Abstract
Purpose: The study investigated the effect of intravitreally administered tanibirumab, a fully human monoclonal antibody against vascular endothelial growth factor receptor 2, in a rat model of laser-induced choroidal neovascularization (CNV).
Methods: CNV was induced by laser photocoagulation on day 0 in the eyes of Brown Norway rats. Intravitreal injection of tanibirumab or phosphate-buffered saline (PBS) was done on day 0 (prevention arm) or day 7 (treatment arm). Seven days after injection, the eyes were enucleated and retinal pigment epithelium-choroid-sclera flat mounts were prepared. Areas of CNV were determined in the flat mounts using tetramethylrhodamine isothiocyanate Bandeiraea simplicifolia (BS) isolectin labeling and intravenously administered fluorescein isothiocyanate–dextran and quantified using an image analysis program.
Results: In the prevention arm, the mean area of CNV measured by BS isolectin labeling was reduced by 28.2% and 53.9% in tanibirumab-treated eyes (20 and 60 μg, respectively) compared with PBS-treated control eyes on day 7 (P=0.038 and P<0.001, respectively). In the treatment arm, the mean area of CNV measured by BS isolectin labeling was reduced by 28.7% and 46.0% in tanibirumab-treated eyes (20 and 60 μg, respectively) compared with PBS-treated control eyes on day 14 (P=0.048 and P<0.001, respectively).
Conclusions: Intravitreally administered tanibirumab partially suppressed the formation of new CNV and partially regressed preformed laser-induced CNV in the rat model. Tanibirumab may be a feasible treatment for CNV associated with age-related macular degeneration or other causes.
Introduction
Age-related macular degeneration (AMD) is the major cause of irreversible visual loss among elderly people worldwide.1,2 Exudative or wet AMD usually gives rise to more severe visual loss compared with nonexudative or dry AMD.1,2 Choroidal neovascularization (CNV) is the hallmark of exudative AMD and it leaks serous fluid, lipids, and blood beneath and into the neural retina with fibrous scarring.3
Among the several factors that stimulate the development of CNV, vascular endothelial growth factor-A (VEGF-A) has been identified as a key proangiogenic and vascular permeability factor.4,5 VEGF-A acts through 2 major receptors, VEGF receptor-1 (VEGFR-1, also known as fms-like tyrosine kinase and Flt-1) and VEGF receptor-2 (VEGFR-2, also known as KDR and Flk1).6 The tyrosine kinase VEGFR-2 is expressed by the vascular endothelial cells and mediates key responses to VEGF-A, such as angiogenesis and vascular hyperpermeability.7,8 The VEGFR-2/VEGF-A axis is crucial in the development of CNV.9,10 Thus, blocking VEGF-A from binding to VEGFR-2 may be an effective and specific treatment strategy for CNV. In addition, VEGF-C and -D were detected in the vitreous of AMD patients and CNV membranes from humans and laser-induced CNV mouse models.11,12 These reports may imply that VEGF-C and -D also contributes to the formation of CNV. Thus, blocking other VEGF family members like VEGF-C and -D from binding to VEGFR-2 might produce a better effect than blocking VEGF-A alone.
Tanibirumab (PharmAbcine, Daejeon, Korea) is a fully human monoclonal antibody against VEGFR-2.13 It is derived from a fully human naive single-chain variable fragment phage library.13 Tanibirumab binds to the VEGF-binding domain of VEGFR-2 and neutralizes the biological activity of VEGFR-2 by blocking the binding of VEGF.13 Tanibirumab reportedly inhibits angiogenesis in various in vitro and in vivo systems and results in potent antitumor activity in colorectal, breast, nonsmall-cell lung cancer, and glioblastoma tumor models.13
In this study, we evaluated the inhibitory effect of intravitreally administered tanibirumab on the formation of CNV and growth of established CNV in a rat model of laser-induced CNV.
Methods
Laser-induced CNV model
Brown Norway rats (Japan SLC, Hamamatsu, Japan) weighing 200–250 g were used as the laser-induced CNV rat models. The animals were cared for in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. All animal experiments were carried out in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Samsung Medical Center. Laser photocoagulation in the rats was performed, as previously described, on day 0.14,15 In brief, after anesthesia and dilatation of the pupils, 6 laser spots were applied (532 nm wavelength, 300 mW power, 100 ms duration, 75 μm spot size) around the optic nerve. Only burns that generated a bubble, implying the rupture of the Bruch membrane, were included in the study. Spots failing to develop a bubble at the laser site or containing hemorrhage were excluded from analysis.
Intravitreal administration of tanibirumab
To investigate the effect of tanibirumab on the formation of CNV in the prevention arm, eyes were randomized into 5 groups and received intravitreal injections of tanibirumab [5, 20, and 60 μg in 2 μL of phosphate-buffered saline (PBS)], the same volume of PBS or human IgG (20 μg in 2 μL of PBS), using a 33-gauge needle (Hamilton, Reno, NV) immediately after laser photocoagulation on day 0. To investigate the effect of tanibirumab on established CNV in the treatment arm, tanibirumab (5, 20, and 60 μg in 2 μL PBS) or the same volume of PBS was injected into the eyes with the same methods on day 7. Only one eye of each animal was selected to be injected with tanibirumab, and the contralateral eye was not included in this study.
Measurement of laser-induced CNV size
On day 7 in the prevention arm and on day 14 in the treatment arm, anesthetized mice were perfused with fluorescein isothiocyanate (FITC)–dextran (MW 2×106; Sigma-Aldrich, St. Louis, MO). The rats were euthanized and eyes were enucleated and fixed in 4% paraformaldehyde for 2 h, after which the anterior segments, vitreous, and retina were removed from the eyecups. Lens opacity was evaluated with light microscopy when the eyes were enucleated. Residual retinal pigment epithelium (RPE)-choroid-sclera cups were incubated in a blocking solution [5% bovine serum albumin (BSA) in PBS] for 1 h. Tetramethylrhodamine isothiocyanate (TRITC)-conjugated Bandeiraea simplicifolia (BS) isolectin B4 (0.2 mg/mL; Sigma-Aldrich) was applied to the eyecups overnight at 4°C in 0.2% BSA in PBS. After extensive washing, the RPE-choroid-sclera cups were flattened using relaxing radial cuts and flat mounted in a mounting medium. The flat mounts were examined using an LSM700 laser confocal microscope (Carl Zeiss, Jena, Germany) and images of the laser spots were captured. The areas of green and red fluorescence were measured using the ImageJ program (National Institute of Health, Bethesda, MD). In brief, the confocal images were converted to 8-bit images and then to binary (black and white) images. Using the analyze particles mode, CNV areas were automatically measured (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jop). The areas of CNV were compared between each dose of tanibirumab-treated and control eyes by the Mann–Whitney U-test. A P value of<0.05 was considered statistically significant.
Evaluation of histological cross sections
On day 7 in the prevention arm, the eyes were enucleated, fixed in 4% paraformaldehyde for 24 h, and embedded in paraffin. Sagittal sections of 5 μm were cut and stained with hematoxylin and eosin (HE). Microscopic images of the stained sections were acquired using a digital camera attached to a light microscope (Olympus BX51; Olympus, Tokyo, Japan). CNV was estimated by the B/C ratio of the thickness from the bottom of the pigmented choroidal layer to the top of the neovascular membrane (B) to the thickness of the intact pigmented choroid adjacent to the lesion (C), as previously described.16 The B/C ratio was compared between the laser-treated spots of tanibirumab (60 μg)-injected and control eyes by the Mann–Whitney U-test. In addition, potential ocular changes, such as retinal thinning and choroidal atrophy, were evaluated on microscopic images.
Results
Effect of tanibirumab on CNV in the prevention arm
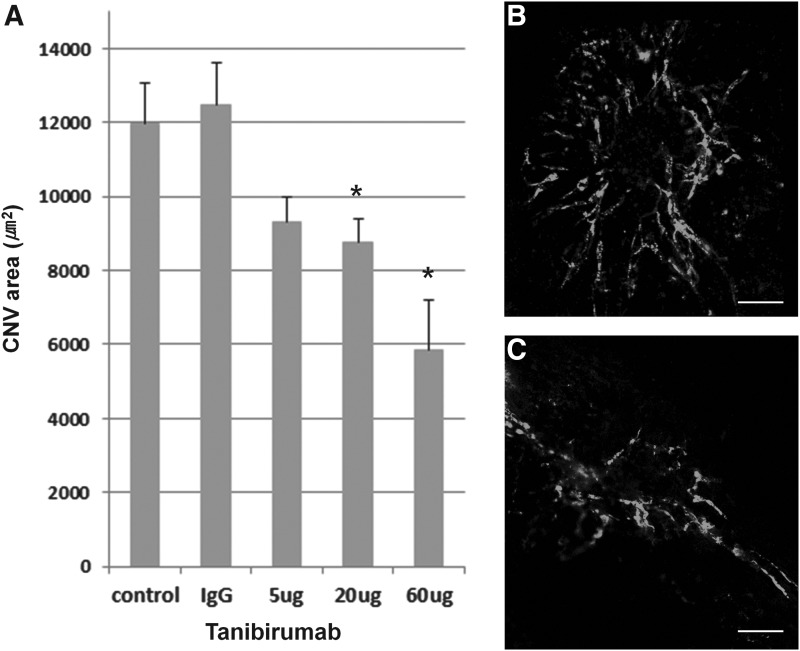
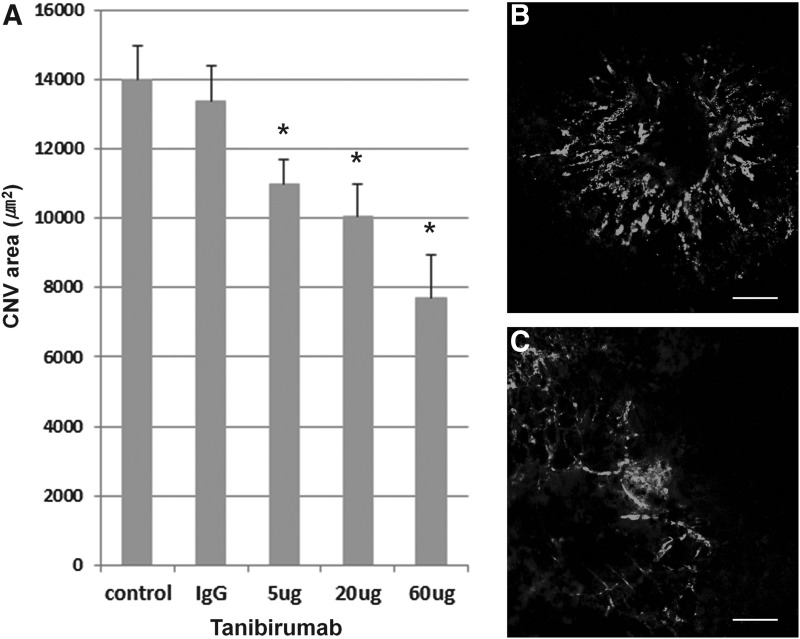
CNV areas of the RPE-choroid-sclera flat mounts were compared between tanibirumab-treated and control eyes in the prevention arm on day 7. The mean CNV areas, as measured by intravenously injected FITC-dextran, were significantly smaller in tanibirumab-treated eyes (20 and 60 μg/2 μL PBS; n=7 and 6, respectively) than in PBS-injected eyes (n=8; P=0.033 and P=0.003, respectively; by Mann–Whitney U-test, Fig. 1). Human IgG-injected eyes showed no difference compared with PBS-injected eyes. However, in eyes treated with 5 μg of tanibirumab (n=8), the mean CNV areas, as measured by intravenously injected FITC-dextran, were not significantly smaller than in PBS-injected eyes (n=8; P=0.063 by Mann–Whitney U-test, Fig. 1). The mean CNV area in tanibirumab-treated eyes decreased by 22.1%, 26.7%, and 51.0%, respectively, compared with PBS-treated control eyes. The mean CNV areas, as measured by BS isolectin B4 labeling, were significantly smaller in eyes treated with tanibirumab (20 and 60 μg/2 μL of PBS) (n=8 and 7, respectively) than in PBS-injected eyes (n=8; P=0.038 and P<0.001, respectively, by the Mann–Whitney U-test, Fig. 2). There was no difference between human IgG-injected eyes and PBS-injected eyes. However, in eyes treated with 5 μg of tanibirumab (n=10), the mean CNV areas, as measured by BS isolectin B4 labeling, were not significantly smaller than in PBS-injected eyes (n=8; P=0.162 by Mann–Whitney U-test, Fig. 2). The mean CNV area in tanibirumab-treated eyes decreased by 21.0%, 28.2%, and 53.9%, respectively, compared with PBS-treated control eyes.
FIG. 1.
Effect of tanibirumab on the formation of laser-induced choroidal neovascularization (CNV) measured by intravenously injected fluorescein isothiocyanate (FITC)-dextran. (A) CNV areas of the retinal pigment epithelium (RPE)-choroid-sclera flat mounts were compared between tanibirumab-treated and control eyes. (B, C) On day 7, the mean CNV areas, as measured by intravenously injected FITC-dextran, were significantly smaller in tanibirumab-treated eyes [20 and 60 μg/2 μL phosphate-buffered saline (PBS); n=7 and 6, respectively] than in PBS-injected eyes (n=8; P=0.033 and P=0.003, respectively, by the Mann–Whitney U-test). Human IgG-injected eyes showed no difference compared with PBS-injected eyes. However, in eyes treated with 5 μg of tanibirumab (n=8), the mean CNV areas, as measured by intravenously injected FITC-dextran, were not significantly smaller than in PBS-injected eyes (n=8; P=0.063 by Mann–Whitney U-test). *P<0.05. Scale bar denotes 100 μm.
FIG. 2.
Effect of tanibirumab on the formation of laser-induced CNV measured by Bandeiraea simplicifolia (BS) isolectin B4 labeling. (A) CNV areas of the RPE-choroid-sclera flat mounts were compared between tanibirumab-treated and control eyes. (B, C) On day 7, the mean CNV areas, as measured by BS isolectin B4 labeling, were significantly smaller in tanibirumab-treated eyes (20 and 60 μg/2 μL of PBS) (n=8 and 7, respectively) than in PBS-injected eyes (n=8; P=0.038 and P<0.001, respectively, by the Mann–Whitney U-test). There was no difference between human IgG-injected eyes and PBS-injected eyes. However, in eyes treated with 5 μg of tanibirumab (n=10), the mean CNV areas, as measured by BS isolectin B4 labeling, were not significantly smaller than in PBS-injected eyes (n=8; P=0.162 by Mann–Whitney U-test). *P<0.05. Scale bar denotes 100 μm.
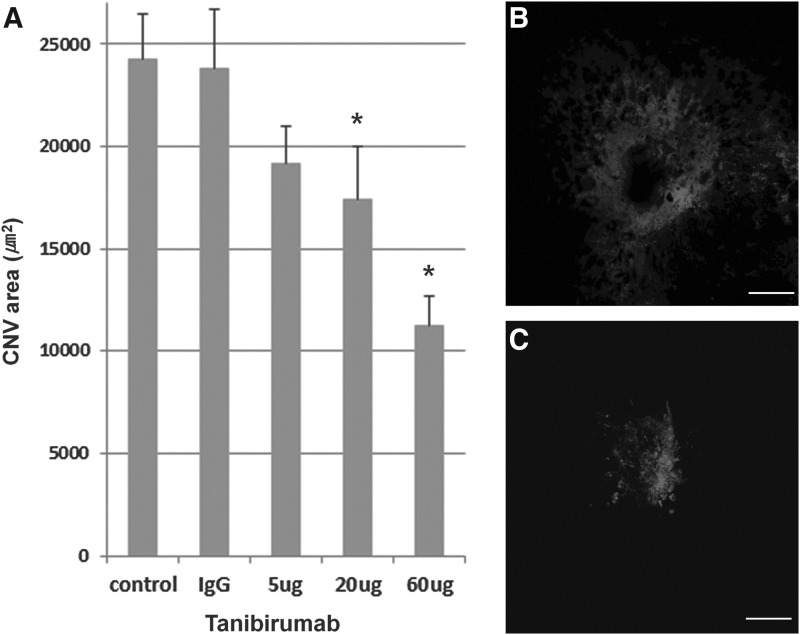
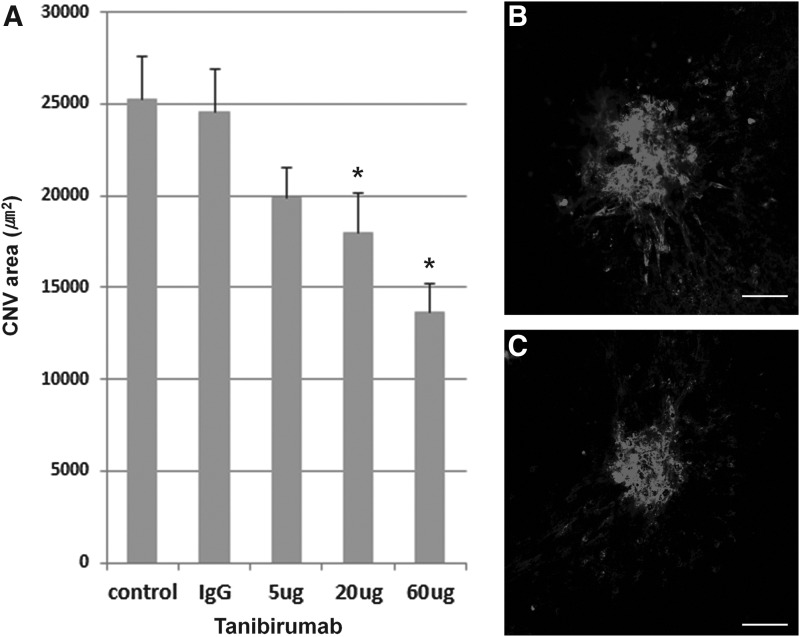
Effect of tanibirumab on CNV in the treatment arm
In the treatment arm, on day 14, the mean CNV areas, as measured by intravenously injected FITC-dextran, were significantly smaller in eyes treated with tanibirumab (5, 20, and 60 μg/2 μL of PBS) (n=8, 8, and 7, respectively) than in PBS-injected eyes (n=8; P=0.038, P=0.003, and P<0.001, respectively, by the Mann–Whitney U-test, Fig. 3). Human IgG-injected eyes showed no difference compared with PBS-injected eyes. The mean CNV area in tanibirumab-treated eyes decreased by 21.5%, 28.3%, and 44.8%, respectively, compared with PBS-treated control eyes. The mean CNV areas, as measured by BS isolectin B4 labeling, were significantly smaller in tanibirumab-treated eyes (20 and 60 μg/2 μL of PBS) (n=8 and 7, respectively) than in PBS-injected eyes (n=8; P=0.048 and P<0.001, respectively, by the Mann–Whitney U-test, Fig. 4). There was no difference between human IgG-injected eyes and PBS-injected eyes. However, in eyes treated with 5 μg of tanibirumab (n=7), the mean CNV areas, as measured by BS isolectin B4 labeling, were not significantly smaller than in PBS-injected eyes (n=8; P=0.132 by the Mann–Whitney U-test, Fig. 4). The mean CNV area in tanibirumab-treated eyes decreased by 21.3%, 28.7%, and 46.0%, respectively, compared with PBS-treated control eyes.
FIG. 3.
Effect of tanibirumab on the regression of laser-induced CNV measured by intravenously injected FITC-dextran. (A) CNV areas of the RPE-choroid-sclera flat mounts were compared between tanibirumab-treated and control eyes. (B, C) On day 14, the mean CNV areas, as measured by intravenously injected FITC-dextran, were significantly smaller in tanibirumab-treated eyes (5, 20, and 60 μg/2 μL of PBS) than in PBS-injected eyes (n=8; P=0.038, P=0.003, and P<0.001, respectively, by the Mann–Whitney U-test). Human IgG-injected eyes showed no difference compared with PBS-injected eyes. *P<0.05. Scale bar denotes 100 μm.
FIG. 4.
Effect of tanibirumab on the regression of laser-induced CNV measured by BS isolectin B4 labeling. (A) CNV areas of the RPE-choroid-sclera flat mounts were compared between tanibirumab-treated and control eyes. (B, C) On day 14, the mean CNV areas, as measured by BS isolectin B4 labeling, were significantly smaller in tanibirumab-treated eyes (20 and 60 μg/2 μL of PBS) (n=8 and 7, respectively) than PBS-injected eyes (n=8; P=0.048 and P<0.001, respectively, by the Mann–Whitney U-test). Human IgG-injected eyes showed no difference compared with PBS-injected eyes. In the 5 μg tanibirumab-treated eyes (n=7), the mean CNV areas, as measured by BS isolectin B4 labeling, were not significantly smaller than in PBS-injected eyes (n=8; P=0.132 by t-test). *P<0.05. Scale bar denotes 100 μm.
Evaluation of histological cross sections
When the eyes were enucleated, the lens opacity was evaluated with light microscopy. No eyes of the prevention and treatment arm showed visible lens opacity.
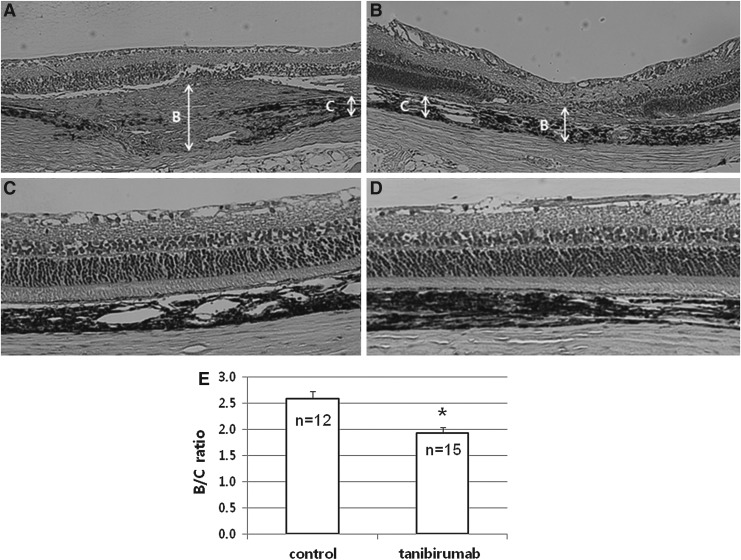
In the prevention arm, the retinal cross sections revealed that the B/C ratios of laser-treated spots were significantly lower in tanibirumab (60 μg)-injected eyes than in control eyes (P<0.001 by Mann–Whitney U-test, Fig. 5E). The representative photos are shown in Figure 5A and B. In addition, HE staining of retinal cross sections showed no retinal thinning or choroidal atrophy in tanibirumab-treated eyes compared with control eyes (Fig. 5C, D).
FIG. 5.
Evaluation of histological cross sections. On retinal cross sections, CNV was estimated by the B/C ratio of the thickness from the bottom of the pigmented choroidal layer to the top of the neovascular membrane (B) to the thickness of the intact pigmented choroid adjacent to the lesion (C). The representative photos are shown in (A) (control eye) and (B) (tanibirumab-injected eye). The B/C ratios of laser-treated spots were significantly lower in tanibirumab-injected eyes than in control eyes (P<0.001 by the Mann–Whitney U-test, E). In addition, retinal cross sections showed no retinal thinning or choroidal atrophy in tanibirumab-treated eyes (D) compared with control eyes (C) *P<0.05.
Discussion
In this study, tanibirumab, a fully human monoclonal antibody against VEGFR-2, inhibited laser-induced CNV in a rat model. Intravitreally administered tanibirumab partially suppressed CNV formation and partially regressed established CNV.
Ranibizumab and bevacizumab are humanized monoclonal antibody fragments designed to bind all isoforms of VEGF-A, thereby blocking angiogenesis and vessel permeability in CNV.17,18 Inhibition of VEGF-mediated signaling pathways by blocking VEGFR also suppresses the formation of laser-induced CNV.10,19,20 SU5416, a novel synthetic compound and an inhibitor of VEGFR-1 and VEGFR-2, suppresses laser-induced CNV in a mouse model, with significant effects produced following both intraperitoneal and intravitreal administration.10,19 Systematic administration of axitinib, which inhibits receptor tyrosine kinases of VEGFR-1, VEGFR-2, and VEGFR-3, reportedly suppressed and regressed laser-induced CNV.20 Systemically administered DC101, a murine monoclonal antibody against VEGFR-2, also suppressed the formation of CNV.21 In our study, intravitreally administered tanibirumab showed regression of preformed laser-induced CNV as well as suppression of CNV formation in the rat model. Furthermore, in the preclinical study for comparing tanibirumab with bevacizumab in the human malignant glioblastoma orthotopic model, as well as lung cancer, breast cancer, colon cancer, and liver cancer models in mice, tanibirumab showed better efficacy in all of the models.22 Thus, intravitreal tanibirumab is a potential therapeutic agent for CNV.
Binding to the VEGF-binding domain of VEGFR-2 with a dissociation constant of 2.3×10−10 M in humans, tanibirumab inhibits VEGF-mediated signaling pathways such as phosphorylation of VEGFR-2 and its downstream signaling molecule, ERK, in a dose-dependent manner.13 Tanibirumab has a possible advantage compared with currently approved antiangiogenic agents in that, it blocks all forms of VEGF from binding to VEGFR-2. VEGF-C and -D reportedly were detected in the vitreous of AMD patients and CNV membranes from humans and laser-induced CNV mouse models. Recently, Lashkari et al. reported that VEGF-C levels are significantly elevated in the plasma of AMD patients and blockade of VEGF-C/-D significantly inhibits laser-induced CNV.12 Therefore, blocking all VEGF family members might produce a better effect than blocking VEGF-A alone.11,12
VEGFRs, especially VEGFR-1, had been reported to be associated with controlling the recruitment and infiltration of inflammatory cells to specific lesions in different disease statuses. Van de Veire et al.23 reported that the deficiency of the VEGF homolog PlGF significantly inhibited infiltration of macrophages into laser-induced CNV. Moreover, it was suggested by Huang et al.24 that circulating cells responding to the CNV lesion with positivity for VEGFR-1 and 2 reacted to the VEGFR antibody therapy and halted the production of cytokines that were needed to recruit the microglia, which had no positivity for VEGFR-1 and -2. In this regard, tanibirumab also has the potential acting mechanism through indirect blockade on the recruitment of inflammatory cells as well as direct inhibitory action on VEGFR-2. Because BS isolectin B4 not only binds vascular endothelial cells but also activated microglia in laser-induced CNV models,21 a decrease in the BS isolectin B4-stained area might imply decreased recruitment of inflammatory cells as well as reduced CNV.
In this study, both FITC-dextran perfusion and BS isolectin B4 labeling were used for visualizing and quantifying morphological changes associated with laser-induced CNV. FITC-dextran perfusion was developed to visualize retinal vessels in the beginning25 and, hereafter, has been used widely for evaluating entirely developed CNV lesions.19,26,27 This technique does not allow consistent visualization of vasculature during the early phase of CNV formation,19,26 and so may provide variable results when used to measure laser-induced CNV.26 On the other hand, BS isolectin B4 labeling allows the visualization of the formation of laser-induced CNV from the beginning.28 This technique enables researchers to avoid the variability involved with FITC-dextran perfusion. The combination of FITC-dextran perfusion and TRITC-BS isolectin B4 labeling is effective for the visualization of nonperfused and perfused newly developed CNV.28 In this study, there was an agreement between FITC-dextran perfusion and BS isolectin B4 labeling on measurement of the mean CNV areas.
This study demonstrated the inhibitory effect of tanibirumab on laser-induced CNV in a rat model. Intravitreally administered tanibirumab showed partial regression of established CNV as well as suppression of CNV formation. Although tanibirumab is a fully human monoclonal antibody, its inhibitory effects on CNV proved to be effective using an in vivo rat model. In murine models, the ability to neutralize murine VEGF by existing anti-VEGF antibody agents, such as ranibizumab and bevacizumab, was reported to be weak.29,30 Therefore, tanibirumab is expected to be useful for further research for antiangiogenesis in rat models.
Despite the contributions of the study discussed here, our study is not without its shortcomings. First, due to the lack of images taken using fluorescent angiography or optical coherence tomography, we could not evaluate leakage or retinal edema associated with CNV. Second, the concentration of tanibirumab resulting in maximal inhibitory effects on the CNV was not confirmed. The maximum concentration that we could obtain was 30 mg/mL, which is similar to the concentration of marketed agent, Avastin® (bevacizumab) (25 mg/mL). Starting from this maximum concentration, we tested lower doses. Finally, the systemic safety of tanibirumab was not reported yet. However, because the clinical phase I trial of tanibirumab in advanced metastatic cancer has been completed on January 2014 and the study results are currently being assessed, the data about adverse events of tanibirumab will be presented, including a comparison with bevacizumab, in the near future. Although none of the eyes showed visible lens opacity, retinal thinning, or choroidal atrophy, it is important to be mindful of the likelihood of side effects from blocking VEGFR-2 in the clinical application of tanibirumab because VEGF signaling through VEGFR-2 is thought to play physiological roles in cell survival, especially in neuronal cells and in developing organs.31,32
In conclusion, intravitreally administered tanibirumab, a fully human monoclonal antibody against VEGFR-2, resulted in the partial inhibition of newly forming and partial regression of established laser-induced CNV in the rat model. Tanibirumab may be a feasible treatment for CNV associated with AMD or other causes.
Supplementary Material
Acknowledgments
This study was supported by the Samsung Medical Center grant (SMR112051) and grant from the PharmAbcine, Inc. (PHX1127081 and PHX1127161). The authors thank Dr. Jin-San Yoo, Dr. Weon Sup Lee, and Dr. Sung-Woo Kim at PharmAbcine for helpful scientific discussions and comments.
Author Disclosure Statement
S.R.S. is a coinventor of a patent for tanibirumab. S.R.S. and S.H.L. are employees of PharmAbcine, Inc.
References
- 1.Lim L.S., Mitchell P., Seddon J.M., et al. Age-related macular degeneration. Lancet. 379:1728–1738, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Jager R.D., Mieler W.F., and Miller J.W.Age-related macular degeneration. N. Engl. J. Med. 358:2606–2617, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Wong T.Y., Chakravarthy U., Klein R., et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 115:116–126, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Folkman J.Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1:27–31, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P., Ferreira V., Breier G., et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 380:435–439, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Shibuya M., Ito N., and Claesson-Welsh L.Structure and function of VEGF receptor-1 and -2. Curr. Topics Microbiol. Immunol. 237:59–83, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T., Yamaguchi S., Chida K., et al. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 20:2768–2778, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waltenberger J., Claesson-Welsh L., Siegbahn A., et al. Different signal transduction properties of KDR and Flt-1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 269:26988–26995, 1994 [PubMed] [Google Scholar]
- 9.Takeda A., Hata Y., Shiose S., et al. Suppression of experimental choroidal neovascularization utilizing KDR selective receptor tyrosine kinase inhibitor. Graefes Arch. Clin. Exp. Ophthalmol. 241:765–772, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Kami J., Muranaka K., Yanagi Y., et al. Inhibition of choroidal neovascularization by blocking vascular endothelial growth factor receptor tyrosine kinase. Jpn. J. Ophthalmol. 52:91–98, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Otani A., Takagi H., Oh H., et al. Vascular endothelial growth factor family and receptor expression in human choroidal neovascular membranes. Microvasc. Res. 64:162–169, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Lashkari K., et al. Association for research in vision & ophthalmology. Invest. Ophthalmol. Vis. Sci. 54:E-Abstract 4999, 2013 [Google Scholar]
- 13.Lee S.H.Tanibirumab (TTAC-0001): a fully human monoclonal antibody targets vascular endothelial growth factor receptor 2 (VEGFR-2). Arch. Pharm. Res. 34:1223–1226, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Kim S.J., Kim J., Lee J., et al. Intravitreal human complement factor H in a rat model of laser-induced choroidal neovascularisation. Br. J. Ophthalmol. 97:367–370, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Edelman J.L., and Castro M.R.Quantitative image analysis of laser-induced choroidal neovascularization in rat. Exp. Eye Res. 71:523–533, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Lambert V., Munaut C., Noël A., et al. Influence of plasminogen activator inhibitor type 1 on choroidal neovascularization. FASEB J. 15:1021–1027, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Presta L.G., Chen H., O'Connor S.J., et al. Humanisation of an anti-VEGF monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 57:4593–4599, 1997 [PubMed] [Google Scholar]
- 18.Lowe J., Araujo J., Yang J., et al. Ranibizumab inhibits multiple forms of biologically active VEGF in vitro and in vivo. Exp. Eye Res. 85:425–430, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Honda M., Asai T., Umemoto T., et al. Suppression of choroidal neovascularization by intravitreal injection of liposomal SU5416. Arch. Ophthalmol. 129:317–321, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Kang S., Roh C.R., Cho W.K., et al. Antiangiogenic effects of axitinib, an inhibitor of vascular endothelial growth factor receptor tyrosine kinase, on laser-induced choroidal neovascularization in mice. Curr. Eye Res. 38:119–127, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Huang H., Shen J., and Vinores S.A.Blockade of VEGFR1 and 2 suppresses pathological angiogenesis and vascular leakage in the eye. PLoS One. 6:e21411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho M., Royston I., and Beck A.2nd PEGS Annual Symposium on Antibodies for Cancer Therapy. MAbs. 4:562–570, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van de Veire S., Stalmans I., Heindryckx F., et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 141:178–190, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Huang H., Parlier R., Shen J.K., et al. VEGF receptor blockade markedly reduces retinal microglia/macrophage infiltration into laser-induced CNV. PLoS One. 8:e71808, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Amato R., Wesolowski E., and Smith L.E.Microscopic visualization of the retina by angiography with high-molecular-weight fluorescein labeled dextrans in the mouse. Microvasc. Res. 46:135–142, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Semkova I., Peters S., Welsandt G., et al. Investigation of laser-induced choroidal neovascularization in the rat. Invest. Ophthalmol. Vis. Sci. 44:5349–5354, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Apte R.S., Barreiro R.A., Duh E., et al. Stimulation of neovascularization by the anti-angiogenic factor PEDF. Invest. Ophthalmol. Vis. Sci. 45:4491–4497, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Campos M., Amaral J., Becerra S.P., et al. A novel imaging technique for experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 47:5163–5170, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lu F., and Adelman R.A.Are intravitreal bevacizumab and ranibizumab effective in a rat model of choroidal neovascularization? Graefes Arch. Clin. Exp. Ophthalmol. 247:171–177, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Yu L., Wu X., Cheng Z., et al. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest. Ophthalmol. Vis. Sci. 49:522–527, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Beazley-Long N., Hua J., Jehle T., et al. VEGF-A165b is an endogenous neuroprotective splice isoform of vascular endothelial growth factor A in vivo and in vitro. Am. J. Pathol. 183:918–929, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokunaga C.C., Mitton K.P., Dailey W., et al. Effects of anti-VEGF treatment on the recovery of the developing retina following oxygen-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 28:1884–1892, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.