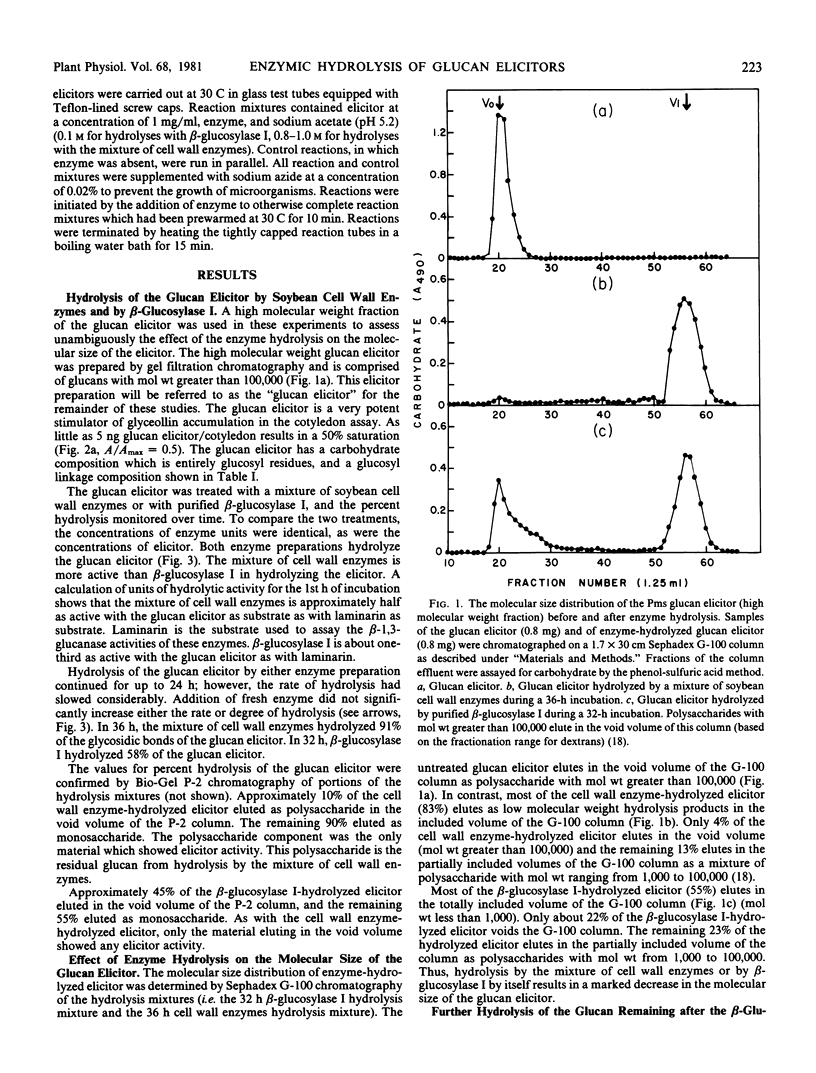
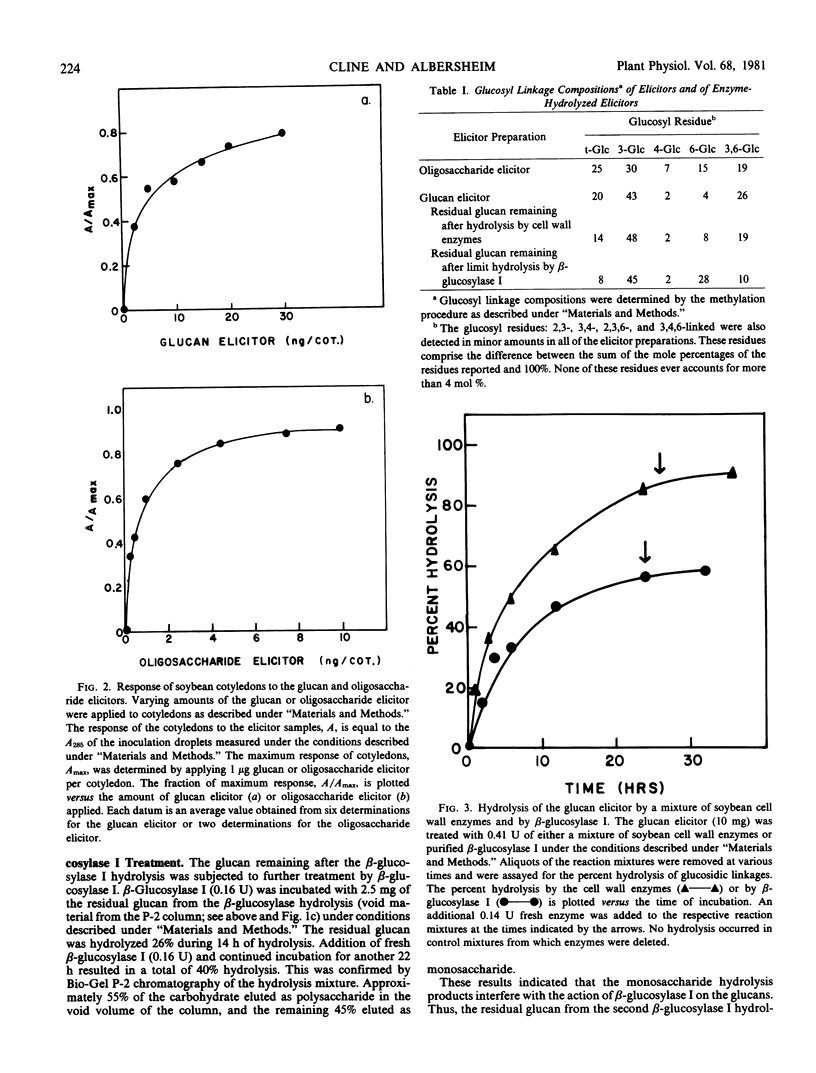
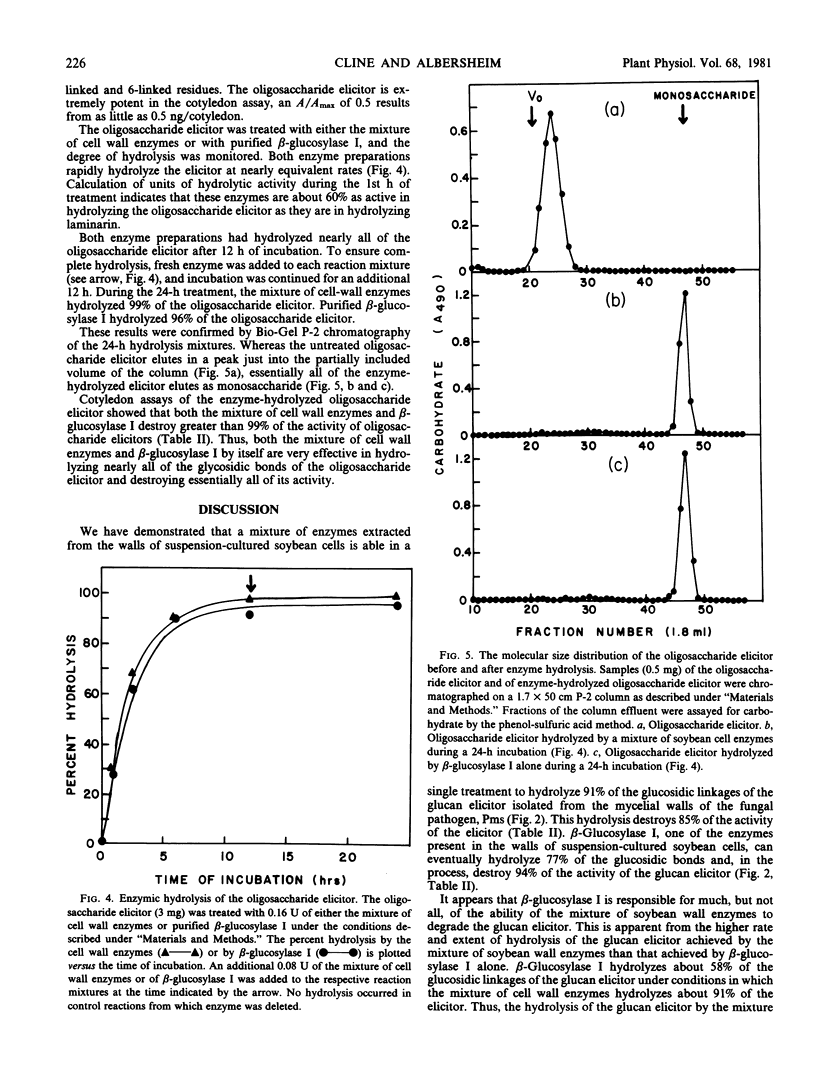
Abstract
The ability of β-glucosylase I, a soybean cell wall β-glucosyl hydrolase, to degrade elicitors of phytoalexin accumulation was studied. Extensive β-glucosylase I treatment of the glucan elicitor isolated from the mycelial walls of Phytophthora megasperma var. sojae results in hydrolysis of 77% of the glucosidic bonds of the elicitor and destruction of 94% of its activity. Soybean cell walls contain some additional factor, probably one or more additional enzymes, which can assist β-glucosylase I in hydrolyzing the glucan elicitor. This was demonstrated by the more rapid hydrolysis of the glucan elicitor by a mixture of soybean cell wall enzymes (containing β-glucosylase I). In a single treatment, the mixture of cell wall enzymes hydrolyzed 91% of the glucosidic bonds and destroyed 85% of the activity of the elicitor. The enzymes from soybean cell walls will also hydrolyze elicitor-active oligoglucosides prepared from the mycelial walls of Phytophthora megasperma var. sojae. The active oligoglucosides are more susceptible than the glucan elicitor to hydrolysis by these enzymes. The mixture of cell wall enzymes or β-glucosylase I, by itself, hydrolyzes more than 96% of the glucosidic bonds and destroys more than 99% of the activity of the oligoglucoside elicitor. Two possible advantages for the existence of these enzymes in the walls of soybean cells are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Finelli F., Berger N., Albersheim P. Host-Pathogen Interactions: IX. Quantitative Assays of Elicitor Activity and Characterization of the Elicitor Present in the Extracellular Medium of Cultures of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):751–759. doi: 10.1104/pp.57.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. A., Skipp R. A. Toxicity of phytoalexins. Ann Appl Biol. 1978 Jul;89(2):354–358. [PubMed] [Google Scholar]
- Boquet P., Pappenheimer A. M., Jr Interaction of diphtheria toxin with mammalian cell membranes. J Biol Chem. 1976 Sep 25;251(18):5770–5778. [PubMed] [Google Scholar]
- Carpita N., Sabularse D., Montezinos D., Delmer D. P. Determination of the pore size of cell walls of living plant cells. Science. 1979 Sep 14;205(4411):1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Cline K., Albersheim P. Host-Pathogen Interactions: XVI. PURIFICATION AND CHARACTERIZATION OF A beta-GLUCOSYL HYDROLASE/TRANSFERASE PRESENT IN THE WALLS OF SOYBEAN CELLS. Plant Physiol. 1981 Jul;68(1):207–220. doi: 10.1104/pp.68.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Darvill A. G., McNeil M., Albersheim P. Structure of Plant Cell Walls: VIII. A New Pectic Polysaccharide. Plant Physiol. 1978 Sep;62(3):418–422. doi: 10.1104/pp.62.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K., Albersheim P. The Involvement of Glycosidases in the Cell Wall Metabolism of Suspension-cultured Acer pseudoplatanus Cells. Plant Physiol. 1970 Jun;45(6):675–678. doi: 10.1104/pp.45.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Lee G., Kohn L. D., Habig W. H., Hardegree M. C. Tetanus toxin interactions with thyroid plasma membranes. Implications for structure and function of tetanus toxin receptors and potential pathophysiological significance. J Biol Chem. 1977 Jun 25;252(12):4049–4055. [PubMed] [Google Scholar]
- Peters B. M., Cribbs D. H., Stelzig D. A. Agglutination of plant protoplasts by fungal cell wall glucans. Science. 1978 Jul 28;201(4353):364–365. doi: 10.1126/science.201.4353.364. [DOI] [PubMed] [Google Scholar]



