody titers reached detectable levels in piglets immunized with pBudCE4.1-ORF2/IL18 2 weeks after initial immunization, and further increases in antibody levels were observed subsequently (Fig. 2), whereas in piglets immunized with pBudCE4.1-ORF2, PCV2-specific antibody could be detected 3 weeks after initial immunization. Higher total levels of PCV2 Ag–specific antibodies were induced by pBudCE4.1-ORF2/IL18 compared with those induced by pBudCE4.1-ORF2, although this difference did not reach the level of statistical significance (p>0.05). No PCV2-specific antibody responses were detected in piglets inoculated with pBudCE4.1 or PBS before the challenge. All groups had increased levels of serum antibodies against PCV2 following the challenge.
FIG. 2.
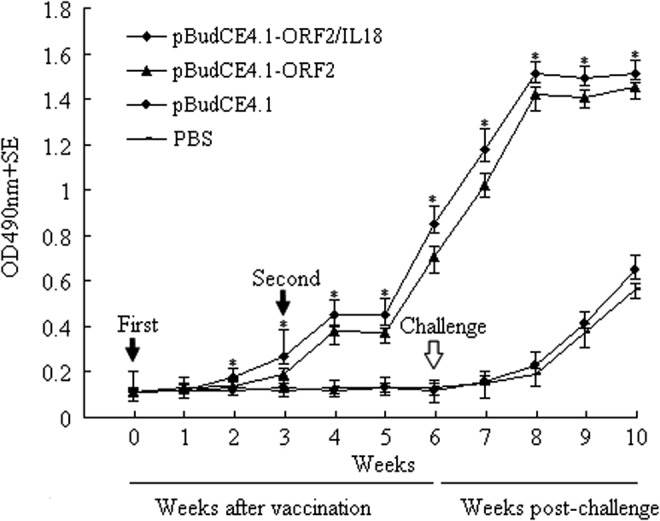
The antibody response to PCV2 assayed by enzyme-linked immunosorbent assay (n=5; i.e., number of pigs analyzed in each experimental group). Piglets were immunized with pBudCE4.1-ORF2/IL18 or pBudCE4.1-ORF2. pBudCE4.1 and phosphate-buffered saline (PBS)–immunized groups were used as negative controls. Three weeks after the first injection, the second injection was offered at the same dose as before. (The time of vaccination is indicated with black arrows.) All piglets from each group were challenged with the virulent PCV2 Wuzhi strain at 42 days (white arrow) after the initial immunization. Sera were collected weekly via the vena cava. Values are expressed as mean absorbance values±standard error. *p<0.05 (compared with pBudCE4.1 or PBS).
Cap-protein–specific T-cell proliferation
To determine whether T-cell proliferation response to the DNA vaccine encoding the Cap protein may be boosted by porcine IL-18, we examined the PBMCs from the vaccinated piglets for antigen-specific T-cell proliferation. As shown in Figure 3, antigen-specific T-lymphocyte proliferation responses in piglets were induced following DNA immunization. There was a significant difference (Fig. 3; p<0.05) between the vaccine groups and the negative control groups (pBudCE4.1 and PBS separately). The SI in the pBudCE4.1-ORF2/IL18 group was higher than that in the pBudCE4.1-ORF2 group (Fig. 3; p<0.05). The Con A control group showed a stimulation index of 4 to 5. These results indicate that the DNA vaccine candidates induced T-lymphocyte proliferation and that the SI could be markedly increased by porcine IL-18.
FIG. 3.
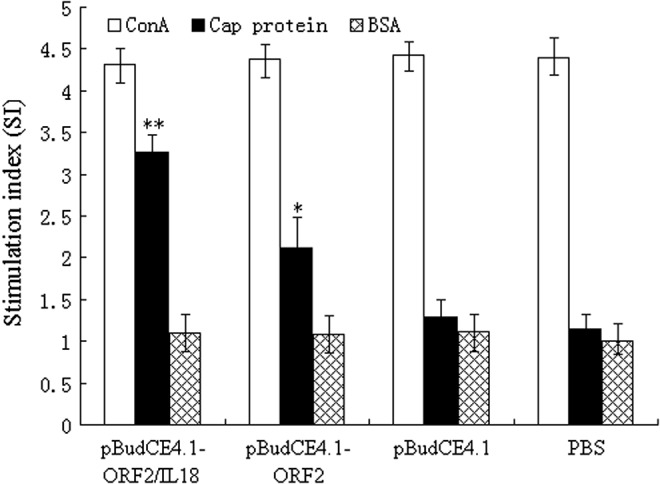
Peripheral blood T-lymphocyte proliferation assay (n=5; i.e., number of pigs analyzed in each experimental group). Five peripheral blood samples from five piglets in each group were collected via the vena cava at 21 days after the boost immunization. Values are expressed as mean SI-value±standard error. *p<0.05 (compared with pBudCE4.1 or PBS); **p<0.05 (compared with pBudCE4.1-ORF2).
Levels of Th1 and Th2 cytokines
The concentrations of all three cytokines increased to varying extents in the vaccine groups compared with controls, as shown in Figure 4. Higher levels of IL-4 were detected in the vaccine groups compared with those in the control groups (Fig. 4; p<0.05), although the levels in the pBudCE4.1-ORF2 and pBudCE4.1-ORF2/IL18 groups were statistically comparable (p>0.05). However, the IL-2 and IFN-γ levels increased significantly following immunization with pBudCE4.1-ORF2/IL18 compared with pBudCE4.1-ORF2 (Fig. 4; p<0.05). This profile of cytokine secretion suggests that porcine IL-18 enhances the induction of immune responses by promoting a Th1-dominant response.
FIG. 4.
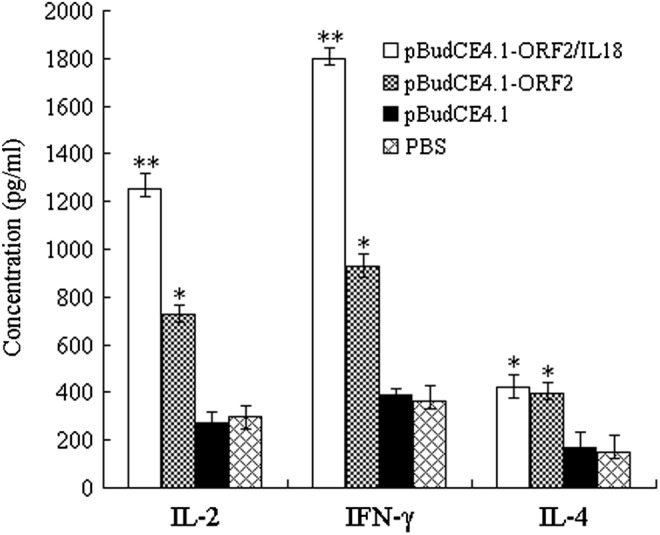
Levels of cytokine production from peripheral blood mononuclear cells after the capsid protein stimulation in vitro (n=5; i.e., number of pigs analyzed in each experimental group). Five peripheral blood samples from five piglets in each group were collected via the vena cava at 21 days after the boost immunization. Values are expressed as mean counts±standard error. *p<0.05 (compared with pBudCE4.1 or PBS); **p<0.05 (compared with pBudCE4.1-ORF2).
Incidence and amount of PCV2 DNA in serum
The genomic DNA of PCV2 in sera was quantified by SYBR green I real-time PCR. PCV2 DNA was not detected in any of the serum samples on the day of the challenge. As shown in Figure 5A, in the pBudCE4.1-ORF2/IL18-immunized group, one out of five piglets had PCV2 viremia at 21 days after the PCV2 challenge, and no viremia was observed at 28 days after the PCV2 challenge, whereas in the pBudCE4.1-ORF2-immunized group, three out of five piglets had PCV2 viremia at 21 days after the PCV2 challenge, and the PCV2 viremia in one out of five piglets persisted for at least 28 days. However, in control groups immunized with either the pBudCE4.1 control vector or PBS, all piglets had PCV2 viremia, which persisted for at least 28 days. In addition, the piglets immunized with either the pBudCE4.1 control vector or PBS exhibited an increase in the level of viremia compared with those immunized with pBudCE4.1-ORF2/IL18 and pBudCE4.1-ORF2 (Fig. 5B), but this difference was not significant (p>0.05).
FIG. 5.
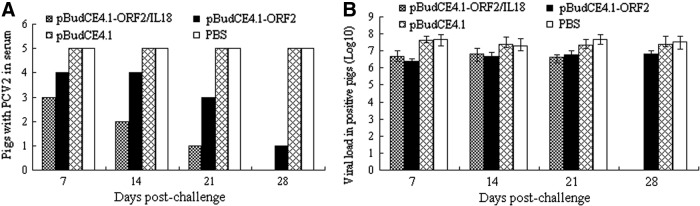
Incidence and amount of PCV2 DNA in serum quantified by SYBR green I real-time polymerase chain reaction. (A) PCV2 prevalence in the serum of pigs following intranasal and intramuscular inoculations with PCV2. (B) The mean viral load of the PCV2-positive pigs (Log10) from different groups. Values are expressed as mean counts±standard error.
Detection of the PCV2 antigen in tissues
The incidences of the PCV2 antigen in tissues at necropsy (DPC 28) were determined by IHC for groups. As shown in Table 2, PCV2 antigen was detected in the lymph nodes and lung from one out of five piglets immunized with pBudCE4.1-ORF2/IL18, whereas it was detected in all the organs from piglets immunized with pBudCE4.1-ORF2, and PCV2 antigen was detected in the lymph nodes from three out of five piglets in the pBudCE4.1-ORF2-immunized group. The amounts of PCV2 antigen in piglets immunized with pBudCE4.1 or PBS were significantly higher than those in the piglets immunized with pBudCE4.1-ORF2/IL18 and pBudCE4.1-ORF2 in the lung and lymph nodes (p<0.05). In addition, compared with piglets immunized with either the pBudCE4.1 control vector or PBS, those immunized with pBudCE4.1-ORF2/IL18 and pBudCE4.1-ORF2 exhibited a reduction in the amounts of PCV2 antigen in the heart, liver and spleen, although these differences were not significant (p>0.05).
Table 2.
Immunohistochemistry Detection Results and Mean Score in the Tissues of Pigs at Necropsy 28 Days Following Intranasal and Intramuscular Inoculations with PCV2
| No. of piglets with IHC detection positive/total | Mean scorea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Heart | Liver | Spleen | Lung | Lymph node | Heart | Liver | Spleen | Lung | Lymph node |
| pBudCE4.1-ORF2/IL18 | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | 0.0±0.00 | 0.0±0.00 | 0.0±0.00 | 0.2±0.45 | 0.4±0.72 |
| pBudCE4.1-ORF2 | 1/5 | 1/5 | 1/5 | 1/5 | 3/5 | 0.2±0.57 | 0.0±0.00 | 0.2±0.75 | 0.4±0.65 | 0.6±0.43 |
| pBudCE4.1 | 3/5 | 3/5 | 4/5 | 4/5 | 5/5 | 0.8±0.39 | 1.0±0.45 | 1.4±0.71 | 1.8±0.39* | 2.6±0.62* |
| PBS | 3/5 | 3/5 | 4/5 | 5/5 | 5/5 | 1.0±0.73 | 1.2±0.55 | 1.6±0.55 | 2.0±0.71* | 2.8±0.63* |
Values are the mean estimated amounts of the PCV2 antigen in the tissues (range: 0, no antigen detected; 3, high amounts of antigen).
p<0.05 (compared with pBudCE4.1-ORF2/IL18 or pBudCE4.1-ORF2).
IHC, immunohistochemistry; PBS, phosphate-buffered saline.
Discussion
Recently, a newly recognized PCV2 variant, genotype PCV2b, and a shift from PCV2a to PCV2b were identified concurrently around the world (14). PCV2a and PCV2b genotypes share an identity of approximately 95% (32). The current commercial vaccines are based on PCV2a genotype. Cross-protection between PCV2a and PCV2b genotypes is further supported by the efficacy of PCV2a-based vaccines under field conditions (5,24,27). However, PCV2-associated diseases (PCVAD) outbreaks in vaccinated herds do occur (25). Thus, a new generation of PCV2 vaccines based on PCV2b genotype is necessary.
IL-18 is an important cytokine with multiple functions in innate and acquired immunity (17). Similar to IL-12, the dominant function of IL-18 is to facilitate Th1 immune responses.
Plasmids expressing IL-18 have been investigated as potential vaccine adjuvants in several studies and have been shown to increase protective immunity by DNA vaccine against pathogens (19,36). Here, we selected porcine IL-18 as an adjuvant to improve the immunogenicity of a PCV2 DNA vector vaccine in a PCV2 challenge model. In this study, the pBudCE4.1-ORF2/IL18 and pBudCE4.1-ORF2 plasmids were constructed containing the ORF2 gene with or without porcine IL-18 based on the plasmid pBudCE4.1. Furthermore, investigation of the protective effects of experimental immunization with recombinant plasmids in a PCV2-challenge model revealed that vaccination with the co-expression pBudCE4.1-ORF2/IL18 plasmid induced stronger immune responses than vaccination with pBudCE4.1-ORF2. Thus, these observations indicate that vaccination with pBudCE4.1-ORF2/IL18 co-expressing the PCV2 Cap protein and IL-18 elicits a potent specific immune response.
The activation and the proliferation of lymphocytes play a critical role in both the humoral and cellular immune responses induced by vaccination. Therefore, the influence of vaccination with pBudCE4.1-ORF2/IL18 and pBudCE4.1-ORF2 on the antigen-specific T-cell proliferation response was investigated. Piglets immunized with pBudCE4.1-ORF2 exhibited a specific T-cell proliferative response. However, response in pBudCE4.1-ORF2/IL18-immunized piglets was significantly higher (p<0.05), suggesting that porcine IL-18 stimulates T-cell proliferation. Similar results were also reported by Yin et al. (36) and Zhu et al. (37). These data clearly show that IL-18 is a strong adjuvant that enhances vaccine potency.
To demonstrate whether the DNA vaccine induces a sufficiently protective immune response, the immune responses of 4-week-old piglets were analyzed by ELISA antibody titers. All DNA vaccine-immunized groups produced PCV2-specific antibodies at 21 days after vaccination, and further increases in antibody levels were observed subsequently (Fig. 2). The level of specific antibodies induced in the pBudCE4.1-ORF2/IL18-immunized group was slightly higher but not significantly different (p>0.05) than that induced in the pBudCE4.1-ORF2 group from the second week after vaccination. However, the pBudCE4.1-ORF2/IL18-immunized group had better inhibition of viruses than the pBudCE4.1-ORF2-immunized group. Furthermore, PCV2 antigen was detected only in the lung and lymph node from one out of five piglets immunized with pBudCE4.1-ORF2/IL18 on day 28 after challenge, whereas for pBudCE4.1-ORF2-immunized piglets, low amounts of PCV2 antigen were detected in all the organs. The results show that the piglets immunized with pBudCE4.1-ORF2/IL18 exhibited a marked inhibition of PCV2 replication compared to the pBudCE4.1-ORF2 group, demonstrating that the absolute levels of antibody cannot be used alone to evaluate the immunoprotective effects of a vaccine. The results suggest that the cellular immunity of PCV2 is also very important for the protection of the pig from the challenge, which is similar to results reported by Fenaux et al. (9).
Viral clearance for PCV2 infection can be mediated by cell-mediated responses. It has become evident that T-cell–mediated immunity via inducing a strong Cap-specific Th1 immune response is essential for effective protection against PCV2 infection (22). The function of IL-18 (also known as IFN-γ inducing factor) is reflected in the enhancement of cell-mediated immunity and in regulating both Th1- and Th2-driven immune responses. Therefore, it can be speculated that the protective immunity resulting from vaccination with pBudCE4.1-ORF2/IL18 can be attributed to enhanced cell-mediated immunity, demonstrated by increased splenocyte proliferation and increased levels of cytokine (IL-2 and IFN-γ) production. In this study, the T-lymphocyte proliferative responses and the profile of cytokine secretion suggest that porcine IL-18 enhances the induction of immune responses by promoting a Th1-dominant response. These findings are consistent with the results of other studies of the use of IL-18 plasmids as adjuvants in DNA vaccines (17,36). Therefore, porcine IL-18 is implicated as a broadly effective Th1 adjuvant suitable for the development of PCV2 vaccines.
We verified the ability of the pBudCE4.1-ORF2/IL18 plasmid to express Cap protein both in vitro and in vivo by demonstrating the induction of antibodies in piglets immunized with the plasmid. Using DNA-based immunization rather than more conventional methods has several advantages. First and foremost, it eliminates the need for performing conventional antigen preparation, which is rather laborious. Furthermore, DNA-based immunization enables the use of a very pure immunogen, since plasmid preparations can readily be purified from protein contaminants. Finally, DNA immunization has been shown to be capable of inducing immunity irrespective of the presence of maternally derived antibodies (4,10). The strong immunogenicity of ORF2/IL18-encoding plasmid in piglets suggests that the pBudCE4.1-ORF2/IL18 plasmid may be useful in the control of PCV2 infections on both an individual and a population basis, even in the presence of maternally derived immunity.
This study demonstrates the induction of both antibody and T-cell responses that provide protection against PCV2 challenge in piglets in response to co-expression of the Cap protein from the PCV2 and porcine IL-18 via the pBudCE4.1-ORF2/IL18 plasmid. These data indicate that the pBudCE4.1-ORF2/IL18 plasmid may be an effective approach for increasing PCV2 DNA vaccine immunogenicity. In the case of PCV2, this would be the ability of the vaccine to reduce viremia and lymphoid tissue lesions. Further studies should be conducted to investigate whether this type of vaccination (DNA) can be utilized in the swine industry.
Acknowledgments
This work was supported by a grant from Important Henan Province Science and Technology Specific Projects (111100110300) and Innovation Scientists and Technicians Troop Construction Projects of Zhengzhou City (10CXTD148).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aravindaram K, Kuo TY, Lan CW, et al. . Protective immunity against porcine circovirus 2 in mice induced by a gene-based combination vaccination. J Gene Med 2009;11:288–301 [DOI] [PubMed] [Google Scholar]
- 2.Baekbo P, Kristensen CS, and Larsen LE. Porcine circovirus diseases: a review of PMWS. Transbound Emerg Dis 2012;17:1865–1682 [DOI] [PubMed] [Google Scholar]
- 3.Blanchard P, Mahe D, Cariolet R, et al. . Protection of swine against post-weaning multisystemic wasting syndrome (PMWS) by porcine circovirus type 2 (PCV2) proteins. Vaccine 2003;21:4565–4575 [DOI] [PubMed] [Google Scholar]
- 4.Bot A, and Bona C. Genetic immunization of neonates. Microbes Infect 2002;4:511–520 [DOI] [PubMed] [Google Scholar]
- 5.Chae C. Commercial porcine circovirus type 2 vaccines: efficacy and clinical application. Vet J 2012;194:151–157 [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Shang YH, Yao HX, et al. . Immune responses of chickens inoculated with a recombinant fowlpox vaccine coexpressing HA of H9N2 avain influenza virus and chicken IL-18. Antiviral Res 2011;91:50–56 [DOI] [PubMed] [Google Scholar]
- 7.Chen HY, Zheng LL, Li XS, et al. . Cloning, in vitro expression, and bioactivity of interleukin-18 isolated from a domestic porcine breed found in Henan. FEMS Immunol Med Microbiol 2009;57:129–135 [DOI] [PubMed] [Google Scholar]
- 8.Dong B, Feng J, Lin H, et al. . Immune responses of mice immunized by DNA plasmids encoding PCV2 ORF 2 gene, porcine IL-15 or the both. Vaccine 2013;31:5736–5744 [DOI] [PubMed] [Google Scholar]
- 9.Fenaux M, Opriessnig T, Halbur PG, et al. . A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J Virol 2004;78:6297–6303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer L, Barzu S, Andreoni C, et al. . DNA vaccination of neonate piglets in the face of maternal immunity induces humoral memory and protection against a virulent pseudorabies virus challenge. Vaccine 2003;21:1732–1741 [DOI] [PubMed] [Google Scholar]
- 11.Fort M, Sibila M, Nofrarias M, et al. . Evaluation of cell-mediated immune responses against porcine circovirus type 2 (PCV2) Cap and Rep proteins after vaccination with a commercial PCV2 sub-unit vaccine. Vet Immunol Immunopathol 2012;150:128–132 [DOI] [PubMed] [Google Scholar]
- 12.Fraile L, Grau-Roma L, Sarasola P, et al. . Inactivated PCV2 one shot vaccine applied in 3-week-old piglets: improvement of production parameters and interaction with maternally derived immunity. Vaccine 2012;30:1986–1992 [DOI] [PubMed] [Google Scholar]
- 13.Fraile L, Sibila M, Nofrarias M, et al. . Effect of sow and piglet porcine circovirus type 2 (PCV2) vaccination on piglet mortality, viraemia, antibody titre and production parameters. Vet Microbiol 2012;161:229–234 [DOI] [PubMed] [Google Scholar]
- 14.Grau-Roma L, Fraile L, and Segales J. Recent advances in the epidemiology, diagnosis and control of diseases caused by porcine circovirus type 2. Vet J 2011;187:23–32 [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Cho D, Hwang SY, and Kim TS. Efficient induction of antigen-specific, T helper type 1-mediated immune responses by intramuscular injection with ovalbumin/interleukin-18 fusion DNA. Vaccine 2001;19:4107–4114 [DOI] [PubMed] [Google Scholar]
- 16.Mankertz A, Domingo M, Folch JM, et al. . Characterisation of PCV-2 isolates from Spain, Germany and France. Virus Res 2000;66:65–77 [DOI] [PubMed] [Google Scholar]
- 17.Marshall DJ, Rudnick KA, McCarthy SG, et al. . Interleukin-18 enhances Th1 immunity and tumor protection of a DNA vaccine. Vaccine 2006;24:244–253 [DOI] [PubMed] [Google Scholar]
- 18.McNeilly F, McNair I, Mackie DP, et al. . Production, characterisation and applications of monoclonal antibodies to porcine circovirus 2. Arch Virol 2001;146:909–922 [DOI] [PubMed] [Google Scholar]
- 19.Mingxiao M, Ningyi J, Juan LH, et al. . Immunogenicity of plasmids encoding P12A and 3C of FMDV and swine IL-18. Antiviral Res 2007;76:59–67 [DOI] [PubMed] [Google Scholar]
- 20.Nawagitgul P, Morozov I, Bolin SR, et al. . Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J Gen Virol 2000;81:2281–2287 [DOI] [PubMed] [Google Scholar]
- 21.O'Neill KC, Shen HG, Lin K, et al. . Studies on porcine circovirus type 2 vaccination of 5-day-old piglets. Clin Vaccine Immunol 2011;18:1865–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh Y, Seo HW, Han K, et al. . Protective effect of the maternally derived porcine circovirus type 2 (PCV2)-specific cellular immune response in piglets by dam vaccination against PCV2 challenge. J Gen Virol 2012;93:1556–1562 [DOI] [PubMed] [Google Scholar]
- 23.Okamura H, Tsutsi H, Komatsu T, et al. . Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995;378:88–91 [DOI] [PubMed] [Google Scholar]
- 24.Opriessnig T, Gerber PF, Xiao CT, et al. . A commercial vaccine based on PCV2a and an experimental vaccine based on a variant mPCV2b are both effective in protecting pigs against challenge with a 2013 U.S. variant mPCV2b strain. Vaccine 2014;32:230–237 [DOI] [PubMed] [Google Scholar]
- 25.Opriessnig T, O'Neill K, Gerber PF, et al. . A PCV2 vaccine based on genotype 2b is more effective than a 2a-based vaccine to protect against PCV2b or combined PCV2a/2b viremia in pigs with concurrent PCV2, PRRSV and PPV infection. Vaccine 2013;31:487–494 [DOI] [PubMed] [Google Scholar]
- 26.Opriessnig T, Patterson AR, Madson DM, et al. . Comparison of efficacy of commercial one dose and two dose PCV2 vaccines using a mixed PRRSV-PCV2-SIV clinical infection model 2–3 months post vaccination. Vaccine 2009;27:1002–1007 [DOI] [PubMed] [Google Scholar]
- 27.Opriessnig T, Ramamoorthy S, Madson DM, et al. . Differences in virulence among porcine circovirus type 2 isolates are unrelated to cluster type 2a or 2b and prior infection provides heterologous protection. J Gen Virol 2008;89:2482–2491 [DOI] [PubMed] [Google Scholar]
- 28.Pogranichnyy RM, Yoon KJ, Harms PA, et al. . Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral Immunol 2000;13:143–153 [DOI] [PubMed] [Google Scholar]
- 29.Segales J, Kekarainen T, and Cortey M. The natural history of porcine circovirus type 2: from an inoffensive virus to a devastating swine disease? Vet Microbiol 2013;165:13–20 [DOI] [PubMed] [Google Scholar]
- 30.Seo HW, Han K, Park C, and Chae C. Clinical, virological, immunological and pathological evaluation of four porcine circovirus type 2 vaccines. Vet J 2014;200:65–70 [DOI] [PubMed] [Google Scholar]
- 31.Shen HG, Zhou JY, Huang ZY, et al. . Protective immunity against porcine circovirus 2 by vaccination with ORF2-based DNA and subunit vaccines in mice. J Gen Virol 2008;89:1857–1865 [DOI] [PubMed] [Google Scholar]
- 32.Trible BR, and Rowland RR. Genetic variation of porcine circovirus type 2 (PCV2) and its relevance to vaccination, pathogenesis and diagnosis. Virus Res 2012;164:68–77 [DOI] [PubMed] [Google Scholar]
- 33.Wei F, Liu Q, Gao S, et al. . Enhancement by IL-18 of the protective effect of a Schistosoma japonicum 26kDa GST plasmid DNA vaccine in mice. Vaccine 2008;26:4145–4149 [DOI] [PubMed] [Google Scholar]
- 34.Wei H, Lenz SD, Thompson DH, and Pogranichniy RM. DNA-epitope vaccine provided efficient protection to mice against lethal dose of influenza A virus H1N1. Viral Immunol 2014;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen J, Hao W, Fan Y, et al. . Co-delivery of LIGHT expression plasmid enhances humoral and cellular immune responses to HIV-1 Nef in mice. Arch Virol 2014;17:17. [DOI] [PubMed] [Google Scholar]
- 36.Yin RL, Li C, Yang ZT, et al. . Construction and immunogenicity of a DNA vaccine containing clumping factor A of Staphylococcus aureus and bovine IL18. Vet Immunol Immunopathol 2009;132:270–274 [DOI] [PubMed] [Google Scholar]
- 37.Zhu M, Xu X, Liu H, et al. . Enhancement of DNA vaccine potency against herpes simplex virus 1 by co-administration of an interleukin-18 expression plasmid as a genetic adjuvant. J Med Microbiol 2003;52:223–228 [DOI] [PubMed] [Google Scholar]


