Abstract
Dietary changes could potentially reduce prostate cancer morbidity and mortality. Transgenic adenocarcinoma of the mouse prostate (TRAMP) prostate tumor responses to a 100 g of fat/kg diet (whole walnuts, walnut oil, and other oils; balanced for macronutrients, tocopherols [α-and γ]) for 18 weeks ad libitum were assessed. TRAMP mice (n=17 per group) were fed diets with 100 g fat from either whole walnuts (diet group WW), walnut-like fat (diet group WLF, oils blended to match walnut's fatty acid profile), or as walnut oil (diet group WO, pressed from the same walnuts as WW). Fasted plasma glucose was from tail vein blood, blood was obtained by cardiac puncture, and plasma stored frozen until analysis. Prostate (genitourinary intact [GUI]) was weighed and stored frozen at −80°C. Plasma triglyceride, lipoprotein cholesterol, plasma multianalyte levels (Myriad RBM Rat Metabolic MAP), prostate (GUI), tissue metabolites (Metabolon, Inc., Durham, NC, USA), and mRNA (by Illumina NGS) were determined. The prostate tumor size, plasma insulin-like growth factor-1 (IGF-1), high density lipoprotein, and total cholesterol all decreased significantly (P<.05) in both WW and WO compared to WLF. Both WW and WO versus WLF showed increased insulin sensitivity (Homeostasis Model Assessment [HOMA]), and tissue metabolomics found reduced glucose-6-phosphate, succinylcarnitine, and 4-hydroxybutyrate in these groups suggesting effects on cellular energy status. Tissue mRNA levels also showed changes suggestive of altered glucose metabolism with WW and WO diet groups having increased PCK1 and CIDEC mRNA expression, known for their roles in gluconeogenesis and increased insulin sensitivity, respectively. WW and WO group tissues also had increased MSMB mRNa a tumor suppressor and decreased COX-2 mRNA, both reported to inhibit prostate tumor growth. Walnuts reduced prostate tumor growth by affecting energy metabolism along with decreased plasma IGF-1 and cholesterol. These effects are not due to the walnut's N-3 fatty acids, but due to component(s) found in the walnut's fat component.
Key Words: : chemoprevention, fat, insulin-like growth factor 1, prostate cancer, transgenic adenocarcinoma of the mouse prostate model, walnut, whole foods
Introduction
Frequent tree nut consumption is associated not only with reduced cardiovascular disease risk factors but also cancer as well.1,2 The recent PREDIMED human intervention study found that increased nut consumption significantly reduced the risk of all-cause mortality along with reduced risk of cancer mortality, an effect specific to walnuts.3 Moreover, a recent, large, epidemiological human cohort study found nut consumption had significant inverse associations with total deaths along with those attributable specifically to cancer, heart disease, and respiratory disease.4
Increasingly, studies using animal models have been used to gain basic insight into the health effects of walnut consumption.5–10 Our previous mouse feeding study showed that walnuts reduced prostate cancer (PCa) tumor growth and used soybean oil as a control because it has virtually the same fatty acid ratios as walnuts.8 However, other studies of PCa and walnuts used corn oil as their control fat despite the differences in fatty acid composition between corn oil and walnut fats.11 These differences in fat composition between the walnut and control diets make it difficult to conclude with certainty that the effects noted ascribed to walnuts are not due simply to the differences in fatty acids. The current study uses the same high-fat diet-fed transgenic adenocarcinoma of the mouse prostate (TRAMP) PCa model, but the control diet (walnut-like fat [WLF]) contained an oil blended from nonwalnut sources to be virtually identical in fatty acids to walnut fat, whereas the fat source of the other two diets was either from whole walnuts (WW) or from walnut oil (WO), which was pressed from the same batch of walnuts as the WW diet. This design allows a more direct assessment of the specific contributions of walnuts and their constituents in altering prostate tumor growth.
Materials and Methods
Animals
Tramp mice [C57BL/6-Tg (TRAMP) 8247Ng/J] were purchased from Jackson Laboratories (JAX Mice and Services, Bar Harbor, ME, USA). A large number of mice required necessitated multiple small cohorts shipped at 6–7 weeks old over the course of 3 months.
Animals were housed in individual cages (USDA, Western Regional Research Center, Albany, CA, USA) in a temperature/humidity controlled room with free access to water and diets. Animals were fed standard rodent chow until the start of the experimental diets at 8 weeks old and randomized into the three diet groups (n=17/group). Feed consumption was recorded twice per week and animals weighed weekly. After 18 weeks of ad libitum feeding, animals were killed under anesthesia by diaphragm puncture. Procedures involving animals followed institutional and national guidelines and were approved by both the Animal Care and Use Committee, USDA Western Regional Research Center (Albany, CA, USA) and the UCDavis Institutional Animal Care and Use Committee.
Diet
The diet composition (100 g fat/kg diet) was based on the high-fat diet previously used.8 The experimental design was specifically modified by having three diet groups: one group of animals (WLF) consumed a high-fat diet compounded from a blend of nonwalnut vegetable oils to match walnut fat; another diet group (WO) consumed a diet formulated with WO pressed from the same batch of walnuts used as the WW group; and finally, a group was fed a high-fat diet from WW. The diets used were custom compounded (Dyets, Inc., Bethlehem, PA, USA) and had the same macronutrient composition (minerals, carbohydrates, proteins, and alpha and gamma tocopherol content). The WLF diet was shipped complete, whereas the other diets were shipped as powders and finalized by the addition of either WW or WO. Walnuts were provided as shelled whole kernels with brown husk by the California Walnut Commission. Diets were stored at−20°C until provided to the animals.
Sampling
Cardiac puncture was used to collect blood into EDTA-rinsed syringes and the plasma was isolated and stored as multiple aliquots at −80°C until analysis. The prostate genitourinary intact (GUI), that is, the bladder, seminal vesicles, and coagulating and ampullary glands were removed and weighed. The GUI was then flash frozen in liquid nitrogen and separately stored at −80°C until analysis. Dissection and analysis of prostate tissue might seem preferable; however, in the later stages of TRAMP tumors, the tissues of the GUI form a solid mass making dissection and identification of specifically the prostate tissue extremely difficult and unreliable. Moreover, the use of the GUI allows assessment of the changes in both the tumor and its microenvironment, both of which are essential for tumor formation and progression.12
Plasma analysis
Plasma glucose was measured using a handheld glucometer (LifeScan, Inc., Milpitas, CA, USA) from blood from the animal's tail vein blood immediately before sacrifice.
Plasma lipoprotein cholesterol was determined by size exclusion chromatography, as previously described (30). Bovine cholesterol lipoprotein standards were used to calibrate the HPLC signal on the basis of peak areas.
Plasma samples were submitted to Myriad/Rules Based Medicine (Myriad RBM, Austin, TX, USA) for analysis using their rodent quantitative protein biomarkers metabolic Multi-Analyte Profile.
Metabolomic analysis
GUI tissues selected at random (n=8–11/diet) were submitted for metabolomic analysis. Analysis used mView, a metabolomic profiling system (Metabolon, Inc., Durham, NC, USA), and compounds were identified by matching with purified standards.
mRNA analysis
mRNA was isolated from whole GUI and then characterized by next-generation sequencing (NGS), a nontargeted approach that can quantify much lower mRNA levels and is not restricted to an arbitrary number of mRNA species. The mRNA was isolated from four individual GUI/diet according to the manufacturer's instructions (TRIzol plus RNA purification kit; Invitrogen, Life Technologies, Carlsbad, CA, USA). PolyA+ RNA was selected [Sera-Mag oligo(dT) beads; Thermo Scientific] and fragmented (Ambion Fragmentation Reagents kit; Ambion, Austin, TX, USA). cDNA synthesis, end repair, A-base addition, and ligation of the Illumina PCR adaptors were performed according to Illumina's protocol. Libraries were then size selected on a 3.5% agarose gel and PCR amplified for 15–18 PCR cycles. PCR products were then purified and libraries sequenced on an Illumina Genome Analyzer I flow cell. Scythe (version 0.981) and Sickle (version 1.2; https://github.com/ucdavis-bioinformatics) were used for Illumina adapter trimming and quality trimming. BWA's short read aligner (version 0.6.2) and SAMtools (version 0.1.18) were used to align reads (15–18 million/sample) to transcript sequences derived from the current mouse genome build (mm10) and generate alignment files for viewing and read counting, which was done using sam2counts (https://github.com/ucdavis-bioinformatics).13
Statistical analysis
Data for each mouse were collected in Excel and statistical analysis was performed using ANOVA (JMP Pro 11 for Macintosh; SAS, Inc., Cary, NC, USA) with differences having a P<.05 considered significant. Metabolomics data were assessed using Welch's two-sample t test. Q values were computed, but no absolute cutoff was set to maximize the power to detect differentially regulated metabolites and those ratios having a P<.05 were considered significant. For NGS data, several packages from Bioconductor (release 2.11; www.bioconductor.org/)—org.Mm.eg.db (for translation of ids) and DESeq—were used to normalize read counts and test for differential expression (DE) between sample groups, producing lists of DE transcripts/genes. In addition, TopHat (version 2.02) and Cufflinks (version 2.02) were used to align reads directly to the genome (mm10) and then test for DE between the sample groups. The mouse annotation package from Bioconductor (org.Mm.eg.db) was used in custom R scripts to translate between transcript and gene ids, as needed.
Results
Tissue weights
The effect(s) of the diets on body weight and prostate weight are presented in Table 1. Body weight did not differ, whereas whole prostate weights were statistically significantly lower in the WW and WO diet groups compared to the WLF diet group. Increases or decreases in the GUI weight have been shown to correlate closely with increased or decreased prostate tumor burden and occurrence.8,14
Table 1.
Body and Prostate (as GUI) Weights
( ±SEM) ±SEM) |
WW | WLF | WO |
|---|---|---|---|
| Body weight | 28.24±1.04 | 31.68±1.09 | 29.44±0.87 |
| GUI weight | 1.24±0.06a | 1.55±0.10b | 1.28±0.06a |
TRAMP mice (n=17/diet, 7–8 weeks old) were allowed ad libitum access for 18 weeks to one of three different diets. The diets were identical in terms of macro and micronutrients and contained 100 g/kg diet of dietary fat provided as WW, WLF (fats blended to mimic walnut fatty acid composition), or WO (pressed from same walnuts as used for WW diets). Animals were weighed, then killed, and GUI was obtained by dissection and weighed.
Mean values with unlike letters are significantly different (P<.05).
GUI, genitourinary intact; WLF, walnut-like fat; WO, walnut oil; WW, whole walnuts.
Plasma analytes, triglycerides, and lipoprotein cholesterol
Plasma glucose, insulin, insulin-like growth factor-1 (IGF-1), leptin, and testosterone resulting from feeding the different diets are presented in Table 2. Homeostasis Model Assessment (HOMA) as a measure of insulin resistance is presented for each diet group in Table 2 as well.
Table 2.
Plasma Analytes and Homeostatic Model Assessment
Plasma analyte ( ±SEM) ±SEM) |
WW (n=15) | WLF (n=16) | WO (n=17) |
|---|---|---|---|
| Glucose (mg/dL) | 162±9b | 192±8a | 172±8a,b |
| Insulin | 3.49±0.19 | 3.65±0.21 | 3.16±0.15 |
| IGF-1 | 54±3a | 69±4b | 58±2a |
| Leptin | 2.5±0.4a | 6.3±0.8b | 4.6±0.7a,b |
| Testosterone | 12.2±3.6 | 4.0±1.3 | 8.2±2.3a |
| HOMA | 25.25±1.90a | 31.11±1.99b | 24.29±1.84a |
TRAMP mice (n=17, 7–8 weeks old) were allowed ad libitum access for 18 weeks to one of three different diets. The diets were identical in terms of macro and micronutrients and contained 100 g/kg diet of dietary fat provided as WW, WLF (fats blended to mimic walnut fatty acid composition), or WO (pressed from same walnuts as used for WW diets). Glucose was measured using tail vein blood and handheld glucometer. Plasma was obtained from blood drawn through cardiac puncture. HOMA was calculated from values obtained to assess insulin sensitivity.
Mean values with unlike letters are significantly different (P<.05).
HOMA, homeostatic model assessment; IGF-1, insulin-like growth factor-1.
Plasma total triglyceride and cholesterol, as well as the distribution of cholesterol among the different plasma lipoprotein fractions are presented in Table 3.
Table 3.
Plasma Triglyceride, Total and Subfraction Cholesterol ( ±SEM)
±SEM)
| WW | WLF | WO | |
|---|---|---|---|
| TG (mmol/L) | 0.74±0.037 | 0.78±0.050 | 0.80±0.055 |
| TC (mmol/L) | 2.27±0.080a | 2.87±0.12b | 2.42±0.11a |
| VLDL (mmol/L) | 0.052±0.005 | 0.063±0.0041 | 0.059±0.0052 |
| LDL (mmol/L) | 0.26±0.021a | 0.51±0.057b | 0.40±0.037a |
| HDL (mmol/L) | 1.96±0.061a | 2.29±0.091b | 1.96±0.075a |
TRAMP mice (n=17, 7–8 weeks old) were allowed ad libitum access for 18 weeks to one of three different diets. The diets were identical in terms of macro and micronutrients and contained 100 g/kg diet of dietary fat provided as WW, WLF (fats blended to mimic walnut fatty acid composition), or WO (pressed from same walnuts as used for WW diets). Plasma was obtained from blood drawn through cardiac puncture. Mean values for plasma total and subfraction cholesterol were determined by size-exclusion liquid chromatography.
Mean values with unlike letters are significantly different (P<.05).
TG, triglyceride; TC, total cholesterol; VLDL, very low density lipoprotein; LDL, low density lipoprotein; HDL, high density lipoprotein.
Metabolomic analysis
The metabolomic results for whole GUI are expressed as ratios of compound amounts in the WO and WW groups compared to the WLF group. Ratios exceeding 1 means the compound's level increased, whereas a ratio less than 1 means it declined relative to that in the WLF. WO and the WW groups showed only a limited number of specific compounds having both a common direction of change and a significant ratio, which are detailed in Table 4.
Table 4.
Metabolomic Analysis
| Compound | WWWLF | WOWLF |
|---|---|---|
| Gamma-glutamylmethionine | 0.65 | 0.79 |
| Glucose-6-phosphate (G6P) | 0.66 | 0.35 |
| Gamma-glutamylphenylalanine | 0.72 | 0.76 |
| 4-Hydroxybutyrate (GHB) | 0.75 | 0.63 |
| Succinylcarnitine | 0.84 | 0.58 |
| Leucylglycine | 1.29 | 1.59 |
| Alanylvaline | 1.36 | 1.39 |
| Scyllo-inositol | 2.25 | 1.71 |
| Suberylglycine | 2.33 | 3.17 |
Significant changes (P<.05) in GUI tissue metabolite levels relative to WLF diet GUI tissue.
TRAMP mice (n=17/diet, 7–8 weeks old) were allowed ad libitum access for 18 weeks to one of three different diets. The diets were identical in terms of macro and micronutrients and contained 100 g/kg diet of dietary fat provided as WW, WLF (fats blended to mimic walnut fatty acid composition), or WO (pressed from same walnuts as used for WW diets). GUI was obtained by dissection, selected at random (n=8–11/diet), and submitted for metabolomic analysis.
NGS-based mRNA analysis
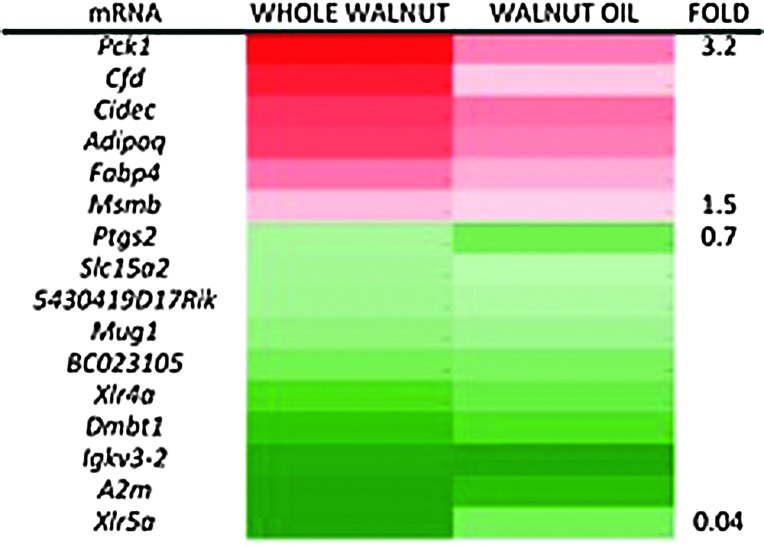
Specific mRNA in the walnut diet groups (WW, WO) was compared to the levels of the same mRNA in the WLF diet group. Specific mRNA in the walnut groups (WW, WO) compared to the levels of same mRNA in the WLF group with adjusted p-values<0.05 was considered differentially expressed.15 Compared to the WLF diet group, the WO diet group had 566 significantly differentially expressed transcripts, whereas the WW diet group had 530 differentially expressed transcripts. The differentially expressed mRNAs in the two groups were then matched by ID and the same direction of change (i.e., both groups' relative levels up or down simultaneously) between WW and WO to produce a list of 16 walnut regulated mRNA transcripts. These are listed along with a fold change heat map in Figure 1.
FIG. 1.
Results of next-generation sequencing (NGS)-based genitourinary intact (GUI) mRNA quantitation statistically significantly increased or decreased specific mRNA abundance ratio compared to walnut-like fat (WLF) for both whole walnut (WW) and walnut-fat diet groups' GUI. TRAMP mice GUI was obtained by dissection and 4/diet were randomly selected for mRNA isolation and NGS sequencing. The NGS data were analyzed and those WW and walnut oil GUI mRNA species whose ratio to the levels found in the WLF was statistically significant are considered differentially expressed and were then matched for having the same direction of change. The results are presented as a list of as well as heat map of walnut diet-affected mRNAs. Color images available online at www.liebertpub.com/jmf
Discussion
The relationship between diet and PCa has been extensively studied, as PCa is the Western male's second leading cause of cancer-related death with the role of dietary fat intake in PCa being of specific interest. However, fat is a caloric component of significant complexity, whose use as a diet composition descriptor (e.g., high fat) renders the identification/attribution of specific effects to dietary fats difficult and misleading. As an example, high fat intake in humans, in some but not all cohorts, has been shown to be a factor in the development and progression of PCa.16–19 In animal studies, the Freedland's group showed using mouse PCa xenograft models that decreased saturated fat diets did not extend survival and that fish oil-fed mice outlived those fed corn oil, olive oil, or animal fat diets.20,21 In our previous study, we found that WW, high-fat diets reduced TRAMP mouse prostate tumor size and growth compared to a soybean oil, high-fat diet.8 Hardman and Ion found that the growth of implanted MDA-MB231 human breast cancer cells in female nude mice and later the growth of large T-antigen-driven mammary tumors in female transgenic mice were slowed by a walnut-enriched diet versus a corn oil-based diet fed either throughout (i.e., in utero and then after birth) or after weaning.6,7 Reiter et al. reported recently that the growth of LNCaP androgen-sensitive prostate adenocarcinoma xenografts in mice was negatively associated with walnut feeding compared to a corn oil control diet.10
The results of the current study reaffirm our findings, that is, reduced prostate tumor growth by walnut-containing diets, but crucially again show that this effect is not an effect of walnut's fatty acids. They also again demonstrate that the dietary fat intake level is not a driver of PCa, given that a reduced GUI size was found in both the WW and WO diet groups despite their high dietary fat levels. In addition, although γ-tocopherol is high in walnuts and has recently been reported to have protective effects on N-methyl-N nitrosourea-induced epithelial dysplasia in rat ventral prostate, neither α- nor γ-tocopherol is responsible for the slowed PCa growth, as all three diets provided equivalent levels of both.22
As noted previously, the animals fed diets containing either WW or WO had significantly reduced plasma cholesterol and altered lipoprotein distributions. High-cholesterol diet-fed TRAMP mice have accelerated prostate tumor development, whereas reduced cholesterol availability induces apoptosis in LNCaP prostate tumor cells.23,24 Importantly, the current study results are not due to decreased plasma testosterone in response to the reduced plasma cholesterol in the WW and WO diet groups. This was a potential issue raised by the previous study as decreased androgen biosynthesis has been linked to lower plasma cholesterol and the TRAMP model depends on testosterone-driven SV40 T-antigen expression.25 In both the WW- and WO-fed groups, plasma testosterone was higher than the WLF group (Table 3). Furthermore, neither fiber nor minerals (e.g., Zn, Mg, and Se) were responsible for any of the effects noted, as these dietary components are either not found in WO diets or higher only in WW diet (Se ∼0.9 mg/kg diet versus ∼0.2 mg Se/kg diet in WLF and WO).
The current study results expand and further define the effect of walnut diets on PCa-related energy metabolism, its signaling, and machinery. That both the walnut-derived diets showed decreased IGF-1 is of note, as elevated IGF-1 is associated with increased risk of PCa and breast/mammary cancer.26 The tumor inhibitory effects of 30% calorie restriction on orthotopically transplanted mammary tumor in mice can be partially counteracted by exogenous IGF-1. This suggests that the slowed prostate tumor growth may be in response to walnut-induced declines in IGF-1.27 Other findings point to altered whole animal energy metabolism in the walnut diet groups as HOMA, a measure of insulin resistance, was lower in the WW and WO groups than the WLF control. The WW and WO diet groups also had increased CIDEC mRNA levels (Fig. 1) that has been linked to increased insulin sensitivity.28 In addition, the WW and WO diet groups had increased PCK1 mRNA expression (Fig. 1) and PCK1 is known for its role in gluconeogenesis and is increased in the livers of rats subjected to 30% CR.29,30
In addition to the mRNA changes already noted, the WW and WO diet groups had increased expression of mRNAs coding for FABP4 (fatty acid binding protein 4), along with mRNAs coding for Cfd (adipsin) and adiponectin as well (Fig. 1). Higher serum adiponectin is associated with a marked reduction in risk of PCa31–33 MSMB mRNA, increased in both WW and WO (Fig. 1) codes for PSP94, a tumor suppressor with lower levels being associated with more aggressive PCa.34,35 Of particular note, COX-2 mRNA was significantly reduced in the WW and WO diet groups (Fig. 1). Increased COX-2 expression levels are associated with PCa, and downregulated COX-2 expression reduced the prostate tumor size in TRAMP mice fed a 1% w/w ursolic acid diet.36
The response of the prostate to the various diets was also assessed by metabolomics profiling. The WW and WO diet groups relative to the WLF group had statistically significant declines in glucose-6-phosphate, succinylcarnitine, and 4-hydroxybutyrate, suggesting effects on cellular energy status/tricarboxylic acid cycle in the GUI (Table 4). Recently, fish oil diet's PCa benefits have been related to changes in the mitochondrial activity and insulin synthesis/secretion.21
Recent work has shown that a high-fat diet can drive prostate differentiation and this can be opposed by thiazolidinedione, a PPARγ agonist.37 In addition, the PPAR activity has been proposed to directly or indirectly modulate the supply of glucose and lipids for prostate metabolism.37 The WW and WO diet groups, as noted earlier, both had increased FABP4 mRNA, a known PPAR target.38 In other studies, PPARγ can be activated by a lipid-rich walnut extract.39
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) is found in walnuts and inhibits TRAMP prostate tumor tissue growth through inhibition of STAT3 and its downstream target genes.40 Both STAT3 and IGF-1 signaling pathways converge on AKT. This may explain the reduced cholesterol levels in the walnut diet group animals, as AKT controls cholesterol synthesis by affecting SREBP-2, a cholesterol supply-related transcription factor.41 Finally, the reduced IGF-1 level noted as well as the gene and metabolic changes found in walnut- and WO-fed animals parallel many of those identified as related to the inhibition of mice breast cancer growth by FGFR4 knockout.42
In summary, the current study provides additional evidence that the PCa health benefits, which accompany walnut consumption, are associated with walnut-driven changes in IGF-1 and cholesterol-related systems along with energy-related metabolic systems, all of which have been shown to be important in human PCa. The use of walnuts either whole or as oil as the sole fat source at high levels in the diets presents potential issues regarding walnuts in plausible human diets. However, the recently reported, walnut-associated cancer-related benefits in human studies suggest that walnut's beneficial effects on human cancer do not require unrealistically high consumption levels.3,4 In addition, these study results also suggest that walnut's beneficial effects with respect to cancer are not lost when walnuts are consumed as part of a typical American diet. More generally, our findings show that the use of total fat content of walnuts specifically or of either a food or a diet when assessing health effects is unwarranted, as it proved a very poor predictor of walnut's health-related effects. Finally, the current study results further highlight the need to continue to improve our current understanding of walnuts' health effects to effectively use them in diets to reduce PCa morbidity and mortality.
Acknowledgments
Hyunsook Kim was supported by the KU-Research Professor Program of Konkuk University. This study was supported by research grants to the University of California, Davis from the American Institute for Cancer Research (award MG10A001) and the California Walnut Board. Neither had input into study data analysis or the contents and conclusions of the manuscript. The manuscript's contents are solely the responsibility of the authors and do not necessarily represent the official view of the United States Department of Agriculture (USDA) Agricultural Research Service (ARS).
Author Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Ibarrola-Jurado N, Bullo M, Guasch-Ferre M, et al. : Cross-sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: The PREDIMED study. PLoS One 2013;8:e57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddad EH, Gaban-Chong N, Oda K, Sabate J: Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr J 2014;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guasch-Ferre M, Bullo M, Martinez-Gonzalez MA, et al. : Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med 2013;11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao Y, Han J, Hu FB, et al. : Association of nut consumption with total and cause-specific mortality. N Engl J Med 2013;369:2001–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis P, Valacchi G, Pagnin E, et al. : Walnuts reduce aortic ET-1 mRNA levels in hamsters fed a high-fat, atherogenic diet. J Nutr 2006;136:428–432 [DOI] [PubMed] [Google Scholar]
- 6.Hardman WE, Ion G: Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr Cancer 2008;60:666–674 [DOI] [PubMed] [Google Scholar]
- 7.Hardman WE, Ion G, Akinsete JA, Witte TR: Dietary walnut suppressed mammary gland tumorigenesis in the C(3)1 TAg mouse. Nutr Cancer 2011;63:960–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis PA, Vasu VT, Gohil K, et al. : A high-fat diet containing whole walnuts (Juglans regia) reduces tumour size and growth along with plasma insulin-like growth factor 1 in the transgenic adenocarcinoma of the mouse prostate model. Br J Nutr 2012;108:1764–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagel JM, Brinkoetter M, Magkos F, et al. : Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition 2012;28:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiter RJ, Tan DX, Manchester LC, et al. : A walnut-enriched diet reduces the growth of LNCaP human prostate cancer xenografts in nude mice. Cancer Invest 2013;31:365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo GL: Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol 2009;77:937–946 [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell 2011;144:646–674 [DOI] [PubMed] [Google Scholar]
- 13.Li H, Handsaker B, Wysoker A, et al. : The sequence alignment/Map format and SAMtools. Bioinformatics 2009;25:2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H: Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res 2004;64:8715–8722 [DOI] [PubMed] [Google Scholar]
- 15.Lee IH, Hong X, Mathur SC, et al. : A detailed analysis of next generation sequencing reads of microRNA expression in Barrett's esophagus: absolute versus relative quantification. BMC Res Notes 2014;7:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolonel LN, Nomura AM, Cooney RV: Dietary fat and prostate cancer: current status. J Natl Cancer Inst 1999;91:414–428 [DOI] [PubMed] [Google Scholar]
- 17.Villeneuve PJ, Johnson KC, Kreiger N, Mao Y: Risk factors for prostate cancer: results from the Canadian National Enhanced Cancer Surveillance System. The Canadian Cancer Registries Epidemiology Research Group. Cancer Causes Control 1999;10:355–367 [DOI] [PubMed] [Google Scholar]
- 18.Kolonel LN: Fat, meat, and prostate cancer. Epidemiol Rev 2001;23:72–81 [DOI] [PubMed] [Google Scholar]
- 19.Chan JM, Gann PH, Giovannucci EL: Role of diet in prostate cancer development and progression. J Clin Oncol 2005;23:8152–8160 [DOI] [PubMed] [Google Scholar]
- 20.Lloyd JC, Antonelli JA, Phillips TE, et al. : Effect of isocaloric low fat diet on prostate cancer xenograft progression in a hormone deprivation model. J Urol 2010;183:1619–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd JC, Masko EM, Wu C, et al. : Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis 2013;16:285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanches LD, Santos SA, Carvalho JR, et al. : Protective effect of gamma-tocopherol-enriched diet on N-methyl-N-nitrosourea-induced epithelial dysplasia in rat ventral prostate. Int J Exp Pathol 2013;94:362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR: Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest 2005;115:959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llaverias G, Danilo C, Wang Y, et al. : A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am J Pathol 2010;177:3180–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED: The TRAMP mouse as a model for prostate cancer. Curr Protoc Immunol 2001;Chapter 20:Unit 20.5 [DOI] [PubMed] [Google Scholar]
- 26.Key TJ: Diet, insulin-like growth factor-1 and cancer risk. Proc Nutr Soc 2011;3:1–4 [DOI] [PubMed] [Google Scholar]
- 27.Nogueira LM, Lavigne JA, Chandramouli GV, Lui H, Barrett JC, Hursting SD: Dose-dependent effects of calorie restriction on gene expression, metabolism, and tumor progression are partially mediated by insulin-like growth factor-1. Cancer Med 2012;1:275–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri V, Ranjit S, Konda S, et al. : Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci USA 2008;105:7833–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tilghman SM, Hanson RW, Reshef L, Hopgood MF, Ballard FJ: Rapid loss of translatable messenger RNA of phosphoenolpyruvate carboxykinase during glucose repression in liver. Proc Natl Acad Sci USA 1974;71:1304–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiba T, Komatsu T, Nakayama M, et al. : Similar metabolic responses to calorie restriction in lean and obese Zucker rats. Mol Cell Endocrinol 2009;309:17–25 [DOI] [PubMed] [Google Scholar]
- 31.Michalakis K, Williams CJ, Mitsiades N, et al. : Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev 2007;16:308–313 [DOI] [PubMed] [Google Scholar]
- 32.Sher DJ, Oh WK, Jacobus S, Regan NM, Lee GS, Mantzoros C: Relationship between serum adiponectin and prostate cancer grade. Prostate 2008;68:1592–1598 [DOI] [PubMed] [Google Scholar]
- 33.Arisan ED, Arisan S, Atis G, Palavan-Unsal N, Ergenekon E: Serum adipocytokine levels in prostate cancer patients. Urol Int 2009;82:203–208 [DOI] [PubMed] [Google Scholar]
- 34.Shukeir N, Arakelian A, Kadhim S, Garde S, Rabbani SA: Prostate secretory protein PSP-94 decreases tumor growth and hypercalcemia of malignancy in a syngenic in vivo model of prostate cancer. Cancer Res 2003;63:2072–2078 [PubMed] [Google Scholar]
- 35.Reeves JR, Dulude H, Panchal C, Daigneault L, Ramnani DM: Prognostic value of prostate secretory protein of 94 amino acids and its binding protein after radical prostatectomy. Clin Cancer Res 2006;12(20 Pt 1):6018–6022 [DOI] [PubMed] [Google Scholar]
- 36.Shanmugam MK, Ong TH, Kumar AP, et al. : Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS One 2012;7:e32476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strand DW, Jiang M, Murphy TA, et al. : PPARy isoforms differentially regulate metabolic networks to mediate mouse prostatic epithelial differentiation. Cell Death Dis 2012;3:e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabre A, Lazaro I, Girona J, et al. : Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis 2007;195:e150–e158 [DOI] [PubMed] [Google Scholar]
- 39.Vanden Heuvel JP, Belda BJ, Hannon DB, et al. : Mechanistic examination of walnuts in prevention of breast cancer. Nutr Cancer 2012;64:1078–1086 [DOI] [PubMed] [Google Scholar]
- 40.Hafeez BB, Zhong W, Mustafa A, Fischer JW, Witkowsky O, Verma AK: Plumbagin inhibits prostate cancer development in TRAMP mice via targeting PKCepsilon, Stat3 and neuroendocrine markers. Carcinogenesis 2012;33:2586–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luu W, Sharpe LJ, Stevenson J, Brown AJ: Akt acutely activates the cholesterogenic transcription factor SREBP-2. Biochim Biophys Acta 2012;1823:458–464 [DOI] [PubMed] [Google Scholar]
- 42.Luo Y, Yang C, Ye M, et al. : Deficiency of metabolic regulator FGFR4 delays breast cancer progression through systemic and microenvironmental metabolic alterations. Cancer Metab 2013;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]