Abstract
Reproducing the native collagen structure and glycosaminoglycan (GAG) distribution in tissue-engineered cartilage constructs is still a challenge. Articular cartilage has a specific nutrient supply and mechanical environment due to its location and function in the body. Efforts to simulate this native environment have been reported through the use of bioreactor systems. However, few of these devices take into account the existence of gradients over cartilage as a consequence of the nutrient supply by diffusion. We hypothesized that culturing chondrocytes in an environment, in which gradients of nutrients can be mimicked, would induce zonal differentiation. Indeed, we show that glucose gradients facilitating a concentration distribution as low as physiological glucose levels enhanced a zonal chondrogenic capacity similar to the one found in native cartilage. Furthermore, we found that the glucose consumption rates of cultured chondrocytes were higher under physiological glucose concentrations and that GAG production rates were highest in 5 mM glucose. From these findings, we concluded that this condition is better suited for matrix deposition compared to 20 mM glucose standard used in a chondrocyte culture system. Reconsidering the culture conditions in cartilage tissue engineering strategies can lead to cartilaginous constructs that have better mechanical and structural properties, thus holding the potential of further enhancing integration with the host tissue.
Introduction
Cartilage is an anisotropic tissue involved in load distribution and facilitation of frictionless movement of joints.1,2 Matrix components are distributed through the tissue in such a way that these functions can be optimally executed. Collagen type II fibers run parallel to the articular cartilage surface, where its concentration is high to absorb load, bend toward the middle zone, and eventually anchor in the subchondral bone in a perpendicular fashion to optimally distribute the load to the underlying bone. Thus, there exists a collagen gradient from a high toward a low concentration starting from the synovial to the subchondral side through cartilage. Also, of the other main component of cartilage matrix, the proteoglycans, which function in attracting water and retaining and transporting growth factors, a concentration gradient of glycosaminoglycans (GAGs) is present in hyaline cartilage.3,4 The amount of GAGs per cell (GAG/DNA) increases from the synovial to the subchondral side.4 Thus, it is the distribution of these components that is important for cartilage function.
Glucose is a precursor of proteoglycans. After conversion to glucose-6-phosphate, it is converted to glucose-1-phosphate instead of entering the glycolysis. From there it is further converted to uridine diphosphate (UDP)-glucose and UDP-glucuronate. This molecule can then be converted in glucuronides, proteoglycans, and GAGs.5 Thus, it can be hypothesized that glucose availability can direct proteoglycan synthesis as it is the starting molecule for carbohydrates present in proteoglycans. Besides this, glucose is also the most important energy source for chondrocytes as reviewed by Mobasheri.6 It has been shown that across cartilage from the synovial side to the subchondral bone, a glucose gradient exists.7 Given the dual role of glucose in cartilage, we hypothesize that glucose gradients are, in part, responsible for establishing the observed GAG gradients in cartilage.
During development, gradients of morphogens guide cellular processes and time-specific organization of cells. Although there has been interest for nutrient gradients, especially oxygen, studies addressing this topic are limited.7–11 Computational models have shown how oxygen and glucose gradients are created in a tissue-engineered construct.7,12 In an environment with a high oxygen (O2) concentration, O2 consumption was inhibited when embedded chondrocytes were cultured in a high glucose concentration.8 In another study, 5% O2 saturation of the medium was suggested to have a protective effect on the energy metabolism and nitric oxide production.13 The same oxygen percentage in the medium (5%) was shown to enhance the chondrogenic capacity in pellet culture of human articular chondrocytes even after preculture in a high-oxygen environment. At the same time, expression and synthesis of catabolic markers were suppressed after culture in 5% O2 saturation.14 In contrast, chondrogenic markers were decreased when chondrocytes were cultured in the presence of a glucose competitor, 2-deoxy-D-glucose. When the same competitor together with insulin was added to healthy (HL) and osteoarthritic (OA) chondrocytes, glucose uptake was improved due to increased glucose transferase expression.15 However, when HL and OA chondrocytes were exposed to 30 mM glucose, both anabolic and catabolic genes were upregulated, even in the presence of a known prochondrogenic growth factor like TGF-β.16 Yu et al. reported recently that, when the glucose uptake was inhibited, chondrocytes lose their native phenotype and started to express catabolic factors.17
The above-described responses to different nutrient concentrations show that their effect on chondrocyte behavior is complex and still poorly understood. By creating glucose gradients, we tested the hypothesis if variations in glucose levels within a cell-laden tissue-engineered construct can contribute to the zonal differentiation of chondrocytes. To test this hypothesis, we cultured cell-laden hydrogels in a bioreactor system, which was designed to create defined and controlled glucose gradients.18
Materials and Methods
Cell sources
Bovine chondrocytes (bCHs) were isolated from femoral articular cartilage by means of collagenase type II digestion (150 U/mL; Worthington) overnight. Subsequently, collagenase suspension was filtered through a 200-μm cell strainer and centrifuged at 300 g for 10 min at 4°C. The pellet was then washed in phosphate-buffered saline (PBS) and centrifuged. This procedure was repeated once before cells were resuspended in the Dulbecco's modified Eagle's medium (DMEM) without glucose (Gibco) and counted using a Burker counting chamber. Cells were used immediately after isolation. In 96-well plates (2D experiments), 10,000 cells/cm2 were seeded, whereas in microwell arrays and agarose hydrogels (3D experiments), 10×106 cells/mL were seeded.
Medium composition
Chondrocytes were cultured in the DMEM supplemented with 100 U/mL penicillin/100 μg/mL streptomycin, 20 mM ascorbic acid, 40 μg/mL proline, 100 μg/mL sodium pyruvate, and 1% insulin–transferrin–selenium premix supplemented with different glucose concentrations. Different glucose concentrations were prepared by mixing DMEM containing 4.5 g/L glucose with DMEM without glucose. Five concentrations were prepared and described as [0], [1.25], [2.5], [5], and [20], referring to 0, 1.25, 2.5, 5, and 20 mM of glucose, respectively The actual starting concentrations are shown in Table 1.
Table 1.
Measured Starting Concentrations in the Bulk Medium in 2D Experiments
| Condition | [glucose] (mM) |
|---|---|
| [0] | 0 |
| [1.25] | 1.48 |
| [2.5] | 2.85 |
| [5] | 5.38 |
| [20] | 23.03 |
Cell seeding
For 2D experiments, a cell suspension was made in five different glucose concentrations (see Table 1) and 200 μL was seeded in each well of a 96-well plate at a density of 10,000 cells/cm2.
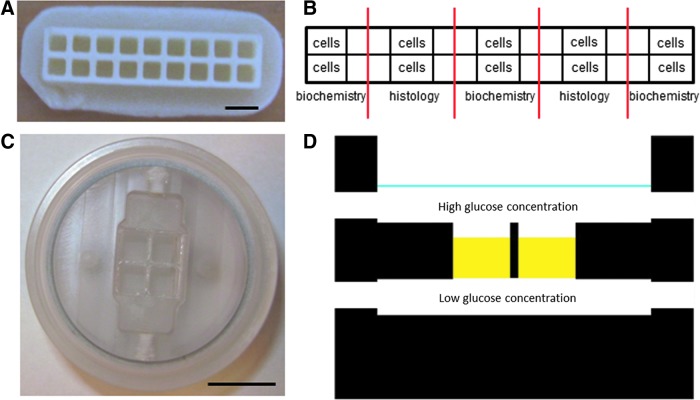
High-density cultures were performed in previously described PolyActive 300/55/45 microwell arrays (Fig. 1A, B; devices kindly provided by Screvo B.V., The Netherlands). Each microwell was seeded with 1 μL cell suspension at a density of 10×106 cells/mL.19 This custom-made array existed of 9×2 wells. The wells had a volume of 1 μL. Before seeding, the arrays were sterilized with ethanol and subsequently washed in PBS to remove residual ethanol. After seeding, the arrays were placed in separate wells of a six-well plate and submerged in 6 mL of DMEM containing 0, 1.25, 2.5, 5, or 20 mM glucose.
FIG. 1.
Culture devices and strategy. (A) Custom-made microwell arrays (scale bar represents 2 mm) and (B) schematic representation of cell seeding and division for analysis. (C) Static bioreactor for creating gradients (scale bar represents 10 mm). (D) Schematic cross section of the bioreactor depicting the different compartments with high and low glucose concentrations. The blue line depicts the height of the medium in the top compartment. The two white openings on both sides depict the in-/outlets. Color images available online at www.liebertpub.com/tea
Cells were embedded into agarose using the following procedure. Aliquots of 40 μL cell suspension (20×106 cells/mL) were prepared in Eppendorf tubes. Then, 40 μL of a 1% UltraPure™ agarose (Invitrogen) suspension in PBS (boiled in a microwave) was added, resulting in a 0.5% agarose gel with 10×106 cells/mL. The mixture was pipetted in custom-made polycarbonate four-chamber inserts (Fig. 1C). After solidification, the inserts were placed into a previously described dual-compartment bioreactor.18 The top compartment was filled with 2 mL of medium and the bottom compartment with 10 mL of medium. Monolayer and microwell array cultures were performed under normoxic (20%O2) and hypoxic (2.5%O2) conditions. A culture period of 7 days was maintained for all experiments. Table 2 shows the glucose concentrations in the top and bottom compartment that were used to create glucose gradients.
Table 2.
Different Glucose Concentrations Used to Create Gradients
| Condition | [glucose] (mM) top compartment | [glucose] (mM) bottom compartment |
|---|---|---|
| 20-0 | 20 | 0 |
| 20-5 | 20 | 5 |
| 20-20 | 20 | 20 |
Computational modeling
Computational fluid dynamics (CFD) of the diffusion in culture conditions was set up and solved in the transport of diluted species module in Comsol Multiphysics version 4.1 software (Comsol).
Figure 1D represents a cross section of the static bioreactor chamber, depicting the top and bottom compartment and the construct explants in yellow.
The Navier–Stokes equation that was solved for incompressible fluid dynamics is defined by Eq. 1 as follows:
 |
where ▽ is the del operator, D is the diffusion coefficient (m2/s), c is the concentration (mol/m3), and R is the reaction constant (mol/[m3·s]).
Glucose concentrations through the constructs were modeled time dependently using the following assumptions. (1) Walls in different conditions were considered rigid and impermeable; (2) no-slip boundary conditions were applied to surfaces; and (3) the glucose diffusion constant (D) in water set to 9.2×10−10 m2/s and in 0.5% agarose was assumed equal.20 The initial concentration of glucose in the top compartment was 20 mM, representing high-glucose DMEM and was 0, 5, or 20 mM in the bottom compartment, representing DMEM without glucose, with a physiological glucose concentration and high-glucose DMEM, respectively. The glucose consumption rate of chondrocytes in the constructs was assumed 1.28×10−7 mol/(L·s) (calculated from the 20 mM condition in Fig. 2A).
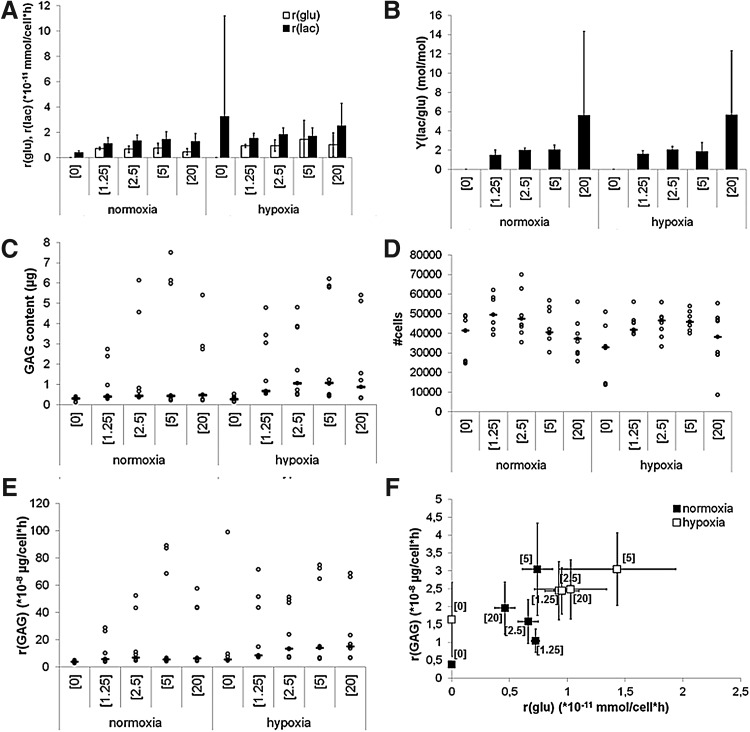
FIG. 2.
Metabolic response of 2D cultured p0 bovine chondrocytes (bCHS) to different glucose and oxygen concentrations. (A) Average glucose consumption and lactate production rates of bCHs and (B) average yield of lactate on glucose. (C) Boxplot with individual data points (gray dots represent the individual data points and the black bars the median) representing total glycosaminoglycan (GAG) production, (D) boxplot with individual data points (gray dots represent the individual data points and the black bars the median) representing the total number of cells, (E) boxplot with individual data points (gray dots represent the individual data points and the black bars the median) representing the GAG production rates, and (F) relationship between the average glucose consumption rate and average GAG production rate (error bars represented as the standard deviation) after 7 days of culture, n=3 donors.
It has to be noted that for glucose, the consumption rate was assumed homogenous throughout the constructs and was in the same range as found in literature.21
Biochemical analysis
Metabolite analysis
After 2D culture, the medium was removed and glucose and lactate concentrations were analyzed using a Vitros DT60II medium analyzer (Ortho-Clinical Diagnostics).
The medium of three wells per condition was analyzed and the consumption and production rates were calculated and correlated with the cell content in each respective well.
GAG content
Before analysis, constructs were incubated in a proteinase K (PK) digestion buffer (1 mg/mL PK, 1μg/mL pepstatin, 18.5μg/mL iodoacetamide) and went through a freeze/thaw cycle. The GAG content was measured with a 1,9-Dimethyl-Methylene Blue (DMMB) assay. Therefore, a 25 μL sample was added to a transparent 96-well plate and 5 μL of a 2.3 M NaCl solution was added. Then, 150 μL of a DMMB solution was added and absorbance was read at 520 nm using a Multiskan GO plate reader (Thermo Scientific). The GAG content was quantified with a chondroitin sulfate standard curve and corrected for DNA content.
DNA quantification
The DNA content was quantified with a CyQuant kit (Invitrogen) according to the manufacturer's protocol, and fluorescence was measured at 480 nm using a spectrophotometer LS50B (Perkin Elmer). DNA concentrations were calculated from a λ DNA standard curve.
Histology
For histology, scaffolds and constructs were dissected from top to bottom, and fixed in 10% buffered formalin. Subsequently, agarose constructs were embedded in cryomatrix, sectioned in a cryotome into 10-μm-thick longitudinal sections, and then mounted onto Superfrost® Plus (Thermo Fisher Scientific) glass slides.
Toluidine blue staining
Glycoasminoglycan deposition in the microwell arrays was visualized by Toluidine blue staining. Therefore, the wells were submerged shortly in a Toluidine blue (TB) solution and subsequently washed with PBS until the residual TB solution was removed.
Staining was visualized with a Nikon stereomicroscope (Nikon). Then, the matrix volume was estimated from these staining pictures by measuring the surface of the TB staining and correcting this for the surface of the well.
Safranin O staining
Sections were hydrated for 10 min in demi water and stained with Fast Green for 3 min, rinsed in 1% acetic acid and subsequently stained with Safranin O for 5 min, and dehydrated in a sequence of 96% EtOH, 100% EtOH, and xylene for 2 min each. Sections were dried and mounted with a Tissue-Tek mounting medium (Sakura Finetek Europe BV).
Slides were scanned with a Nanozoomer 2,0 RS (Hamamatsu) and pictures were captured with its proprietary NDP.Scan software (Hamamatsu).
Results
Chondrogenic response on tissue culture plastic in different glucose concentrations.
bCHs were cultured on tissue culture plastic using different glucose concentrations in 20% O2 (normoxia) and 2.5% O2 (hypoxia). Glucose levels in the medium were measured at the end of the culture period, and the consumption rate was calculated as the amount of glucose consumed per cell per hour (r[glu]). Under normoxic conditions in 1.25, 2.5, and 5 mM glucose, glucose consumption rates during 2D culture were comparable, but decreased in 20 mM (Fig. 2A). However, lactate production rates in these conditions were similar for all settings (Fig. 2A). This indicated that glucose uptake was affected by the high glucose concentration. Under hypoxic conditions, glucose consumption rates were similar and they tended to be higher compared to their normoxic equivalents. The same was observed for the lactate production rates (Fig. 2A). This could indicate that hypoxia regardless of the glucose concentration, maintains the native chondrocyte phenotype.22 When the yield of lactate on glucose (Y[lac/glu]) is considered (Fig. 2B), it could be observed that in all concentrations, the Y(lac/glu) is around 2. This is to be expected when an anaerobic metabolism is maintained.
Under normoxic conditions, the total GAG production did not seem to be affected by different glucose concentrations (Fig. 2C). However, under hypoxic conditions, the 2.5, 5, and 20 mM showed a higher GAG content compared to their normoxic equivalents, whereas the hypoxic 0 and 1.25 mM condition showed similar levels of GAG deposition (Fig. 2C). Proliferation was minimal in all conditions when compared to the initial number of cells seeded (±39,000 cells). This could indicate that cells use the energy they produce for matrix production and maintenance instead of proliferation. However, it could be observed that cell numbers tended to be higher under lower glucose concentrations—except for the condition without glucose—regardless of the oxygen concentration (Fig. 2D).
The GAG production rate (rGAG) showed a similar trend as the GAG content. Under normoxic conditions, there was no influence of the glucose concentration on the r(GAG), whereas under hypoxic conditions, this rate showed an increase up to the setting with 2.5 mM glucose and stabilized at higher glucose concentrations (Fig. 2E). When the r(GAG) is considered as a function of the glucose consumption rate (r[glu]), it can be observed that highest rGAG was obtained at a glucose concentration of 5 mM, although the r(glu) in this condition was lower compared to the condition with 20 mM under hypoxic conditions (white squares in Fig. 2F). Under normoxic conditions, rGAG seemed more optimal following culture in 5 mM glucose compared to culturing in 20 mM glucose (black squares in Fig. 2F).
From these data, it can be concluded that glucose consumption, as well as lactate and GAG production by chondrocytes in 2D culture are slightly affected by the bulk glucose concentrations, especially under low concentrations. The GAG production rate seemed optimal at 5 mM glucose.
Tissue formation in microwell arrays showed an optimum under hypoxic conditions
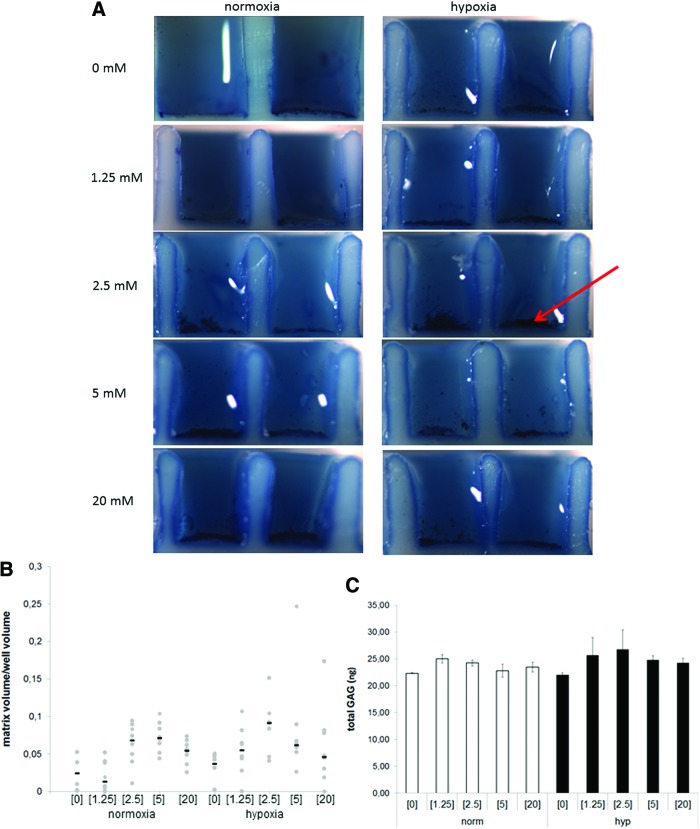
To investigate the influence of glucose concentration on matrix production of chondrocytes seeded in high density, cells were cultured in a microwell array system and the same glucose concentrations as described above were used for cultivation. After 7 days of culture in normoxic conditions, glucose concentrations of 0 and 1.25 mM resulted in minimal Toluidine blue (TB) staining, which increased with increasing glucose concentrations. Under low oxygen tensions, a similar trend was observed and the staining seemed more intense compared to high oxygen conditions (Fig. 3A). Our staining observations were validated by comparing the matrix area/well area ratio in each condition. This showed similar trends as described for 2D culture. In the 0 and 1.25 mM conditions, this ratio was lower compared to the other three glucose conditions in both oxygen tensions. It was also observed that in a setting with 20 mM glucose, the ratio decreased compared to 2.5 and 5 mM glucose regardless of the oxygen concentration—with the highest ratio observed in 2.5 mM (Fig. 3B). Quantification of the GAG content in the wells showed no obvious differences between glucose concentrations under high oxygen levels. Under hypoxic conditions, the GAG content seemed highest under 2.5 mM glucose confirming the staining (Fig. 3C).
FIG. 3.
Chondrogenic response in printed microwells under different glucose concentrations. (A) Microtissues stained with Toluidine blue after 7 days (arrow indicates location of cells and matrix). (B) Boxplot with individual data points quantifying the Toluidine blue staining after 7 days, n=8 wells of two donors. (C) Total GAG in printed microwells. Color images available online at www.liebertpub.com/tea
These data correlated to the results shown in Figure 2 indicating that the matrix formation in a 3D environment could be enhanced by using physiological glucose concentrations.
Glucose gradients induced zonal differences in matrix distribution
Computational modeling of the glucose gradient with and without cellular consumptions in the hydrogel constructs cultured between two medium compartments with distinct glucose concentrations showed that after 1 h of glucose diffusion without the presence of cells, a glucose gradient could be observed from top to bottom in the 20-0 and 20-5 condition. As assumed, the gradient in the 20-0 condition was steeper (a higher concentration difference between top and bottom) compared with the 20-5 condition (Fig. 4A, B, left). A different concentration profile was predicted in the 20-20 condition, where the lowest concentration was observed in the middle of a construct (Fig. 4C, left). When a consumption rate of 1.28e-7 mol·m3·s−1 (calculated from the normoxic-20 mM glucose condition in Fig. 2) was assumed, the glucose gradient in the 20-0 condition became steeper, whereas the gradient in the 20-5 condition did not seem to change notably compared with the gradient without consumption. In the 20-20 condition, it was observed that the width of the low-concentration zone in the middle of the construct increased when compared to the gradient without consumption. At 24 h, when in all modeled conditions the gradient became stable (data not shown), a homogeneous glucose distribution was observed in the 20-20 condition, even when glucose was consumed (Fig. 4C, right). This means that the consumption rate was too small compared to the diffusion coefficient to induce changes in the glucose gradient. The gradients in the 20-0 and 20-5 conditions became less steep after 24 h compared with the gradients after 1 h. Although a change in the concentration distribution was observed in the 20-0 condition after 1 h of consumption compare to no consumption, this was not observed after 24 h when a cellular consumption rate was introduced in the model (Fig. 4A, right). In the 20-5 condition, no change in the gradient was observed after consumption (Fig. 4B, right). In the 20-0 condition, the concentration distribution at equilibrium ranged from 19.7 to 0.2 mM. In the 20-5 condition, this range was from 19.7 to 5.1 mM.
FIG. 4.
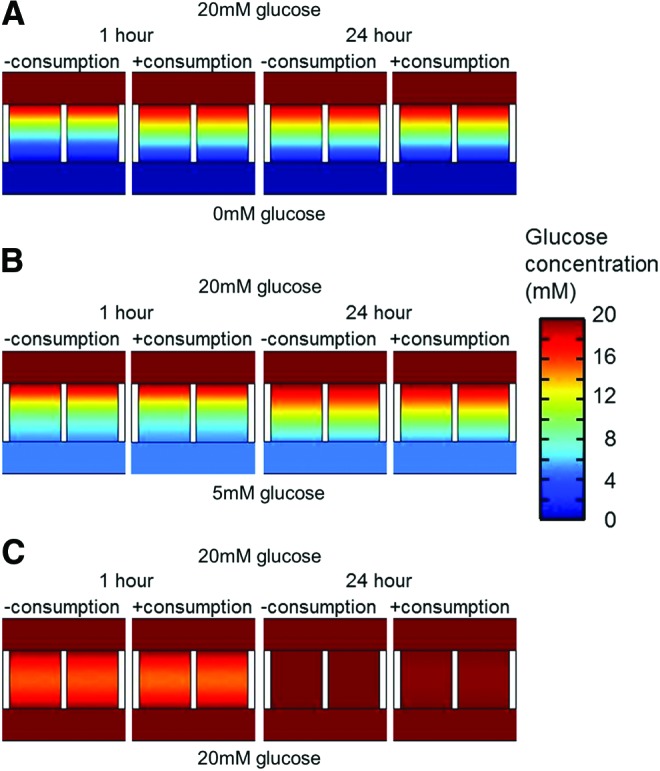
Modeled glucose gradients with and without consumption through constructs between different glucose concentrations. (A) Predicted glucose gradients without and with cellular glucose consumption after 1 h (left) and 24 h (right) after culture between 20 and 0 mM glucose. (B) Predicted glucose gradients without and with cellular glucose consumption after 1 h (left) and 24 h (right) after culture between 20 and 5 mM glucose. (C) Predicted glucose gradients without and with cellular glucose consumption after 1 h (left) and 24 h (right) after culture between 20 and 20 mM glucose. Color images available online at www.liebertpub.com/tea
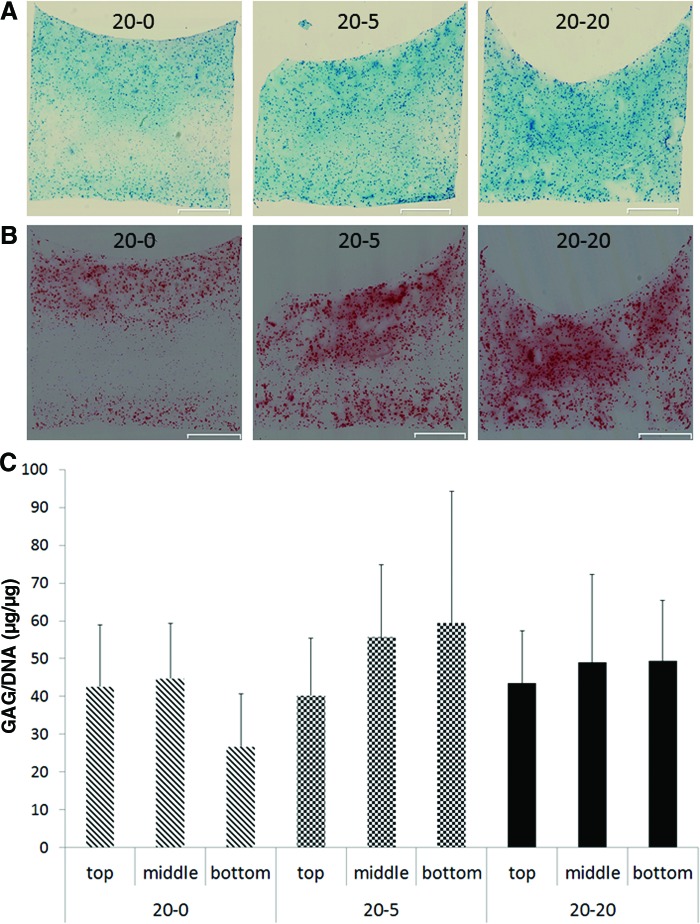
The effect of these gradients on cartilaginous matrix production over time is shown in Figure 5. After 7 days of culture, 10 μm sections were stained with Alcian blue (AB, Fig. 5A) and Safranin O (Saf-O, Fig. 5B) to visualize GAG distribution. This revealed zonal differences in matrix deposition in the 20-0 and 20-5 condition, whereas distribution in the 20-20 condition seemed homogeneous. In the 20-0 gradient, the intensity of both AB and Saf-O staining decreased from the top to the middle of the construct where the latter was almost absent. In the bottom zone, the staining intensity increased compared with the middle zone. In the 20-5 condition, the AB staining appeared to be homogeneous (Fig. 5A), but the nonstained Saf-O zone decreased compared to the 20-0 condition and the staining appeared more homogeneously distributed (Fig. 5B).
FIG. 5.
Chondrogenic response of p0 bCHs embedded in agarose cultured in different glucose gradients. Constructs were cultured between media containing 20 mM glucose and 0, 5, or 20 mM glucose in the top and bottom compartment, respectively. (A) Alcian blue staining after 7 days, n=2, scale bar represents 1 mm. (B) Safranin O staining after 7 days, n=2, scale bar represents 1 mm. (C) Zonal GAG/DNA ratios, data represent the mean of five cubes. Color images available online at www.liebertpub.com/tea
Evaluating the chondrogenicity (GAG/DNA) of the different zones showed that in the 20-5 condition, an increasing trend in GAG/DNA was observed, whereas GAG/DNA was equal in all zones in the 20-20 condition. The 20-0 condition showed as expected a decrease in GAG/DNA from middle to bottom zones (Fig. 5D). Comparing the predicted gradients to the biological response of chondrocytes showed that in a gradient, which includes subphysiological concentrations (<5 mM), the matrix production seemed to be inhibited, but also hyperphysiological concentrations (20 mM) seemed to decrease matrix deposition compared to superphysiological glucose levels (5 mM<[glucose]<10 mM). Thus, between the gradients tested in this study, the 20-5 condition appeared to be the best candidate to conduct further experiments to determine the exact influence of long-term cultures in nutrient gradients.
Discussion
Morphogen gradients are known to play important roles in cell migration, proliferation, and terminal differentiation.23–25 However, the influence of nutrient gradients on differentiation and tissue formation is still largely unknown. Heywood has studied the influence of the glucose concentration on chondrocyte behavior in a 3D environment.8 Glucose is one of the most important energy sources for chondrocytes as reviewed by Mobasheri.6 Mathematically, glucose and oxygen gradients have been modeled assuming an equal consumption rate throughout a construct.7,12 In our computational models, the consumption rate of the 20 mM condition was considered as it could not be estimated from which depth chondrocytes would exert a different consumption rate. However, our results suggest that bCHs, even in a population in which chondrocytes from all zones were mixed, consume glucose at a higher rate at lower availability of this nutrient like they do with oxygen.26,27 This is in accordance with earlier reports.28 Furthermore, we found that the matrix production rates and, in particular, GAG production improved under moderate glucose concentrations when compared to high and low concentrations and were independent from the oxygen concentration. Thus, it can be concluded that culturing in a low glucose concentration creates an environment that is energetically more favorable for maintenance of the chondrocyte phenotype. A limitation in our study is that we have only measured the effect on GAG production and not on the production of collagens. This issue needs further study. The exact influence on the energy metabolism in a physiological glucose environment in vitro is not completely clear.28,29 Although chondrocytes predominantly exhibit an anaerobic metabolism, adenosine triphosphate is produced by chondrocyte mitochondria.30 In this context, mitochondrial dysfunction has been associated with degenerative cartilage diseases, such as Kashin-Beck disease and osteoarthritis, as reviewed by Blanco et al.31,32
In the presence of a glucose gradient that induced a distribution to concentrations as low as physiological concentrations (20-5 condition), GAG matrix production was enhanced in the regions in which the glucose concentration approached physiological levels. It was assumed that in those regions also, the oxygen concentration (O2) was lowest as the polycarbonate plates are impermeable for oxygen. In constructs that had a high glucose concentration in those regions with a low O2, the chondrogenic capacity (GAG/DNA) decreased compared to the 20-5 condition. In addition, in a gradient in which the bottom zone was deprived of glucose (20-0 condition), GAG/DNA values further decreased in those regions. This confirms and extends the above-discussed hypothesis that low glucose concentrations create an environment to which chondrocytes can adapt more easily in vitro. We showed that with a glucose gradient, the chondrocyte GAG production behavior can be influenced.
The variations in glucose concentration tested in this study may have also effected the osmolarity of the culture medium. Although the effect of osmolarity on chondrocyte behavior has been investigated,33–35 the effect of a gradient in osmolarity on matrix deposition has not been touched upon. In our experiments, only the concentration of glucose varied and not the concentration of the other medium components. Consequently, the effect of varying glucose concentrations between 0 and 20 mM glucose on osmolarity will be relatively small (a maximum change of 20mOsm, whereas reported changes in matrix production were induced at differences of 50mOSm or greater33–35). It seems unlikely that this small change is having a strong effect on the establishment of the observed zonal differences, but we cannot exclude the role of subtle changes in osmolarity.
In this article, we show that a zonal distribution of GAG/DNA can be achieved by culturing bCHs in a glucose gradient and the GAG/DNA distribution of native cartilage can be recreated.28 However, the function of a cartilaginous construct is not only defined by the GAG/DNA gradient. The zonal distribution and architecture of collagen fibers are also important to reconstitute proper function. Collagen production has been reported to be upregulated under mechanical stimulation in bioreactor cultures.36,37 A zone-specific protein that is also upregulated by mechanical stimulation is lubricin, which is excreted to the synovial fluid to make it more viscous.38 To determine the progression in the zonal development of a construct, gene upregulation and production of other zone-specific proteins, such as superficial zone protein, cartilage intermediate layer protein, and cartilage oligomeric matrix protein, should be considered as a next step in this analysis.2,39,40 Furthermore, even endochondral ossification could be a target to improve construct integration upon implantation.
To summarize, we show in this proof of principle study how nutrient gradients can be used for zonal differentiation of chondrocytes. For further development of a cartilaginous construct, these gradients can be combined with growth factor gradients to induce the upregulation of zone-specific proteins, and further cellular maturation can be established by mechanical compression. This is expected to enhance collagen production and zonal structure. In addition, a more in-depth study has to reveal if the indications on the effect of glucose gradients described in this article have the same effect in a culture system with human chondrocytes. The aforementioned additional steps will be the focus of future work.
Acknowledgments
This research forms part of the Project P2.02 OAcontrol of the research program of the BioMedical Materials institute, cofunded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation. Lorenzo Moroni and Carlos Mota acknowledge funding from the Preseed grant number 93611002 of the Netherlands Genomics Initiative. Also, the authors thank Dr. Natalie Fekete for the Nanozoomer scans.
Author Contributions
Study design: T.S. and M.K. Experimentation and data interpretation: T.S., C.M., S.U., B.S., D.M., L.M., and M.K. C.M. and L.M. microwell array development. T.S. wrote the article with the help of all authors. M.K. supervised the study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Huang C.Y., et al. Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng 125,84, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Schumacher B.L., et al. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys 311,144, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Ito T., et al. Hyaluronan regulates transforming growth factor-beta1 receptor compartmentalization. J Biol Chem 279,25326, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Woodfield T.B., et al. Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Eng 11,1297, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Prydz K., and Dalen K.T.Synthesis and sorting of proteoglycans. J Cell Sci 113(Pt 2),193, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Mobasheri A.Glucose: an energy currency and structural precursor in articular cartilage and bone with emerging roles as an extracellular signaling molecule and metabolic regulator. Front Endocrinol (Lausanne) 3,153, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou S., Cui Z., and Urban J.P.Nutrient gradients in engineered cartilage: metabolic kinetics measurement and mass transfer modeling. Biotechnol Bioeng 101,408, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Heywood H.K., Bader D.L., and Lee D.A.Glucose concentration and medium volume influence cell viability and glycosaminoglycan synthesis in chondrocyte-seeded alginate constructs. Tissue Eng 12,3487, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Malda J., et al. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng 86,9, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Marcus R.E.The effect of low oxygen concentration on growth, glycolysis, and sulfate incorporation by articular chondrocytes in monolayer culture. Arthritis Rheum 16,646, 1973 [DOI] [PubMed] [Google Scholar]
- 11.Marcus R.E., and Srivastava V.M.Effect of low oxygen tensions on glucose-metabolizing enzymes in cultured articular chondrocytes. Proc Soc Exp Biol Med 143,488, 1973 [DOI] [PubMed] [Google Scholar]
- 12.Sengers B.G., et al. Computational study of culture conditions and nutrient supply in cartilage tissue engineering. Biotechnol Prog 21,1252, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fermor B., Gurumurthy A., and Diekman B.O.Hypoxia, RONS and energy metabolism in articular cartilage. Osteoarthritis Cartilage 18,1167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strobel S., et al. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res Ther 12,R34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosa S.C., et al. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthritis Cartilage 19,719, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Rosa S.C., et al. Role of glucose as a modulator of anabolic and catabolic gene expression in normal and osteoarthritic human chondrocytes. J Cell Biochem 112,2813, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Yu S.M., Kim H.A., and Kim S.J.2-Deoxy-D-glucose regulates dedifferentiation through beta-catenin pathway in rabbit articular chondrocytes. Exp Mol Med 42,503, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitters T.W., et al. A dual flow bioreactor with controlled mechanical stimulation for cartilage tissue engineering. Tissue Eng Part C Methods 19,774, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Higuera G.A., et al. In vivo screening of extracellular matrix components produced under multiple experimental conditions implanted in one animal. Integr Biol (Camb) 5,889, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Allhands R.V., Torzilli P.A., and Kallfelz F.A.Measurement of diffusion of uncharged molecules in articular cartilage. Cornell Vet 74,111, 1984 [PubMed] [Google Scholar]
- 21.Obradovic B., et al. Gas exchange is essential for bioreactor cultivation of tissue engineered cartilage. Biotechnol Bioeng 63,197, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Markway B.D., Cho H., and Johnstone B.Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res Ther 15,R92, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho H.S., et al. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med 31,212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H.K., Oxendine I., and Kamiya N.High-concentration of BMP2 reduces cell proliferation and increases apoptosis via DKK1 and SOST in human primary periosteal cells. Bone 54,141, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Zhang J., and Li Y.Fibroblast growth factor 21, the endocrine FGF pathway and novel treatments for metabolic syndrome. Drug Discov Today 19,579, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Heywood H.K., Knight M.M., and Lee D.A.Both superficial and deep zone articular chondrocyte subpopulations exhibit the Crabtree effect but have different basal oxygen consumption rates. J Cell Physiol 223,630, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Rajpurohit R., et al. Adaptation of chondrocytes to low oxygen tension: relationship between hypoxia and cellular metabolism. J Cell Physiol 168,424, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Heywood H.K., et al. Cellular utilization determines viability and matrix distribution profiles in chondrocyte-seeded alginate constructs. Tissue Eng 10,1467, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Heywood H.K., Bader D.L., and Lee D.A.Rate of oxygen consumption by isolated articular chondrocytes is sensitive to medium glucose concentration. J Cell Physiol 206,402, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Martin L.J.Biology of mitochondria in neurodegenerative diseases. Prog Mol Biol Transl Sci 107,355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanco F.J., Rego I., and Ruiz-Romero C.The role of mitochondria in osteoarthritis. Nat Rev Rheumatol 7,161, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Li S., et al. Proteoglycan metabolism, cell death and Kashin-Beck disease. Glycoconj J 29,241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jablonski C.L., et al. Integrin alpha1beta1 participates in chondrocyte transduction of osmotic stress. Biochem Biophys Res Commun 445,184, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spillekom S., et al. Increased osmolarity and cell clustering preserve canine notochordal cell phenotype in culture. Tissue Eng Part C Methods, 2014[Epub ahead of print]; DOI: 10.1089/ten.tec.2013.0479 [DOI] [PubMed] [Google Scholar]
- 35.Huttu M., et al. Effects of medium and temperature on cellular responses in the superficial zone of hypo-osmotically challenged articular cartilage. J Funct Biomater 3,544, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buschmann M.D., et al. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci 108(Pt 4),1497, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Kock L.M., et al. Tuning the differentiation of periosteum-derived cartilage using biochemical and mechanical stimulations. Osteoarthritis Cartilage 18,1528, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Grad S., et al. Chondrocyte gene expression under applied surface motion. Biorheology 43,259, 2006 [PubMed] [Google Scholar]
- 39.Bernardo B.C., et al. Cartilage intermediate layer protein 2 (CILP-2) is expressed in articular and meniscal cartilage and down-regulated in experimental osteoarthritis. J Biol Chem 286,37758, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaucke F., et al. Cartilage oligomeric matrix protein (COMP) and collagen IX are sensitive markers for the differentiation state of articular primary chondrocytes. Biochem J 358(Pt 1),17, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]