Abstract
Obesity and diabetes are growing health problems worldwide. In this study, dietary provision of Chinese ginseng (0.5 g/kg diet) prevented body weight gain in high-fat (HF) diet-fed mice. Dietary ginseng supplementation reduced body fat mass gain, improved glucose tolerance and whole body insulin sensitivity, and prevented hypertension in HF diet-induced obese mice. Ginseng consumption led to reduced concentrations of plasma insulin and leptin, but had no effect on plasma adiponectin levels in HF diet-fed mice. Body temperature was higher in mice fed the ginseng-supplemented diet but energy expenditure, respiration rate, and locomotive activity were not significantly altered. Dietary intake of ginseng increased fatty acid oxidation in the liver but not in skeletal muscle. Expression of several transcription factors associated with adipogenesis (C/EBPα and PPARγ) were decreased in the adipose tissue of HF diet-fed mice, effects that were mitigated in mice that consumed the HF diet supplemented with ginseng. Abundance of fatty acid synthase (FASN) mRNA was greater in the adipose tissue of mice that consumed the ginseng-supplemented HF diet as compared with control or un-supplemented HF diet-fed mice. Ginseng treatment had no effect on the expression of genes involved in the regulation of food intake in the hypothalamus. These data suggest that Chinese ginseng can potently prevent the development of obesity and insulin resistance in HF diet-fed mice.
Keywords: : fat, ginseng, insulin resistance, obesity, mice
Introduction
Ginseng root has been used in oriental countries for thousands of years as a fatigue and weakness remedy. Today, it is one of the best-selling plant-derived supplements in the world, with the market value estimated at $2084 million.1 Recent studies have shown that ginseng may possess several beneficial health properties. For example, ginseng has been shown to be effective in the management of menopause symptoms,2 some neurological disorders,3 cardiovascular disease (CVD),4 and hyperglycemia in type 2 diabetes (T2D),5 as well as improving immune function.6
Obesity is a growing health problem worldwide. In the United States, 35.7% of adults and 16.9% of children are considered to be obese.7 Obesity is an established risk factor for the pathogenesis of various chronic diseases such as T2D, CVD, and cancer,8 some of the leading causes of preventable deaths. Several studies showed that ginseng therapy reduced fasting blood glucose and body weight in patients with T2D.9–11 In mouse and rat models, dietary provision of ginseng extracts for 4–8 weeks was associated with reduced high-fat (HF) diet-induced body weight gain, altered expression of lipogenesis-related genes,12–14 increased glucose transporter-4 and insulin receptor protein expression,13,14 and reduced angiogenesis.15 While many studies are focused on the short-term effects of ginseng on obesity, there are few studies that investigate the metabolic and gene expression profile of animals after long-term consumption of a ginseng-supplemented HF diet. The objective of this study was to determine whether relatively long-term dietary supplementation of ginseng can prevent obesity and metabolic syndrome in HF diet-fed mice. We show that dietary intake of ginseng for 15 weeks reduced body weight gain and fat mass of mice that consumed the HF, although food intake and energy expenditure were not altered. In addition, ginseng treatment improved blood glucose homeostasis, insulin sensitivity, and blood pressure. These favorable changes in obese mice are associated with altered hepatic fatty acid metabolism and expression of adipogenesis-related genes in white adipose tissue.
Materials and Methods
Animals
Eight-week-old, male C57BL/6J mice (NCI, NIH) were housed in an animal room maintained on a 12-h light/dark cycle under constant temperature (22–25°C) with ad libitum access to food and water. Before the experiment, fasting blood glucose and body weight were measured. Mice were then divided into three groups (n=15) of similar blood glucose concentrations and body weights, and were fed either a standard control diet with 10% of calories derived from fat, a HF diet (Research Diets, Inc., New Brunswick, NJ, USA) with 58% of calories from fat, or HF diet supplemented with ginseng (HF+G, 0.5 g/kg diet) for 15 weeks. Body weight and food intake were recorded weekly throughout the study. The study protocol and procedures performed in this study were reviewed and approved by the Institutional Animal Care and Use Committee at Virginia Tech.
Measurement of blood parameters
The fasting and nonfasting blood glucose concentrations were measured using a glucometer (The Kroger Co., Cincinnati, OH, USA) at the beginning of the experiment to ensure that the mice were euglycemic. Fasting and nonfasting blood glucose concentrations were then measured bi-weekly throughout the study. Fasting blood glucose was measured after a 16-h food withdrawal as previously described.16 Following 14 week of dietary treatment, blood pressure was determined in conscious mice using the Kent CODA 2 series computerized noninvasive blood pressure system (Kent Scientific, Torrington, CT, USA) as we previously described.17 At the end of the experiment, mice were fasted overnight and anesthetized for collecting blood samples by cardiac puncture. Plasma samples were collected by centrifugation at 16,000 g for 15 min.18 Plasma insulin was determined with a mouse ELISA kit (Mercodia, Inc., Uppsala, Sweden). Plasma total cholesterol, high density lipoprotein (HDL)-cholesterol, and triglycerides were measured using a CardioChek blood analyzer (Polymer Technology Systems, Indianapolis, IN, USA). Plasma leptin and adiponectin were measured with mouse ELISA kits according to the manufacturer's protocols (EMD Millipore Corporation, Billerica, MA, USA).
Glucose tolerance and insulin tolerance tests
For the intraperitoneal glucose tolerance test (IPGTT), mice at 15 weeks of age (n=8 per group) were fasted for 16 h and then injected intraperitoneally (IP) with a single dose of glucose (2 g/kg body weight). Blood glucose was measured before and at 15, 30, 60, and 120 min after glucose injection. An insulin tolerance test (ITT) was performed with the same mice at 24 weeks of age. After an overnight fast, mice were injected IP with a single dose of human insulin (0.75 U/kg body weight; Eli Lilly, Indianapolis, IN, USA), and blood glucose was measured before and at 15, 30, 60, and 120 min after insulin administration.
Body composition and energy expenditure measurements
Body composition of the mice (n=10 per group) was evaluated using an LF-90 instrument (Bruker Optics, Inc., Billerica, MA, USA) at the end of the feeding experiment. The LF-90 body composition instrument is based on Time Domain Nuclear Magnetic Resonance (TD-NMR) technology, which provides an in vivo measurement of lean tissue, body fat, and body fluid in live mice without anesthesia. Body temperature was measured using a thermometer probe placed at a 2.5 cm depth in the rectum. Following these procedures, 10 mice per group were used for simultaneous assessments of whole body metabolic profile using an indirect calorimetry system as described.19 Briefly, mice were individually placed in a TSE LabMaster Calorimetry System cage (Columbus Instruments, Columbus, OH, USA). Following acclimation for 48 h, mice were linked to the TSE LabMaster System, in a closed chamber that allows metabolic sampling. Mice had free access to diet and water for the duration of this study. The rates (mL/kg/h) of oxygen consumption (VO2) and carbon dioxide production (VCO2) for each mouse were recorded at 20-min intervals for 48 h. The respiratory exchange ratio (RER=VCO2/VO2) was then calculated by calorimetry software. RER estimates the proportion of energy generated from fat versus carbohydrate oxidation, with an RER of 0.70 indicating that fat is the pure source of energy, and a value of 1.0 meaning that carbohydrate is the only fuel source. Total energy expenditure was calculated as EE=VO2×(3.815+(1.232×RER)) according to the manufacturer's protocol and normalized for lean body mass (kcal/kg/h).
Palmitate and glucose oxidation assays in liver and skeletal muscle
Liver and skeletal muscle from the gastrocnemius and quadriceps were assayed for fatty acid and glucose oxidation. Palmitate oxidation was assessed by measuring and summing 14CO2 production and 14C-labeled acid soluble metabolites (ASM) from the oxidation of [1-14C] palmitic acid (American Radiolabeled Chemicals, St. Louis, MO, USA), as previously described.20 Glucose oxidation was assessed by measuring 14CO2 production from the oxidation of [U-14C] glucose (American Radiolabeled Chemicals).21
Gene expression analysis in hypothalamus, liver, and adipose tissue
Hypothalamus was isolated as previously described.22 Tissues were excised from each animal, snap-frozen in liquid nitrogen, and stored at −80°C. Tissues were added to a tube containing a 5 mm stainless steel bead (Qiagen, Valencia, CA, USA) and 1 mL of Isol Lysis reagent (5-Prime, Gaithersburg, MD, USA) and homogenized 2×2 min at 20 Hz with a Tissue Lyser II (Qiagen). After centrifugation at 12,000 g for 10 min at 4°C, total RNA was separated following the manufacturer's instructions (5-Prime). Following the addition of 70% ethanol, mixtures were transferred to spin columns and total RNA purified (RNeasy Mini Kit; Qiagen), including the optional on-column RNase-free DNase I step (Qiagen). Total RNA samples were evaluated for integrity by agarose-formaldehyde gel electrophoresis and concentration and purity assessed by spectrophotometry with a Nanophotometer™ Pearl (Implen, Westlake Village, CA, USA).
Single-strand cDNA was synthesized from 200 ng total RNA in 20 μL reactions with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), following the manufacturer's instructions. Reactions were performed under the following conditions: 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min. Primers for real-time polymerase chain reaction (PCR) were designed with Primer Express 3.0 software (Applied Biosystems) (Table 1) and amplification efficiency validated for all primer pairs before use (95–100% efficiency). Real-time PCR was performed in duplicate in 10 μL volume reactions that contained 5 μL Fast SYBR Green Master Mix (Applied Biosystems) and 3 μL of 10-fold diluted cDNA using a 7500 Fast Real-Time PCR System (Applied Biosystems). PCR was performed under the following conditions: 95°C for 20 sec and 40 cycles of 90°C for 3 sec plus 60°C for 30 sec. A dissociation step consisting of 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec, and 60°C for 15 sec was performed at the end of each PCR reaction to ensure specificity.
Table 1.
Primers Used for Real-Time Polymerase Chain Reaction
| Genea | Accession No. | Sequences (forward/reverse) 5′ to 3′ |
|---|---|---|
| C/EBPα | NM_007678.3 | CAGTTGGGCACTGGGTGGGC/CCGCGGCTCCACCTCGTAGAAG |
| C/EBPβ | NM_009883.3 | CGCAACACACGTGTAACTGTCA/AACAACCCCGCAGGAACAT |
| C/EBPδ | NM_007679.4 | TCCAACCCCTTCCCTGATC/CCCTGGAGGGTTTGTGTTTTC |
| SREBP-1 | NM_011480.3 | GCCTAGTCCGAAGCCGGGTG/GGAGCATGTCTTCGATGTCGTTCA |
| PPARγ | NM_001127330.1 | GCCTGCGGAAGCCCTTTGGT/AAGCCTGGGCGGTCTCCACT |
| FASN | NM_001146708.1 | TGCCAACCTGAAAACTAGGCTGAG/TACCCACCCCACCCCCTTCTC |
| GPDH | NM_001145820.1 | AGAGCTGCAGGCCGAGTCCC/GCTCAGCCTGATCACCCGTCGC |
| DGAT1 | NM_010046.2 | CGTGGGCGACGGCTACT/TGAGCTGAACAAAGAATCTTGCA |
| NPY | NM_023456.2 | CAGAAAACGCCCCCAGAAC/TTTCATTTCCCATCACCACATG |
| AGRP | NM_001271806.1 | AGCTTTGGCGGAGGTGCTA/GCGACGCGGAGAACGA |
| POMC | NM_001278584.1 | GACTAGGCCTGACACGTGGAA/GGCCCCTGAGCGACTGTA |
| 18S | NR_003278.3 | ACCTGGTTGATCCTGCCAGTAG/TTAATGAGCCATTCGCAGTTTC |
Primers were designed for a variety of genes associated with adipogenesis. The CCAAT/enhancer-binding protein (C/EBP) α and β activate expression of PPARγ and are required for preadipocyte differentiation, while C/EBP/δ and sterol regulatory element-binding protein-1 (SREBP-1) accelerate but are not required for differentiation. Peroxisome proliferator-activated receptor γ (PPARγ) is the master transcriptional regulator of adipogenesis and is involved in the growth arrest that is required for differentiation. Also investigated in this study was expression of fatty acid synthase (FASN), a key enzyme in de novo lipogenesis that catalyzes the synthesis of saturated fatty acids, and glycerol-3-phosphate-dehydrogenase (GPDH), an enzyme that catalyzes the reversible conversion of dihydroxyacetone phosphate to sn-glycerol-3-phosphate. Neuropeptide Y (NPY), agouti-related peptide (AGRP), and pro-opiomelanocortin (POMC) are appetite-associated factors. The 18S ribosomal subunit served as the endogenous control.
Statistical analysis
All data were analyzed with analysis of variance using JMP Pro V. 10 (SAS, Inc., Cary, NC, USA) and are expressed as mean±standard error of mean (SEM) or least square mean±pooled SEM, where appropriate. Treatment differences were subjected to Tukey's test. A P<.05 was considered significant. Real-time PCR data were analyzed using the ΔΔCT method, where 18S RNA served as the endogenous control and fat from control mice served as the calibrator sample. The ΔCT=CT target gene − CT 18S, and ΔΔCT=ΔCT target sample − ΔCT calibrator.23 Relative quantities, calculated as 2−ΔΔCT, were used for statistical analysis. The statistical model included the main effects of diet group (control, HF, or HF+G), tissue (hypothalamus, liver or fat), and the interaction between them.
Results
Food intake, body weight, and body composition
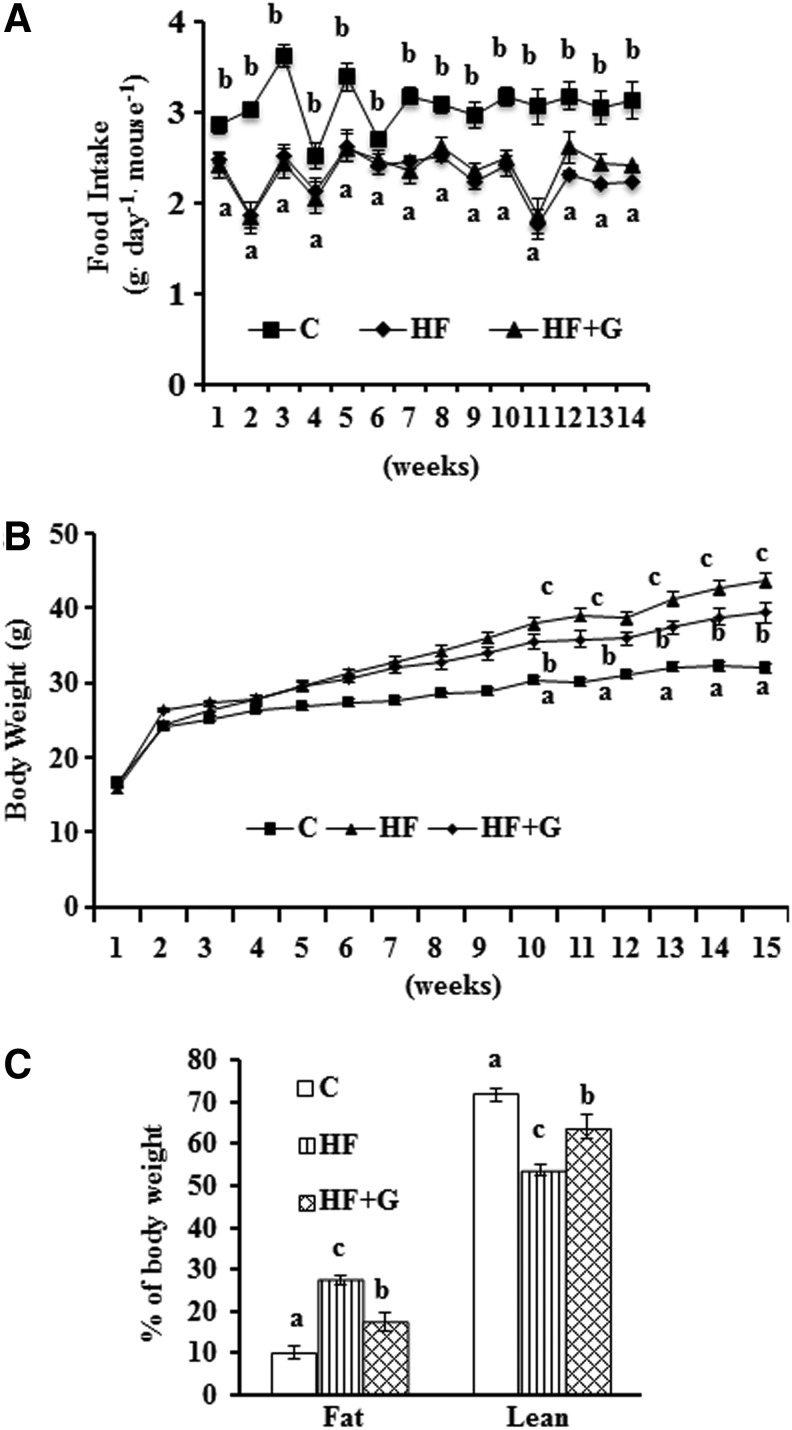
Ginseng supplementation (0.5 g/kg diet) did not alter the amount or pattern of food consumption compared with the HF group (Fig. 1A). There was reduced food intake in mice that were fed the HF diet as compared with those that consumed the control diet. Mice that consumed the HF diet gained more weight than control mice from week 3 to the end of the study. Although mice given the HF+G diet gained more weight than mice fed the control diet from week 2 to 14, they weighed less than mice that were fed the HF diet from week 10 to 14. At the end of the study, mice in the HF+G group (39.4±1.35 g) weighed significantly less than HF diet-fed mice (43.57±0.99 g, P<.05), although they were still more than the control mice (31.92±0.68 g, P<.05) (Fig. 1B). Results from body composition measurements showed that body fat mass in HF+G group was reduced (17.28%±0.36%) as compared with mice fed the HF diet (27.40%±0.16%, P<.05), but was still more than in the control mice (10.03%±0.21%, P<.05). Remarkably, mice fed ginseng also had ∼10% more lean mass (64.02%±0.46%) compared with mice fed the un-supplemented HF diet (53.73%±0.20%, P<.05) (Fig. 1C).
FIG. 1.
Ginseng supplementation had no influence on food consumption, but reduced body weight gain and fat mass. (A) Food intake was recorded weekly and the average daily food intake was calculated on a weekly basis. (B) Body weights were measured each week. (C) Body composition was measured following 14 weeks of HF diet feeding. Data are shown as means±SEM (n=15). Means with different superscript letters indicate significant differences (P<.05). C, control diet; HF, high-fat; HF+G, HF diet supplemented with ginseng; SEM, standard error of mean.
Fasting blood glucose and plasma insulin
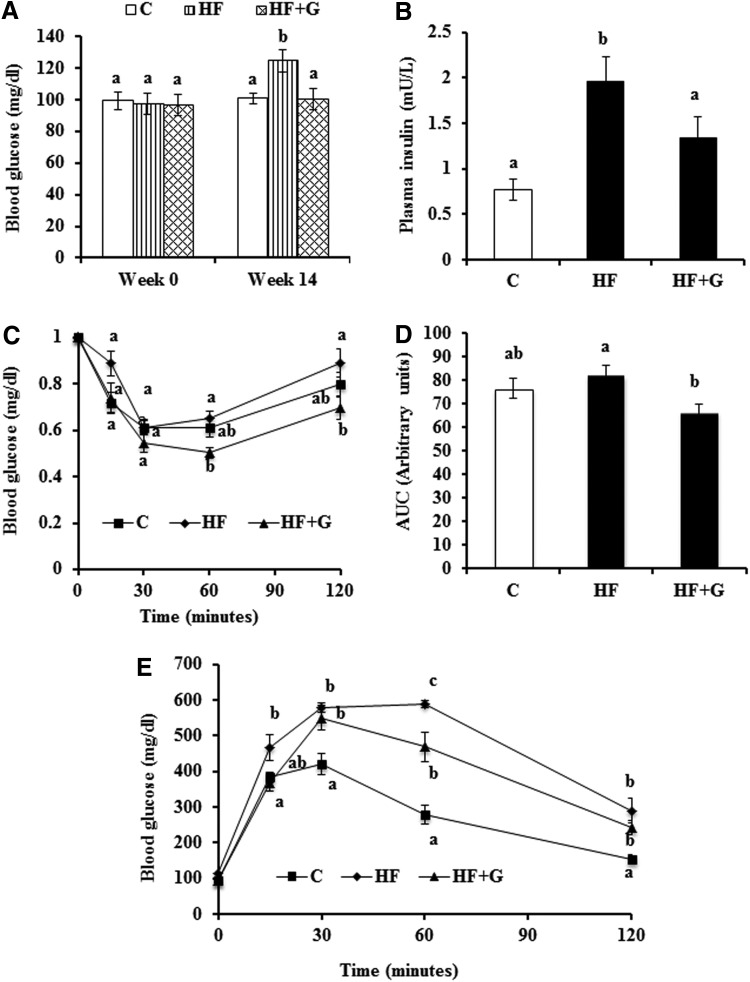
Mice had similar fasting blood glucose concentrations at week 0 (Fig. 2A). At week 14, mice fed the HF diet displayed significantly elevated fasting blood glucose concentrations (124.73±5.69 mg/dL) compared with mice that consumed the control diet (100.93±3.34 mg/dL, P<.05). However, dietary supplementation of ginseng normalized blood glucose (100.31±6.99 mg/dL) to levels that were comparable to the control group (Fig. 2A). There were no differences in nonfasting blood glucose between groups (data not shown). The plasma insulin levels in HF diet-fed mice (1.96±0.27 mU/L) were almost two fold higher than in mice fed the control diet (0.77±0.12 mU/L, P<.05). Mice that consumed the ginseng-supplemented diet had reduced plasma insulin (1.33±0.24 mU/L, P<.05) as compared with HF diet-fed mice (Fig. 2B).
FIG. 2.
Ginseng supplementation reduced blood glucose and plasma insulin concentrations, and improved insulin sensitivity in HF diet-fed mice. (A) Fasting blood glucose levels measured before and after 14 weeks of dietary treatment. (B) Plasma insulin levels were measured using an ELISA kit. ITT (C) and IPGTT (E) were performed as described in the Materials and Methods section. Data of ITT are expressed as percent of glucose levels at time 0. (D) The area under the curve (AUC) for ITT was calculated. Data are shown as means±SEM (n=8). Means with different superscript letters indicate significant differences (P<.05). C, control diet; IPGTT, intraperitoneal glucose tolerance test; ITT, insulin tolerance test.
ITT and IPGTT
ITT data were analyzed by comparing the blood glucose levels after injection of insulin with blood glucose levels at time point 0 within mice. The results showed that for the first 30 min, no difference in insulin sensitivity was observed. Sixty and 120 min after insulin injection, the percentage of blood glucose compared to time point 0 in HF+G group mice (0.50±0.02 and 0.69±0.05, respectively) was significantly lower than in the HF group (0.0.65±0.03 and 0.89±0.06). No difference was observed between the C group and HF group or between the C group and HF+G group (Fig. 2C). Area under the curve (AUC) was calculated using the ITT blood glucose data from all time points. Results showed that the HF+G group had significantly lower AUC than the HF group (P<.05). No difference was observed between the C group and HF group or between the C group and HF+G group (Fig. 2D). For the IPGTT, the blood glucose levels of mice from the three treatment groups were similar at baseline. However, the HF group had elevated blood glucose (466.75±35.65 mg/dL) at 15 min postglucose injection, compared with mice in the C group (383.63±15.25 mg/dL, P<.05). At 30 and 120 min postinjection, both the HF group and HF+G group had significantly higher blood glucose levels than the C group (P<.05). Ginseng supplementation was associated with reduced blood glucose at 60 min (469.71±41.05 mg/dL) compared with the HF diet group (589.63±10.38 mg/dL, P<.05), but concentrations that were still greater than the control group (278.75±27.38 mg/dL, P<.05) (Fig. 2E).
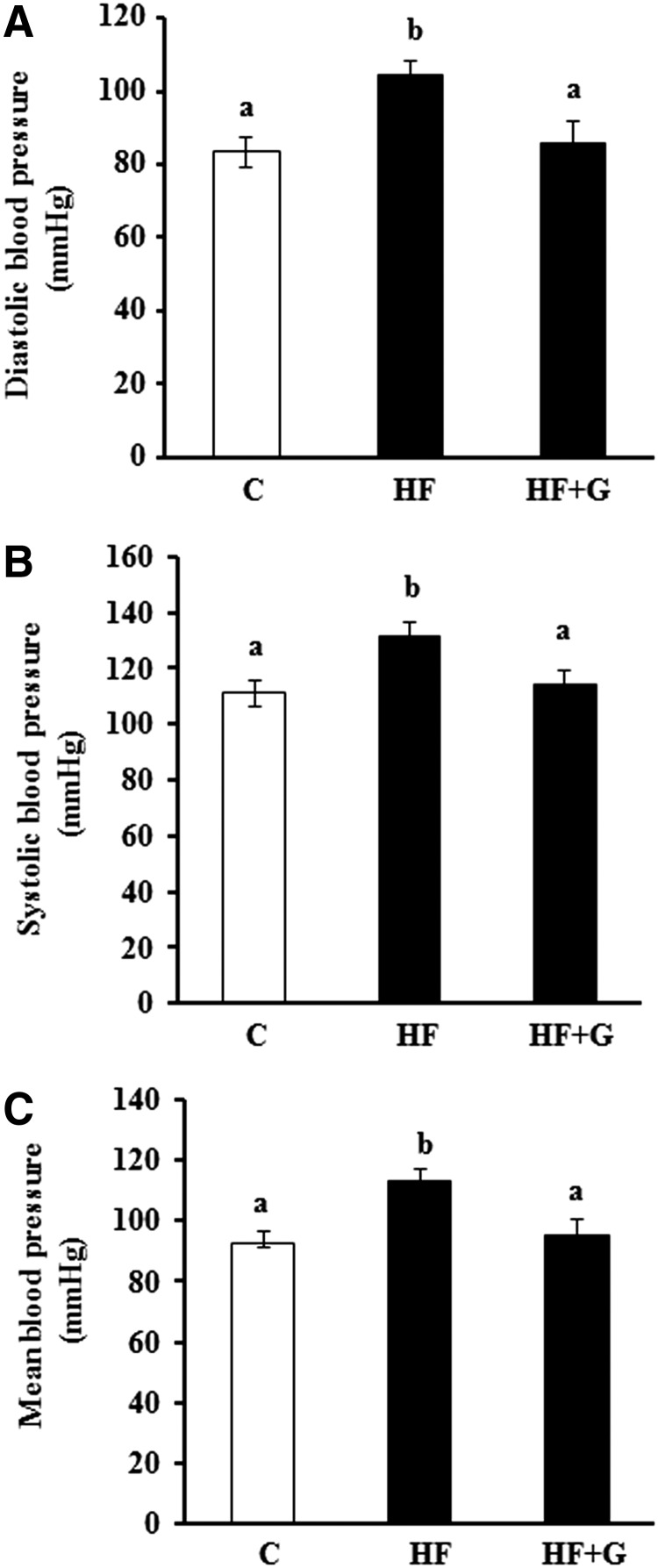
Blood pressures
Both diastolic (Fig. 3A), systolic (Fig. 3B), and mean (Fig. 3C) blood pressures were significantly higher in obese mice following consumption of the HF diet for 14 weeks as compared with the low-fat diet-fed mice controls. However, dietary supplementation of ginseng completely normalized systolic, diastolic, and mean blood pressures in HF diet-induced obese mice.
FIG. 3.
Ginseng supplementation prevented hypertension in HF diet-induced obese mice. Diastolic (A), systolic (B), and mean (C) blood pressures were measured in mice fed a normal chow diet (C), high fat (HF) diet, or HF+G for 14 weeks. Values are means±SEM, n=9–10 mice per group. Means with different superscript letters indicate significant differences (P<.05).
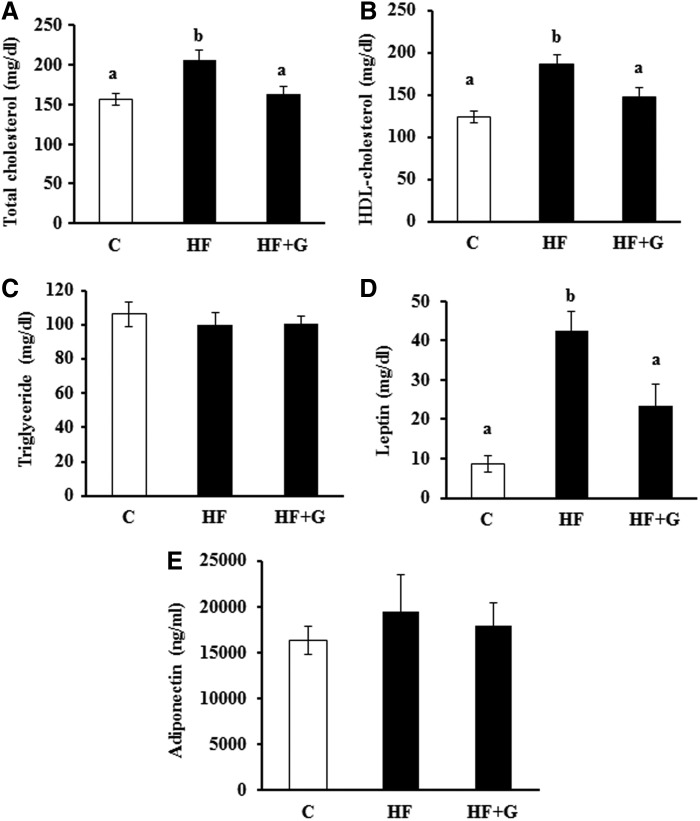
Plasma lipid profile, leptin, and adiponectin
Total plasma cholesterol levels (163.10±8.78 mg/dL) in the HF+G group were significantly lower as compared with the HF diet-fed mice (206.2±11.89 mg/dL, P<.05) (Fig. 4A). Mice fed the HF+G diet also had lower HDL-cholesterol levels (147.3±10.78 mg/dL) than those fed the HF diet (186.7±10.13 mg/dL) (Fig. 4B). However, ginseng supplementation had no effect on plasma triglyceride concentrations (Fig. 4C). After 16 week of dietary treatment, plasma leptin levels in HF diet-fed mice were elevated by 4-fold over those of controls (42.48±4.88 vs. 8.57±2.14 mg/dL, respectively, P<.05). Ginseng supplementation greatly reduced the elevated plasma leptin concentrations that were associated with HF feeding (23.38±5.49 vs. 42.48±4.88 mg/dL, P<.05) (Fig. 4D). There were no significant differences in plasma adiponectin levels among dietary groups (Fig. 4E).
FIG. 4.
Ginseng supplementation decreased circulating levels of total cholesterol, HDL-cholesterol, and leptin, but had no effect on triglyceride and adiponectin concentrations in HF diet-fed mice. At the end of the experiment, fasting plasma total cholesterol (A), HDL-cholesterol (B), triglycerides (C), leptin (D), and adiponectin (E) concentrations were measured in duplicated samples by using mouse ELISA kits. Data are shown as mean±SEM (n=10). Means with different superscript letters indicate significant differences (P<.05). C, control diet; HDL, high density lipoprotein.
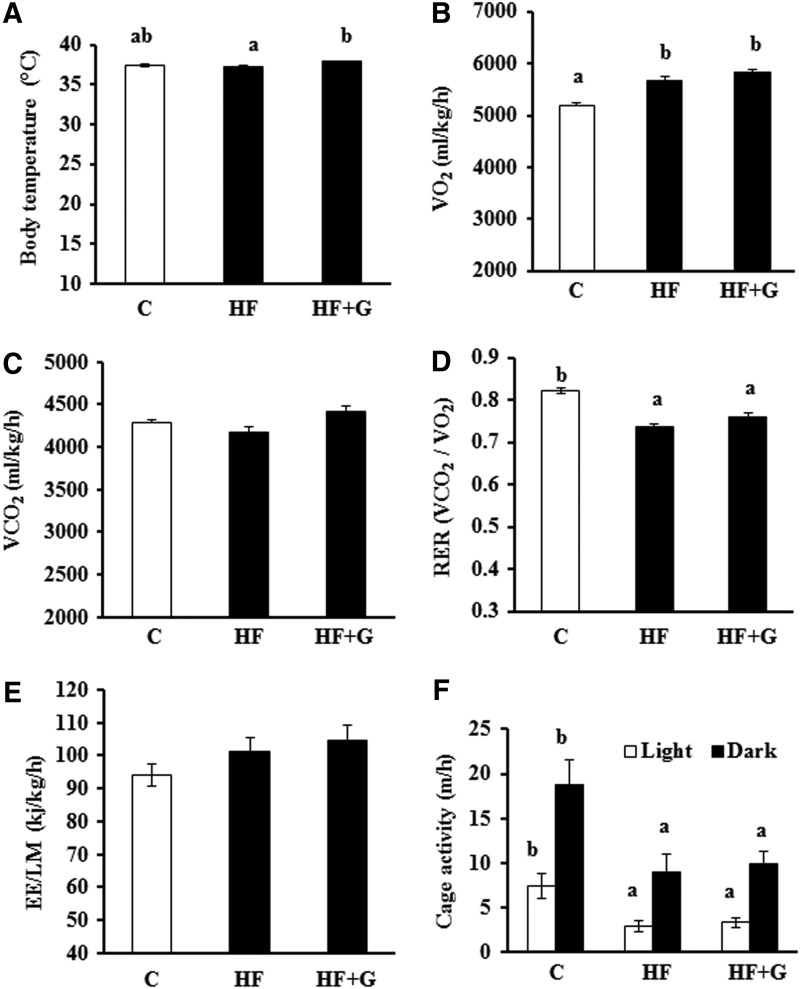
Body temperature, respiration rate, energy expenditure, and cage activity
Mice fed HF+G diet had significantly higher body temperatures (37.85°C±0.23°C) than mice given the control (37.38°C±0.17°C) or HF (37.22°C±0.10°C) diet (Fig. 5A). Energy expenditure was evaluated by indirect calorimetry at 15 weeks of treatment. Obese mice displayed an increase in VO2 as compared with lean mice (Fig. 5B). Mice treated with HF+G tended to have higher VO2 and VO2 production than the HF-fed mice (Fig. 5C). Mice fed the HF diet had significantly lower RER as compared with the control mice, which were not significantly improved by dietary ginseng supplementation (Fig. 5D). Compared with HF-fed control mice, ginseng-fed mice showed slightly higher energy expenditure, as assessed using data recorded over 48 h, but this difference was not statistically significant (Fig. 5E). In addition, ginseng treatment for 15 week did not alter locomotive activity both during the day and night period (Fig. 5F).
FIG. 5.
Ginseng supplementation increased the body temperature of mice, but had no significant effect on the respiration rate, energy expenditure, or cage activity. Body temperature (A), the rates of oxygen consumption (VO2) (B) and carbon dioxide production (VCO2) (C), respiration exchange ratio (RER) (D), energy expenditure normalized to lean mass of body weight (EE/LM) (E), and cage activity (F) were measured as described in the Materials and Methods section following 15 weeks of dietary treatment. Data are shown as means±SEM (n=10). Means with different superscript letters indicate significant differences (P<.05). C, control diet.
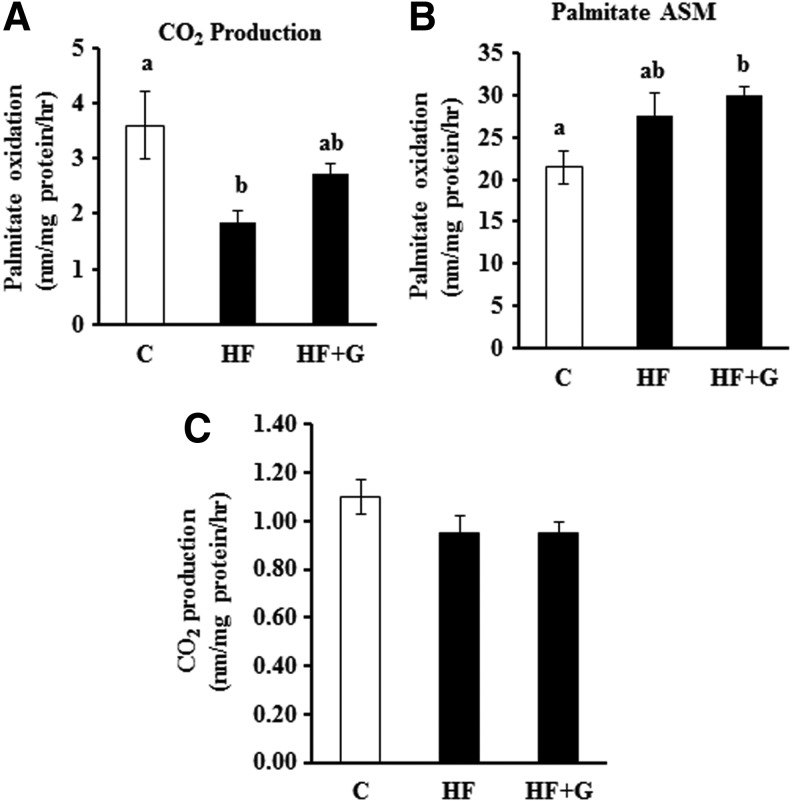
Fatty acid and glucose oxidation in the liver and muscle
The rates of ex vivo palmitate oxidation into CO2 in the liver was significantly lower in HF diet-fed mice (1.85±0.22 vs. 3.60±0.61 nm/mg protein/h, P<.05), suggesting that hepatic fatty acid oxidation was impaired by chronic HF diet feeding. However, ginseng supplementation partially reversed this adverse effect (Fig. 6A). In addition, ginseng treatment increased the rate of palmitate oxidation into ASM (29.98±0.91 nm/mg protein/h) as compared with control mice (21.46±1.93 nm/mg protein/h, p<.05), but there was no significant difference in the production of ASM between HF control and HF+G-fed mice (Fig. 6B). Neither HF feeding or ginseng supplementation altered the rates of ex vivo glucose oxidation in the liver (Fig. 6C). In addition, no differences in ex vivo palmitate or glucose oxidation in the skeletal muscle were observed (data not shown).
FIG. 6.
Ginseng supplementation improved palmitate oxidation to CO2 (A), but had no significant effect on the oxidation of palmitate to ASM (B) and glucose oxidation (C) in the liver. Data are shown as means±SEM (n=10). Means with different superscript letters indicate significant differences (P<.05). ASM, acid soluble metabolites; C, control diet.
The mRNA abundance of appetite and fat metabolism-associated genes
The neuropeptide Y (NPY), agouti-related peptide (AGRP) and pro-opiomelanocortin (POMC) mRNA were not detected in adipose tissue or liver, thus the dietary effects were only analyzed in hypothalamus. There were no effects of diet on mRNA abundance of NPY (P=.21; C, HF-G and HF means±pooled SEM were 1.34, 0.78, and 0.85±0.25, respectively), AGRP (P=.44; C, HF-G and HF means±pooled SEM were 5.99, 1.82 and 1.1±2.9, respectively), and POMC (P=.39; C, HF-G and HF means±pooled SEM were 1.17, 1.85 and 1.4±0.34, respectively) in the hypothalamus. These data are consistent with there being no effect of the HF diet on overall food intake.
For C/EBPα and PPARγ, there were interactions of dietary group and tissue, where in the adipose tissue, mRNA abundance of both genes was greater (P<.05) in mice that consumed the control diet than in mice that consumed the HF diet, with intermediate expression of both genes in mice that consumed the HF+G diet (Table 2). Expression of both genes was almost two fold greater in control mice as compared with HF diet-fed mice (P<.05), with mice that consumed the HF+G diet having intermediate expression of these genes. No treatment differences were detected for these two genes in any of the other tissues examined.
Table 2.
Least Square Means±Pooled Standard Error of Mean of Relative Differences in mRNA Abundance of Lipogenesis- and Adipogenesis-Associated Genes
| Gene1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Effect | C/EBPα | C/EBPβ | C/EBPδ | FASN | GPDH | DGAT | SREBP-1 | PPARγ |
| Diet2 | ||||||||
| Control | 1.05a | 1.02 | 1.14a | 0.95b | 1.03 | 1.03a | 1.02 | 1.20a |
| HF-G | 0.85ab | 1.10 | 0.78b | 1.30a | 1.03 | 0.79b | 1.04 | 0.83b |
| HF | 0.67b | 1.19 | 0.65b | 0.86b | 0.75 | 0.80b | 0.94 | 0.62b |
| SEM | 0.05 | 0.13 | 0.09 | 0.07 | 1.5 | 0.04 | 0.05 | 0.10 |
| P value | .0003 | .6931 | .0021 | .0005 | .991 | .0021 | .3676 | .0004 |
| Tissue3 | ||||||||
| Fat | 1.05a | 1.02a | 1.14a | 0.95a | 1.03b | 1.03a | 1.02a | 1.20a |
| Liver | 0.35b | 1.50a | 0.25b | 0.31b | 0.16b | 0.10b | 0.46b | 0.03b |
| Hypo | 0.005c | 0.18b | 0.31b | 0.13b | 19.79a | 0.14b | 0.44b | 0.006b |
| SEM | 0.05 | 0.13 | 0.09 | 0.07 | 1.5 | 0.04 | 0.05 | 0.10 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Interaction4 | ||||||||
| C Fat | 1.05a | 1.02 | 1.14 | 0.95ab | 1.04 | 1.03 | 1.02 | 1.20a |
| HF-G Fat | 0.85ab | 1.10 | 0.78 | 1.30a | 1.03 | 0.79 | 1.04 | 0.83ab |
| HF Fat | 0.67b | 1.19 | 0.65 | 0.86b | 0.76 | 0.80 | 0.94 | 0.62b |
| C Liver | 0.35c | 1.49 | 0.25 | 0.31c | 0.16 | 0.102 | 0.46 | 0.03c |
| HF-G Liver | 0.36c | 1.69 | 0.17 | 0.30c | 0.19 | 0.102 | 0.46 | 0.03c |
| HF Liver | 0.38c | 1.73 | 0.22 | 0.32c | 0.17 | 0.103 | 0.44 | 0.04c |
| C Hypo | 0.005d | 0.18 | 0.31 | 0.13c | 19.8 | 0.13 | 0.44 | 0.006c |
| HF-G Hypo | 0.005d | 0.14 | 0.18 | 0.06c | 17.7 | 0.11 | 0.39 | 0.004c |
| HF Hypo | 0.005d | 0.14 | 0.16 | 0.08c | 18.4 | 0.11 | 0.39 | 0.004c |
| SEM | 0.05 | 0.13 | 0.09 | 0.08c | 1.5 | 0.04 | 0.05 | 0.09c |
| P value | .0137 | .8452 | .1895 | .0141 | .965 | .0961 | .8722 | .0206c |
The CCAAT/enhancer-binding protein (C/EBP) α and β activate expression of PPARγ and are required for preadipocyte differentiation, while C/EBP/δ and sterol regulatory element-binding protein-1 (SREBP-1) accelerate but are not required for differentiation. Peroxisome proliferator-activated receptor γ (PPARγ) is the master transcriptional regulator of adipogenesis and is involved in the growth arrest that is required for differentiation. Also investigated in this study was expression of fatty acid synthase (FASN), a key enzyme in de novo lipogenesis that catalyzes the synthesis of saturated fatty acids, and glycerol-3-phosphate-dehydrogenase (GPDH), an enzyme that catalyzes the reversible conversion of dihydroxyacetone phosphate to sn-glycerol-3-phosphate, an indirect indicator of adipogenesis. Diglyceride acyltransferase (DGAT) mediates the formation of triglycerides from diacylgycerol and acyl CoA. Means with different superscript letters within a column and effect indicate significant differences (P<.05; Tukey's pairwise comparisons).
Dietary groups included the control (standard rodent chow), high-fat (HF; 60% of calories from lard), and high-fat diet containing ginseng (HF-G).
Tissues analyzed in this study included gonadal fat (fat), liver, and hypothalamus (hypo).
Interaction refers to the two-way interaction of diet and tissue, with means and pooled SEM displayed for all groups.
SEM, standard error of mean.
In all tissues, quantities of C/EBPδ, DGAT, and PPARγ mRNA were greater (P<.05) in control mice as compared with mice in either of the HF groups. Expression of FASN was greater in mice that consumed the HF+G diet than in mice that consumed the control diet or un-supplemented HF diet (Table 2).
Discussion
As an important risk factor for T2D and CVD,8,24 obesity is often associated with hyperlipidemia, elevated blood glucose and insulin, and impaired insulin sensitivity and glucose tolerance.25 HF diets are commonly used as a strategy to induce fat deposition and obesity in animal models that often lead to the development of chronic metabolic disorders,12,26 such as insulin27 and leptin 6 resistance. Insulin resistance is an important risk factor for the development of T2D.28 In the present study, we show that relative long-term provision of Chinese ginseng in the diet prevented obesity in the HF diet-fed mice, which is consistent with findings from several other studies.12–14 In addition, ginseng reduced body fat mass while increasing body lean mass, confirming that ginseng exerts an anti-obesity effect. We further show that dietary supplementation of ginseng prevented insulin resistance, lowered blood pressure, and decreased plasma glucose and insulin concentrations in HF diet-fed mice. Importantly, these beneficial metabolic effects elicited by dietary intake of ginseng are not due to alteration in food intake. Given that obesity and insulin resistance are risk factors for developing T2D, ginseng could be an effective dietary supplement that is capable of preventing the pathogenesis of T2D.
It is known that increased triglyceride and free fatty acids, and decreased HDL-cholesterol with HDL dysfunction represent dyslipidemia that is typical of obesity.25 Our data showed that consumption of the HF diet had no impact on plasma triglycerides, but increased plasma HDL-cholesterol. In addition, plasma total cholesterol concentrations were increased with the consumption of the HF diet. Recent research on ginseng extracts showed that ginseng may exhibit its anti-obesity effect by regulating the expression of lipogenesis-related genes in white adipose tissue and delaying the absorption of lipid in the intestine.12 Because food intake was not affected by ginseng supplementation, the decrease in total cholesterol in the HF-fed mice in our study may be caused by the regulation of lipogenesis-related genes and/or the reduced absorption of lipid. Further experiments are needed to determine how ginseng causes a reduction in circulating cholesterol levels in HF diet-fed mice. It is interesting to note that mice that were fed the HF diet had considerably higher HDL-cholesterol than control mice, but ginseng-fed mice displayed HDL-cholesterol levels that were comparable to those in control mice, consistent with its effect on total cholesterol levels. These results suggest that HF diet-fed mice may be still relatively healthy and are able to increase HDL levels in response to the elevated circulating cholesterol concentrations. There is also possibility that delayed plasma clearance in obese mice may contribute to the elevated HDL-cholesterol levels observed.29
It is well established that obesity results from energy imbalance. As ginseng treatment did not alter calorie intake in mice during the course of this study, we then assessed energy expenditure to determine whether the reduced body fat mass and body weight in ginseng-fed mice is due to its effect on energy expenditure. It is worth noting that in the present study energy expenditure was normalized to lean mass instead of body weight, because fat tissue may contribute comparatively less to the total energy expenditure compared with lean mass due to its relatively low metabolic activity.30 While we did not observe that there was a significant difference in energy expenditure between HF-fed and HF+G-treated mice, as assessed using data obtained over 48 h, ginseng intake slightly increased energy expenditure. However, this small difference in energy expenditure as shown within 48 h between the control and ginseng-treated mice could lead to the large differences in the accumulation of fat mass over 15 week of HF-diet feeding. Indeed, it was estimated that, given the identical calorie intake, a 5% difference in daily energy expenditure in HF diet-fed mice can result in a about 10-g difference in body weight over 4–6 months.30
Insulin resistance and hyperglycemia are related with the dysfunctions of several metabolic pathways including impaired glucose transport and oxidation and glycogen synthesis, increased glucose output by the liver, and altered lipogenesis and lipolysis.31 While data from the present study show that ginseng supplementation improved insulin sensitivity, glucose homeostasis, and blood pressure, these beneficial effects may be the secondary effects whereby long-term intake of ginseng prevented obesity in mice fed a HF diet, given that obesity is a major cause of insulin resistance and T2D. Indeed, ginseng treatment had no significant effect on glucose oxidation in the liver and muscle. Nevertheless, these results suggest that dietary ginseng can potentially lower the risk of developing metabolic syndrome.
Leptin is an adipokine that plays an important role in control of body weight through regulating energy intake and expenditure while adiponectin promotes insulin sensitivity via enhancing fatty acid oxidation and glucose uptake and inhibiting hepatic glucose production.32,33 It has been shown that a combination of physiological doses of adiponectin and leptin, but not either hormone alone, completely reversed insulin resistance in lipoatrophic mice,34 suggesting that leptin and adiponectin exert synergistic metabolic effects. It was shown that obesity and overfeeding can cause leptin resistance in both humans and experimental animals,35 which may contribute to the disturbed fatty acid metabolism and the development of metabolic syndrome.36 In our study, the plasma leptin concentrations of mice in the HF diet group increased dramatically when compared with mice in the control group, indicating leptin resistance or compromised leptin action in obese mice.35 Interestingly, dietary supplementation of ginseng significantly attenuated this harmful effect in obese mice. This result is consistent with previous findings in rats.37 However, it is unclear from the present study whether ginseng directly acts on adipose tissue to modulate leptin production or if this effect is simply a consequence of reduced adiposity and body weight gain by ginseng. Further experiments need to be conducted to investigate the potential mechanisms of ginseng's effects on leptin resistance.
In the present study, we further assessed ex vivo glucose and fatty acid oxidation in liver and skeletal muscle homogenates from ginseng-treated and un-treated mice. Compared to the standard diet-fed mice, HF-diet feeding had no effect on glucose oxidation, but obese mice displayed lower rates of palmitate oxidation to CO2 and accumulated more ASM in the liver, as determined by directly measuring the rate of conversion of radiolabeled palmitate into CO2 or ASM, which provide the index of complete and incomplete oxidation of fatty acids, respectively. These results suggest that the ability of mitochondria to oxidize fatty acids may be impaired after 15 week of HF-diet feeding. While ginseng had no significant effect on the hepatic production of ASM, it partially reversed the impaired fatty acid oxidation to CO2. As hepatic steatosis is highly correlated with reduced lipid oxidation,38 it is expected that dietary intake of ginseng may have a protective effect against obesity-induced steatosis, which is intriguing for further investigation.
On a molecular level, we observed differences in gene expression of adipogenesis and lipogenesis-associated factors in the adipose tissue and liver. Interestingly, expression of the master adipogenic transcription factor PPARγ in fat was reduced in response to consumption of the HF diet, but increased in mice that consumed HF diet supplemented with ginseng. These data are consistent with previous reports of various sources of ginseng upregulating PPARγ expression in liver and adipose tissue of mice.39 Similarly, treatment with Ginsenoside Rb1 was associated with enhanced adipocyte differentiation and increased expression of PPARγ and CEBP/α.40,41 It is well known that PPARγ activity is associated with enhanced insulin signaling, reduced blood glucose concentrations, and reduced ectopic lipid accumulation in various organs. In adipose tissue, PPARγ is also the target of TZDs, a class of T2D drugs. It is possible that in HF diet-fed mice, ginseng supplementation led to enhanced PPARγ activation and hence increased adipogenesis and lipogenesis, thus lowering blood glucose and improving insulin sensitivity. Because the ginseng supplementation was also associated with less body weight gain and reduced body fat composition, it would be informative in future studies to assess rates of fat oxidation in adipose tissue, and rates of triglyceride synthesis and lipolysis, to determine whether ginseng supplementation is associated with changes in adipose tissue dynamics that improve whole body glucose regulation and body composition.
In summary, we provide evidence that long-term dietary supplementation of Chinese ginseng prevents HF diet-induced obesity in mice, which is associated with improved insulin and leptin sensitivity, glucose tolerance, blood pressure, hepatic fatty acid oxidation, and plasma lipid profiles. These results indicate that ginseng, an ancient Asian medicine, can be used as a dietary supplement to prevent obesity and related metabolic disorders. Further studies are needed to fully elucidate the molecular mechanisms underlying the beneficial metabolic effects of ginseng. Moreover, because effects of ginseng may be dependent on ginsenoside profile, further studies should explore the effects of specific ginsenosides on glucose regulation and energy metabolism in adipose tissue, muscle, and liver.
Acknowledgments
The work was supported by grants from the American Diabetes Association (7-11-BS-84 to D. Liu), Diabetes Research and Action Education Foundation (to D. Liu), and National Center for Complementary and Alternative Medicine of National Institutes of Health (1R01AT007077 to D. Liu). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.Baeg IH, So SH: The world ginseng market and the ginseng (Korea). J Ginseng Res 2013;37:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MS, Lim HJ, Yang HJ, Lee MS, Shin BC, Ernst E: Ginseng for managing menopause symptoms: a systematic review of randomized clinical trials. J Ginseng Res 2013;37:30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HJ, Kim P, Shin CY: A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res 2013;37:8–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmazyn M, Moey M, Gan XT: Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs 2011;71:1989–2008 [DOI] [PubMed] [Google Scholar]
- 5.Mucalo I, Rahelic D, Jovanovski E, Bozikov V, Romic Z, Vuksan V: Effect of American ginseng (Panax quinquefolius L.) on glycemic control in type 2 diabetes. Coll Antropol 2012;36:1435–1440 [PubMed] [Google Scholar]
- 6.Kang S, Min H: Ginseng, the ‘Immunity Boost’: the effects of panax ginseng on immune system. J Ginseng Res 2012;36:354–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM: Prevalence of Obesity in the United States. National Center for Health Statistics, Data Brief 2012;82:1-8 [PubMed] [Google Scholar]
- 8.Haslam DW, James WP: Obesity. Lancet 2005;366:1197–1209 [DOI] [PubMed] [Google Scholar]
- 9.Sotaniemi EA, Haapakoski E, Rautio A: Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care 1995;18:1373–1375 [DOI] [PubMed] [Google Scholar]
- 10.Uzayisenga R, Ayeka PA, Wang Y: Anti-diabetic potential of panax notoginseng saponins (PNS): a review. Phytother Res 2014;28:510–516 [DOI] [PubMed] [Google Scholar]
- 11.Oh JS, Lee SR, Hwang KT, Ji GE: The anti-obesity effects of the dietary combination of fermented red ginseng with levan in high fat diet mouse model. Phytother Res 2014;28:617–622 [DOI] [PubMed] [Google Scholar]
- 12.Lee YS, Cha BY, Yamaguchi K, et al. : Effects of Korean white ginseng extracts on obesity in high-fat diet-induced obese mice. Cytotechnology 2010;62:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollah ML, Kim GS, Moon HK, et al. : Antiobesity effects of wild ginseng (Panax ginseng C.A. Meyer) mediated by PPAR-gamma, GLUT4 and LPL in ob/ob mice. Phytother Res 2009;23:220–225 [DOI] [PubMed] [Google Scholar]
- 14.Lim S, Yoon JW, Choi SH, et al. : Effect of ginsam, a vinegar extract from Panax ginseng, on body weight and glucose homeostasis in an obese insulin-resistant rat model. Metabolism 2009;58:8–15 [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Park D, Yoon M: Korean red ginseng (Panax ginseng) prevents obesity by inhibiting angiogenesis in high fat diet-induced obese C57BL/6J mice. Food Chem Toxicol 2013;53:402–408 [DOI] [PubMed] [Google Scholar]
- 16.Gilbert ER, Fu Z, Liu D: Development of a nongenetic mouse model of type 2 diabetes. Exp Diabetes Res 2011;2011:416254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Si H, Liu D: Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J Nutr 2008;138:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Z, Zhang W, Zhen W, et al. : Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 2010;151:3026–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson JM, Aja S, Wei Z, Wong GW: CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J Biol Chem 2012;287:1576–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisard MI, McMillan RP, Marchand J, et al. : Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am J Physiol 2010;298:E988–E998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson AS, Roberts PC, Frisard MI, et al. : Metabolic changes during ovarian cancer progression as targets for sphingosine treatment. Exp Cell Res 2013;319:1431–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith ML, Prall B, Nandar W, Cline MA: Beta-melanocyte-stimulating hormone potently reduces appetite via the hypothalamus in chicks. J Neuroendocrinol 2008;20:220–226 [DOI] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ: Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008;3:1101–1108 [DOI] [PubMed] [Google Scholar]
- 24.Bastard JP, Maachi M, Lagathu C, et al. : Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006;17:4–12 [PubMed] [Google Scholar]
- 25.Klop B, Elte JW, Cabezas MC: Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013;5:1218–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, Nolte LA, Hansen PA, et al. : High-fat diet-induced muscle insulin resistance: relationship to visceral fat mass. Am J Physiol 2000;279:R2057–R2065 [DOI] [PubMed] [Google Scholar]
- 27.Trajcevski KE, O'Neill HM, Wang DC, et al. : Enhanced lipid oxidation and maintenance of muscle insulin sensitivity despite glucose intolerance in a diet-induced obesity mouse model. PLoS One 2013;8:e71747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boden G: Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 2011;18:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishina PM, Lowe S, Wang J, Paigen B: Characterization of plasma lipids in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism 1994;43:549–553 [DOI] [PubMed] [Google Scholar]
- 30.Butler AA, Kozak LP: A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 2010;59:323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bray GA: Medical consequences of obesity. J Clin Endocrinol Metab 2004;89:2583–2589 [DOI] [PubMed] [Google Scholar]
- 32.Diez JJ, Iglesias P: The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 2003;148:293–300 [DOI] [PubMed] [Google Scholar]
- 33.Nedvidkova J, Smitka K, Kopsky V, Hainer V: Adiponectin, an adipocyte-derived protein. Physiol Res 2005;54:133–140 [PubMed] [Google Scholar]
- 34.Yamauchi T, Kamon J, Waki H, et al. : The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941–946 [DOI] [PubMed] [Google Scholar]
- 35.Myers MG, Jr., Leibel RL, Seeley RJ, Schwartz MW: Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 2010;21:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang R, Barouch LA: Leptin signaling and obesity: cardiovascular consequences. Cir Res 2007;101:545–559 [DOI] [PubMed] [Google Scholar]
- 37.Megan Janina Migchels WI, Jason MM, Ciriello J: Ingestion of North American ginseng decreases circulating levels of leptin and insulin. FASEB J 2011;25:8 [Google Scholar]
- 38.Hirschey MD, Shimazu T, Goetzman E, et al. : SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010;464:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park MY, Lee KS, Sung MK: Effects of dietary mulberry, Korean red ginseng, and banaba on glucose homeostasis in relation to PPAR-alpha, PPAR-gamma, and LPL mRNA expressions. Life Sci 2005;77:3344–3354 [DOI] [PubMed] [Google Scholar]
- 40.Shang W, Yang Y, Jiang B, et al. : Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARgamma2 and C/EBPalpha gene expression. Life Sci 2007;80:618–625 [DOI] [PubMed] [Google Scholar]
- 41.Chan LS, Yue PY, Kok TW, Keung MH, Mak NK, Wong RN: Ginsenoside-Rb1 promotes adipogenesis through regulation of PPARgamma and microRNA-27b. Horm Metab Res 2012;44:819–824 [DOI] [PubMed] [Google Scholar]