Abstract
Individuals with medically diagnosed knee osteoarthritis (OA) participated in a randomized, double-blind study to investigate the effects of a high-rosmarinic acid (rosA) spearmint tea. Sixty-two participants were randomized by sex and screening pain score to consume tea brewed from a high-rosA spearmint variety or a commercially available spearmint twice daily for 16 weeks. Pain, quality of life (QoL), and physical function at baseline and week 16 were assessed using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Short-Form 36-item Health Survey (SF-36), 6-minute walk test (6MWT), and stair climb test (SCT). Data from 46 participants (mean age=60.7; BMI=32.9 kg/m2) were analyzed. Pain score significantly decreased from week 0 to 16 for the high-rosA group but not for the control group and scores for stiffness and physical disability significantly decreased from week 0 to 16 for both groups. Increased QoL score on the bodily pain index in the SF-36 was observed at week 16 within the high-rosA group only, although no significant differences were observed between the groups. A nonsignificant improvement was observed in the 6MWT at week 16 in the high-rosA group only. There were no changes in the SCT for either group. Therefore, 16-week daily consumption of the high-rosA and commercial spearmint teas significantly improved stiffness and physical disability scores in adults with knee OA, but only the high-rosA tea significantly decreased pain. Consumption of high-rosA tea warrants further consideration as a potential complementary therapy to reduce pain in OA. Clinical Trial Registration Number: NCT01380015.
Key Words: : 6-minute walk test, knee osteoarthritis, pain, rosmarinic acid, spearmint tea, WOMAC
Introduction
Osteoarthritis (OA) is a disease characterized by the degeneration of articular cartilage, manifesting as joint pain, stiffness, and impaired function leading to physical disability and decreased quality of life (QoL).1 Knee OA is one of the most common chronic diseases with an estimated prevalence of 24% among North American adults.2 The pathogenesis of OA is complex and not fully understood, but is associated with an imbalance of anabolic and catabolic activities in the cartilage in which degenerative processes prevail, leading to the loss of cartilage.3 There is no treatment that slows cartilage breakdown, so treatment is focused on decreasing pain and increasing function with nonpharmacological interventions like exercise and assistive devices, as well as nonsteroidal anti-inflammatory drugs and acetaminophen.4 While these pharmaceuticals offer acute-pain management for some individuals, serious gastrointestinal and cardiac side effects limit their long-term use.5 It is reported that 30–45% of individuals with OA also use supplements to manage the disease,6–8 with glucosamine being the most common. However, even for glucosamine, controversy remains surrounding efficacy, after a large National Institutes of Health, randomized, double-blind glucosamine trial failed to show any significant improvements in OA patients.9 Therefore, further research into safe, complementary therapies for OA is required.
Rosmarinic acid (rosA) is a polyphenolic compound naturally present in spearmint, peppermint, fennel, and other species of the Lamiaceae family and that has been hypothesized to be of potential benefit for OA.10–13 In vitro, rosA has been shown to have anti-inflammatory, antioxidant, immunosuppressant, and antibacterial activities.11 Through selective breeding techniques, a spearmint plant containing ∼20 times more rosA than native spearmint was developed (high-rosA spearmint plant clone 700B).12 In porcine cartilage explants, a high-rosA spearmint extract reduced the expression of lipopolysaccharide (LPS)–induced prostaglandin E2 (PGE2) and nitric oxide release and also inhibited glycosaminoglycan (GAG) release, suggesting a potential chondroprotective effect.13 Also, healthy, mature standardbred horses were fed hay and sweet feeds containing either no (n=4) or 54 mg/kg body weight of the high-rosA spearmint (n=4) for 24 days.14 Following intercarpal LPS injections to induce inflammation, the horses consuming high-rosA spearmint had reduced synovial fluid PGE2 and GAG levels compared with the control horses. These results demonstrate the anti-inflammatory and potential chondroprotective actions of the high-rosA spearmint and support its use as a complementary therapy for the management of knee OA. Therefore, a study was conducted to examine the effects of daily consumption of tea brewed from the high-rosA spearmint plant on measures of pain, stiffness, QoL, and physical function in adults with OA of the knee.
Materials and Methods
Study design
This randomized, parallel-arm, double-blind study was conducted at the Human Nutraceutical Research Unit (HNRU) of the University of Guelph. The University of Guelph Human Research Ethics Board approved the study protocol (REB No. 11JA040), which was registered in the NIH clinical trial registry (Protocol No. NCT0138001) and all participants provided written informed consent.
Recruitment and screening
Adult (>18 years old) men and women were recruited from Guelph, ON, and the surrounding communities from June 2011 to June 2012 and were eligible for study inclusion if they were nonsmokers, had medically diagnosed OA of the knee, and a screening Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score of>125. Exclusion criteria included other systemic or rheumatic arthritis; concomitant inflammatory processes; upcoming knee replacement surgery; chemical, radiologic, or surgical synovectomy in any large joint within the previous 3 months; gastrointestinal ulcers; clinically significant, uncontrolled cardiovascular, hepatic, or renal disorder; any serious medical condition within 6 months, such as heart attack, stroke, cancer, or diabetes; known allergy or hypersensitivity to mint or other food allergies; smoking; alcohol consumption >14 drinks per week; recreational drug use; participation in a clinical trial within the previous 6 months; and pregnancy (or intention to become pregnant), <6 months postpartum, lactating or <6 months postlactation.
Study treatment tea, tea protocol and blinding
The study treatment tea was brewed from the high-rosA spearmint plant (Clone 700B)12 and the control tea was brewed from commercially available spearmint tea (Distinctly Teas, Inc., Stratford, ON, Canada). Dried spearmint leaves were blended on low in a food-grade blender for 30 sec. Three grams of plant material was transferred into individual Teeli®flip tea bags (Riensch & Held, Hamburg, Germany), which were stapled. The identical control and high-rosA tea bags were placed in vacuum sealed bags, coded by a person external to the study team, and stored at room temperature until use. All participants and study team members were blinded throughout data collection and analysis.
Participants were instructed to consume two cups of tea per day in a provided 300-mL study mug for a 16-week period. Brewing instructions detailed that one tea bag was to be steeped in boiling water for 5 min with occasional stirring and the addition of milk, cream, sugar, or sweetener was not allowed. Three grams of high-rosA spearmint in 300 mL of water for 5 min was verified by high-performance liquid chromatography to provide 130–150 mg of rosA per cup compared with ∼13 mg rosA from the control tea (data not shown). Therefore, participants in the control and high-rosA groups consumed 26 versus 280 mg rosA per day, respectively. Analysis of brewed tea samples by Maxxam Analytics (Mississauga, ON, Canada) ensured that pesticide, microbiological, and mycotoxin levels were below the NHP Directorate Accepted Tolerance Limits.15 Participants were asked to not consume any herbal teas for the duration of the study. Consumption of coffee as well as black and green tea was allowed. Participants recorded the times at which they consumed the study tea in a daily study diary as a measure of compliance.
Treatment randomization
Participants were block randomized into treatment groups based on sex and screening WOMAC pain score. Covariate adaptive randomization16 was performed by an individual not associated with the study. Sequentially numbered sheets of paper were used to implement the allocation sequence.
Outcome measures
Outcome measures were assessed at baseline, week 8, and week 16. At baseline and after completion of the 16-week protocol, anthropometric measurements were taken. Phone check-in conversations occurred at weeks 2, 6, 10, and 14 to discuss compliance, medications, physical activity, and any other issues. Safety of tea consumption was assessed by recording adverse events. Participants were asked to maintain their regular medication and supplement use throughout the study period but were also advised that they could increase or decrease their use of pain medication as they felt necessary. They were asked to track all medications and supplements in their daily study diary. Changes in pain medication consumption were quantified by calculating total number of tablets taken per week.17
Anthropometric measurements
Height was measured to the nearest millimeter using a stadiometer (Model 217, SECA®; Hanover, NH, USA) and body weight was measured to the nearest 0.05 kg on a digital scale (SVI-200F; Acculab®, Barrie, Canada). Two measurements were averaged for each of waist and hip circumference determined over clothing with a tape measure (Model 201, SECA). Blood pressure and body composition were determined with an Omron® digital blood pressure monitor and bioelectrical impedance analysis machine (Bodystat®1500; Bodystat®, Isle of Man, United Kingdom), respectively. All anthropometric measurements were completed by the same trained study coordinator.
Western Ontario and McMaster Universities Osteoarthritis Index
The WOMAC is a validated, standardized 24-item questionnaire that assesses pain, disability, and joint stiffness associated with OA.18 In the presence of a study coordinator, the 100-mm visual analog scale version of the WOMAC was administered and scored according to the WOMAC® Osteoarthritis Index User Guide IX.19
Short-Form General Health Survey
The Medical Outcome Study 36-item Short-Form General Health Survey (SF-36) is a self-administered questionnaire that assesses eight components of health-related QoL (i.e., physical function, physical role, bodily pain, vitality, social functioning, role emotional, mental health, and general health) and two composite scores (i.e., physical component score and mental component score).20 In the presence of a study coordinator, participants completed the questionnaire that was scored using Medical Outcomes software.
Physical function tests
The 6-minute walk test (6MWT) and stair climb test (SCT) are performance-based measures used to assess physical function. Briefly, the walk test was conducted by marking off a 20-m distance in a straight and flat interior hallway and asking participants to walk as quickly as possible for 6 min, with standardized, verbal encouragement given at every minute, as adapted from the American Thoracic Society21 and Enright.22 Participants were permitted to rest, as necessary, and to use any mobility aids, as per their normal use. The total distance walked was determined to the nearest centimeter. The SCT involved participants ascending and descending a flight of seven stairs as quickly as was safely possible. The time required for the total ascent/descent was recorded to the nearest millisecond.
Statistical analysis
All statistical analyses were performed using the Statistical Analysis System (version 9.3; Cary, NC, USA) with P<.05 considered statistically significant. A sample size calculation based on the outcome of total WOMAC score, using a significance level of 0.05 and power of 80%, indicated that 25 participants per group were required. Individual WOMAC pain, stiffness, and function subscales were converted to normalized units (0–100).23 Data normality was confirmed using stem leaf diagrams and box plots. Baseline anthropometric data and outcome scores were compared between the high-rosA and control groups using unpaired t-tests. Analysis for efficacy was done using the per protocol population. Baseline data for all outcome measures were compared against weeks 8 and 16 within the high-rosA and control groups using unpaired Student's t-tests. Between high-rosA and control group differences (weeks 0–8 and weeks 0–16) were analyzed for all outcome measures using repeated measures analysis of variance (ANOVA). Differences in pain medication intake between groups were assessed using repeated measures ANOVA.
Results
Participant flow, withdrawals, and exclusions
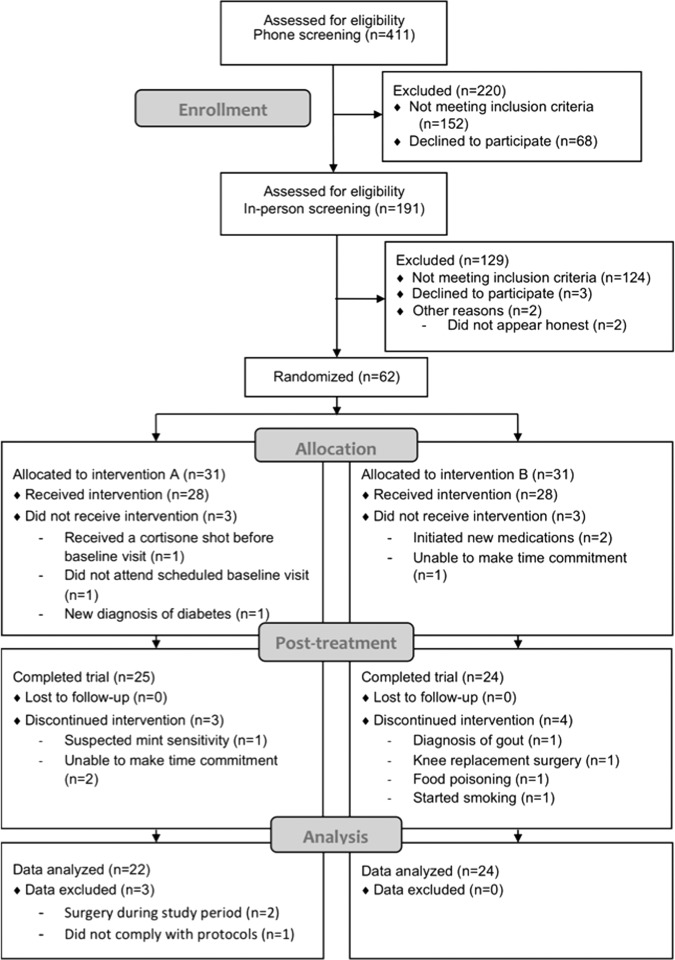
A total of 411 individuals completed the phone screening questionnaire, 191 individuals completed the in-person screening questionnaire, and 62 individuals were randomized to treatment (Fig. 1). Twenty-eight participants in each group received the intervention, and two participants in the high-rosA group withdrew due to time commitment issues and one due to a previously unidentified mint sensitivity. One participant in the control group withdrew due to a diagnosis of gout, one due to knee replacement surgery, one from prolonged nonrelated food poisoning illness, and one participant was excluded because they began smoking. In the high-rosA group, data from two participants were removed from analysis due to arthroscopic surgery after 12 weeks of participation in the study and one for noncompliance with study protocols. Complete data were collected and analyzed for the remaining 46 participants.
FIG. 1.
Participant flow through the trial (CONSORT diagram).
Participant characteristics
Participant characteristics and baseline outcome measures were not significantly different between the high-rosA (n=22) and control (n=24) groups at baseline (Table 1). Participant characteristics and anthropometric measurements were not significantly different between groups at any time during the study (data not shown). Tea consumption compliance was 96.8% and 94.8% for the high-rosA and control tea groups, respectively, as self-reported in daily study diaries. This was consistent with study coordinator tracking of dispensed and returned tea bags. There were no significant differences in pain medication tablets consumed per week between the groups or over time (data not shown).
Table 1.
Participant Characteristics and Outcome Measures at Baseline
| High-rosA (n=22) | Control (n=24) | |
|---|---|---|
| Participant characteristics | ||
| Age (years) | 60.5±11.1 | 60.8±12.1 |
| Sex (n) (males/females) | 8/14 | 6/18 |
| Height (cm) | 167.8±9.4 | 164.3±8.8 |
| Weight (kg) | 96.8±28.3 | 85.2±19.7 |
| BMI (kg/m2) | 34.3±8.6 | 31.5±6.9 |
| Waist circumference (cm) | 65.6±18.2 | 58.4±13.0 |
| Hip circumference (cm) | 49.9±13.2 | 45.0±9.8 |
| Blood pressure SBP/DBP (mmHg) | 135.4/83.8 | 132.3/80.7 |
| Pulse (bpm) | 73.9±11.5 | 73.8±12.2 |
| Body fat (%) | 37.8±12.0 | 38.0±9.6 |
| Lean body weight (kg) | 57.0±16.1 | 51.8±10.9 |
| WOMAC scores | ||
| Pain | 39.6±13.9 | 36.1±22.2 |
| Stiffness | 54.9±19.6 | 52.5±23.2 |
| Physical disability | 36.8±17.7 | 39.0±22.1 |
| Total score | 131.3±42.4 | 127.5±60.5 |
| SF-36 scores | ||
| Mental component summary score | 56.4±9.0 | 52.0±10.4 |
| Physical component summary score | 38.6±6.6 | 39.3±7.3 |
| Physical function tests | ||
| 6MWT (m) | 448.4±73.8 | 487.1±87.2 |
| SCT (sec) | 11.4±3.0 | 12.9±4.2 |
| Serum measurements | ||
| C-Reactive protein (mg/L) | 4.1±4.2 | 4.7±6.2 |
Values are mean±standard deviation.
6MWT, six-minute walk test; DBP, diastolic blood pressure; rosA, rosmarinic acid; SBP, systolic blood pressure; SCT, stair climb test; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Adverse events
There were no serious adverse events reported during the study. Participants in the high-rosA group reported headache (n=2), constipation (n=3), and loose bowel movements (n=1). Participants in the control group reported dry mouth (n=1), itchy skin (n=1), and staining of dentures (n=1). All reported adverse events were transient and short term.
Outcome measures
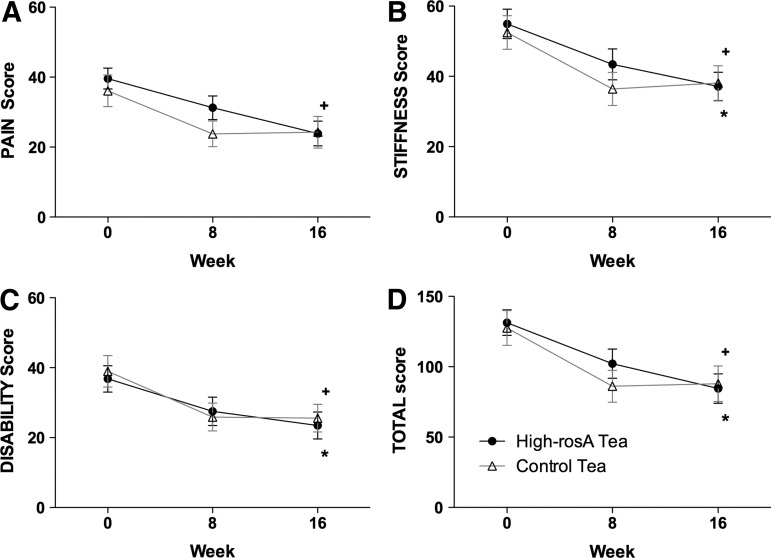
WOMAC pain score significantly decreased from baseline within the high-rosA group to week 16 (P=.002) (Fig. 2A). In the control group, WOMAC pain score also decreased from baseline; however, it was significant at week 8 (P=.04) but not at week 16 (P=.07). There were no significant differences in WOMAC pain score between the groups at any time points in the study. WOMAC stiffness significantly decreased from baseline to week 16 within the high-rosA (P=.004) and control (P=.04) groups, although there was no difference between groups (P=.37) (Fig. 2B). WOMAC physical disability score significantly decreased from baseline to week 16 within the high-rosA (P=.02) and the control (P=.03) groups but no significant differences were observed between the groups at any time point (Fig. 2C). Generally, WOMAC scores on all scales did not significantly differ from week 8 to 16 within or between the treatment groups.
FIG. 2.
Mean scores and standard error are presented for (A) WOMAC pain scores, (B) WOMAC stiffness scores, (C) WOMAC physical function score, and (D) WOMAC total scores for the high-rosA group (circles) and the control group (triangles). The change within groups from baseline to week 16 was examined; significant differences within the high-rosA group are indicated with a cross (+) and significant differences within the control group are indicated with an asterisk (*). rosA, rosmarinic acid; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
In the SF-36, the only significant change observed was in the QoL score for bodily pain, with an increase observed from baseline to week 16 within the high-rosA group (Table 2). There were no other significant changes observed for SF-36 scores either within or between the high rosA and control groups (Table 2).
Table 2.
SF-36 Scores for Weeks 0 and 16 for the High-rosA and Control Groups
| High-rosA | Control | ||||
|---|---|---|---|---|---|
| Week 0 | Week 16 | Week 0 | Week 16 | Canadian norma | |
| Physical function | 55.7±19.8 | 59.8±23.5 | 55.4±20.9 | 63.6±19.9 | 82.3±19.3 |
| Physical role | 63.1±19.9 | 76.1±24.1 | 61.2±27.7 | 70.1±21.4 | 81.3±33.1 |
| Bodily pain | 46.6±7.6 | 58.1±22.1b | 49.7±17.8 | 55.7±14.6 | 74.9±23.7 |
| General health | 70.7±12.7 | 70.8±18.4 | 69.3±19.0 | 74.0±14.1 | 74.8±19.4 |
| Vitality | 60.5±15.6 | 63.1±19.9 | 51.9±19.6 | 58.6±17.7 | 68.3±17.7 |
| Social functioning | 86.9±21.0 | 89.2±22.9 | 76.0±25.3 | 81.3±20.9 | 88.1±18.8 |
| Role emotional | 86.4±19.0 | 87.9±23.5 | 78.8±24.8 | 83.3±20.0 | 87.8±28.3 |
| Mental health | 80.0±13.4 | 80.2±23.4 | 76.8±19.3 | 79.0±13.7 | 79.5±14.7 |
| Physical component score | 38.6±6.6 | 42.5±9.0 | 39.3±7.3 | 42.9±7.2 | 49.0±9.2 |
| Mental component score | 56.4±8.9 | 55.8±13.0 | 51.4±10.9 | 53.1±10.2 | 53.7±8.2 |
Values are mean±standard deviation.
For healthy Canadians aged 55–64 years.27
Change from baseline to week 16, P<.05.
The 6MWT total distance travelled did not significantly differ from baseline to week 16 either between (P=.12) or within the high-rosA (P=.94) or control groups (P=.96) (Table 3). The total time taken to complete the SCT was not significantly different from baseline to week 16 either within the high-rosA (P=.43) or the control group (P=.44) or between the groups (P=0.9).
Table 3.
Six-Minute Walk Test Distances (m) for Weeks 0, 8, and 16 for the High-rosA and Control Groups
| High-rosA (m) | Change from baselinea(m) | Control (m) | Change from baselinea(m) | |
|---|---|---|---|---|
| Week 0 | 451.5±73.6 | 439.4±87.2 | ||
| Week 8 | 451.7±92.9 | +0.2 | 438.3±84.5 | −1.1 |
| Week 16 | 473.8±72.3 | +22.3 | 439.5±88.2 | +0.1 |
Values are mean±standard deviation.
Difference from week 0.
Discussion
This was the first human intervention study to examine the effects of daily consumption of tea brewed from a novel high-rosA spearmint plant on markers of pain, stiffness, and physical function in adults with knee OA. The study was also unique in that it utilized a pragmatic design by allowing participants to maintain their normal pain medication routine. This was intended to explore the potential of the high-rosA tea as a complementary OA therapy.
Anthropometrics did not change over the treatment period, which is important, as weight loss is known to improve OA symptoms.24,25 Although the pain scores were never significantly different between groups, the significant decrease during the treatment period within the high-rosA group is an important finding. In the control group, pain scores only significantly decreased from week 0 to 8 with no further significant decreases, suggesting a placebo effect. Placebo analgesia is a well-known phenomenon in OA and pain research,26 which possibly contributed to the lack of significant difference between the groups. Because of this known placebo effect, we prioritized running a blinded study. Therefore, a commercial spearmint tea, identical in appearance and flavor to the high-rosA tea but that contained a small amount of rosA (∼13 mg per 300 mL), was used as a control. As the decreases in WOMAC scores ceased at week 8 in the control group, but continued to decline in the high-rosA group, we hypothesize that lengthening the treatment period could result in significant difference between the groups.
The SF-36 questionnaire was created to address the importance of patient perspective in the assessment of healthcare outcomes.20 The significant increase in QoL bodily pain score in high-rosA group suggests that reduced pain led to an increase in QoL in this group. This is in agreement with the decrease in WOMAC pain score observed for the high-rosA group. As expected, the SF-36 scores for physical function, physical role, bodily pain, and physical component score were all below the Canadian norms at all time points studied and in both groups (Table 2).27 Individuals with musculoskeletal disorders tend to report lower physical functioning scores compared with the general population and of those patients, OA patients generally have worst pain and physical functioning scores.28–30
The study participants demonstrated impaired physical function compared with average healthy Canadians of comparable age in the 6MWT. In the current study (mean age=61 years), the average distance walked in the 6MWT at baseline was 445.5 m (range=312.0–611.9 m), which falls below the average distance of 672 m for men and 611 m for women (range=416–888 m) for healthy Canadians with an average age of 65.31 This is not surprising, as individuals with arthritis, as well as overweight/obese individuals, have been documented to walk shorter distances in the 6MWT.22
Although no significant differences were observed in the 6MWT, the high-rosA group walked 22 m further at week 16 than at baseline. The control group only walked 0.1 m further at week 16 compared with baseline. We postulate that, at week 16, the high-rosA group was starting to experience improvements in physical function related to their decreased pain. While not statistically significant, the improvement in walk distance could be meaningful as even small increases in walking distance are important to individuals with OA.32 The SCT is useful as it measures the ability to negotiate stairs and this is a common challenge for individuals with OA.33 Heiberg et al. reported that individuals with OA completed an 8-SCT within the range of 10–14 sec.34 This is similar to the range of 10–12 sec recorded in the present study where seven stairs were used.
In the current study, compliance to drinking the tea was high, which reflects our participants' willingness to consume spearmint tea as a treatment for OA. This is important, given the lack of effective OA treatment options4 and reliance on pain medications to which adherence rates are low.35 Failure to adequately manage OA pain and symptoms can negatively impact social interactions and QoL and leads to increased personal, healthcare, and economic costs.36 In this study, individuals with OA of the knee who consumed 600 mL of high-rosA spearmint tea daily for 4 months showed significant improvements in pain scores and physical function. Therefore, adults with knee OA may benefit from including high-rosA spearmint tea in their daily routine. Larger and longer-term studies are required to validate the high-rosA tea as a complementary OA treatment.
Acknowledgments
The research was sponsored by the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA No. 200121). The authors would like to thank all the study participants. Additionally, we thank Dr. Andrew Chow for his insights during the study design and recruitment stages; Dr. Forrest Caldwell, Premila Sathasivam, and Mehrnoosh Kashani for their assistance with sampling; and the many undergraduate research students and volunteers who helped with the study.
Author Disclosure Statement
L.K. has a patent on the high-rosA spearmint plant, clone 700B. No competing financial interests exist for any other authors.
References
- 1.Martel-Pelletier J, Pelletier JP: Is osteoarthritis a disease involving only cartilage or other articular tissues? Eklem Hastalik Cerrahisi 2010;21:2–14 [PubMed] [Google Scholar]
- 2.Bijlsma JW, Berenbaum F, Lafeber FP: Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–2126 [DOI] [PubMed] [Google Scholar]
- 3.Loeser RF: Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage 2009;17:971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Moskowitz RW, Nuki G, et al. : OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;6:137–162 [DOI] [PubMed] [Google Scholar]
- 5.Pincus T, Koch GG, Sokka T, et al. : A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen in patients with osteoarthritis of the hip or knee. Arthritis Rhuem 2001;44:1587–1598 [DOI] [PubMed] [Google Scholar]
- 6.Lawson B, Putnam W, Nicol K, Archibald G, Mackillop J, Conter H: Managing osteoarthritis, medication use among seniors in the community. Can Fam Physician 2004;50:1664–1670 [PMC free article] [PubMed] [Google Scholar]
- 7.Lapane KL, Sands MR, Yang S, McAlindon TE, Eaton CB: Use of complementary and alternative medicine among patients with radiographic-confirmed knee osteoarthritis. Osteoarthritis Cartilage 2012;20:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh SR, Levine MA: Natural health product use in Canada: analysis of the national population health survey. Can J Clin Pharmacol 2006;13:240–250 [PubMed] [Google Scholar]
- 9.Clegg DO, Reda DJ, Harris CL, et al. : Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006;23:795–808 [DOI] [PubMed] [Google Scholar]
- 10.Petersen M, Simmonds MS: Rosmarinic acid. Phytochemistry 2003;62:121–125 [DOI] [PubMed] [Google Scholar]
- 11.Youn J, Lee KH, Won J, Huh SJ, Yun HS, Cho WG, Palik DJ: Beneficial effects of rosmarinic acid on suppression of collagen induced arthritis. J Rheumatol 2003;30:1203–1207 [PubMed] [Google Scholar]
- 12.Fletcher RS, McAuley C, Kott LS: Novel Mentha spicata Clones with enhanced rosmarinic acid and antioxidant activity. Acta Hort 2005;6:31–36 [Google Scholar]
- 13.Pearson W, Fletcher RS, Kott LS, Hurtig MB: Protection against LPS-induced cartilage inflammation and degradation provided by a biological extract of Mentha spicata. BMC Complement Altern Med 2010;11:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson W, Fletcher RS, Kott LS: Oral rosmarinic acid-enhanced Mentha spicata modulates synovial fluid biomarkers of inflammation in horses challenged with intra-articular LPS. J Vet Pharmacol Ther 2012;35:495–502 [DOI] [PubMed] [Google Scholar]
- 15.Natural Health Products Directorate: Draft: Quality of Natural Health Products Guide. Health Canada, Ottawa, Canada, 17, 2012, Report No.: Version 3.0. [Google Scholar]
- 16.Kang M, Ragan BG, Park JH: Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train 2008;43:215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constant F, Guillemin F, Herbeth B, Collin JF, Boulange M: Measurement methods of drug consumption as a secondary judgment criterion for clinical trials in chronic rheumatic diseases. Am J Epidemiol 1997;145:826–833 [DOI] [PubMed] [Google Scholar]
- 18.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW: Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–1840 [PubMed] [Google Scholar]
- 19.Bellamy N: WOMAC Osteoarthritis Index: User guide IX. 9th ed. Queensland, Australia, 2009 [Google Scholar]
- 20.Maruish M, DeRosa M: A Guide to the Integration of Certified Short Form Survey Scoring and Data Quality Evaluation Capabilities. Quality Metric Incorporated, Lincoln, RI, 2009 [Google Scholar]
- 21.American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories: ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117 [DOI] [PubMed] [Google Scholar]
- 22.Enright PL: The six-minute walk test. Respir Care 2003;48:783–785 [PubMed] [Google Scholar]
- 23.Bellamy N, Bell MJ, Goldsmith CH, et al. : The effectiveness of hylan G-F 20 in patients with knee osteoarthritis: an application of two sets of response criteria developed by the OARSI and one set developed by OMERACT-OARSI. Osteoarthritis Cartilage 2005;13:104–110 [DOI] [PubMed] [Google Scholar]
- 24.Gudbergsen H, Boesen M, Lohmander LS, et al. : Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthritis Cartilage 2012;20:495–502 [DOI] [PubMed] [Google Scholar]
- 25.Riddle DL, Stratford PW: Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: a cohort study. Arthritis Care Res 2013;65:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty M, Dieppe P: The “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartilage 2009;17:1255–1262 [DOI] [PubMed] [Google Scholar]
- 27.Hopman WM, Towheed T, Anastassiades T, et al. : Canadian normative data for the SF-36 health survey. CMAJ 2000;163:265–271 [PMC free article] [PubMed] [Google Scholar]
- 28.Kosinski M, Keller SD, Ware JE, Hatoum HT, Kong SX: The SF-36 health survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: relative validity of scales in relation to clinical measures of arthritis severity. Med Care 1999;37(Suppl 5):23–39 [DOI] [PubMed] [Google Scholar]
- 29.Picavet HS, Hoeymans N: Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum 2004;63:723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawson J, Linsell L, Zondervan K, Rose P, Randall T, Carr A, Fitzpatrick R: Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology 2004;43:497–504 [DOI] [PubMed] [Google Scholar]
- 31.Hill K, Wickerson LM, Woon LJ, Abady AH, Overend TJ, Goldstein RS, Brooks D: The 6-min walk test: responses in healthy Canadians aged 45 to 85 years. Appl Physiol Nutr Metab 2011;36:643–649 [DOI] [PubMed] [Google Scholar]
- 32.Frestedt JL, Kuskowski MA, Zenk JL: A natural seaweed derived mineral supplement (aquamin F) for knee osteoarthritis: a randomized, placebo controlled pilot study. Nutr J 2009;8:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennell K, Dobson F, Hinman R: Measures of physical performance assessments: self-paced walk test (SPWT), stair climb test (SCT), six-minute walk test (6MWT), chair stand test (CST), timed up & go (TUG), sock test, lift and carry test (LCT), and car task. Arthritis Care Res 2011;63(Suppl 11):50–70 [DOI] [PubMed] [Google Scholar]
- 34.Heiberg KE, Bruun-Olsen V, Ekeland A, Mengshoel AM: Effect of a walking skill training program in patients who have undergone total hip arthroplasty: follow up one year after surgery. Arthritis Care Res 2012;64:415–423 [DOI] [PubMed] [Google Scholar]
- 35.Sale JE, Gignac M, Hawker G: How “bad” does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Rheum 2006;55:272–278 [DOI] [PubMed] [Google Scholar]
- 36.Davis GC, Hiemenz ML, White TL: Barriers to managing chronic pain of older adults with arthritis. J Nurs Scholarsh 2002;34:121–126 [DOI] [PubMed] [Google Scholar]