Abstract
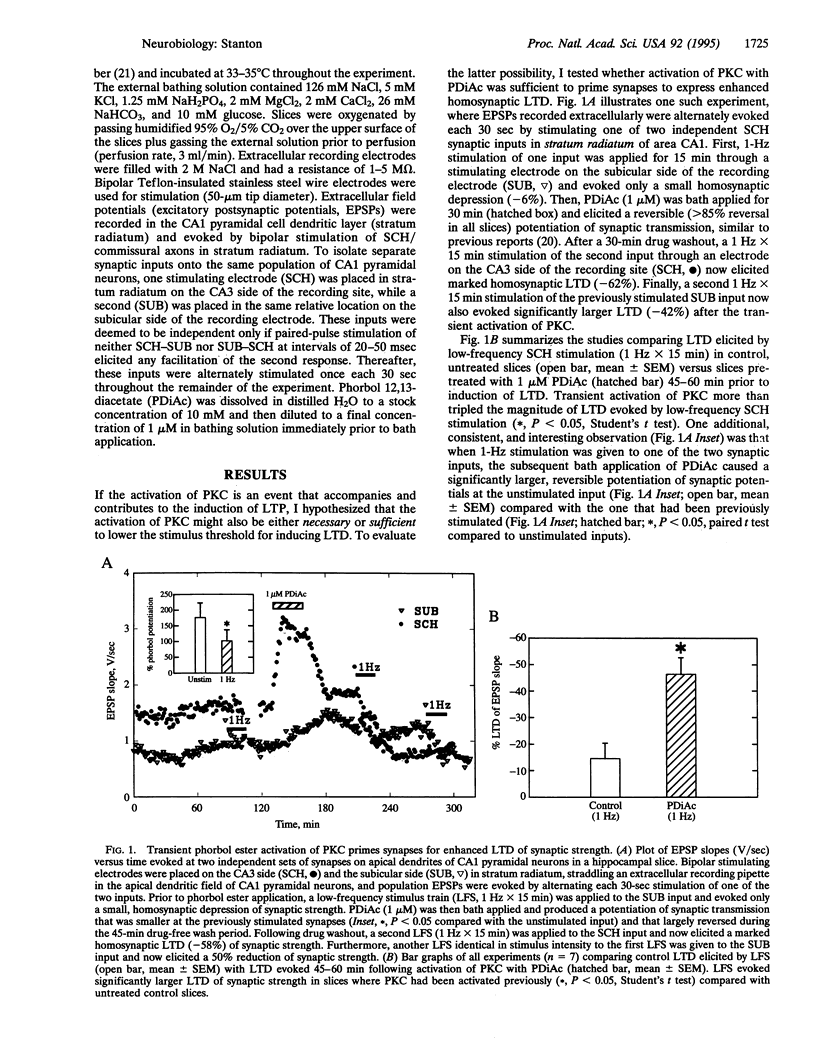
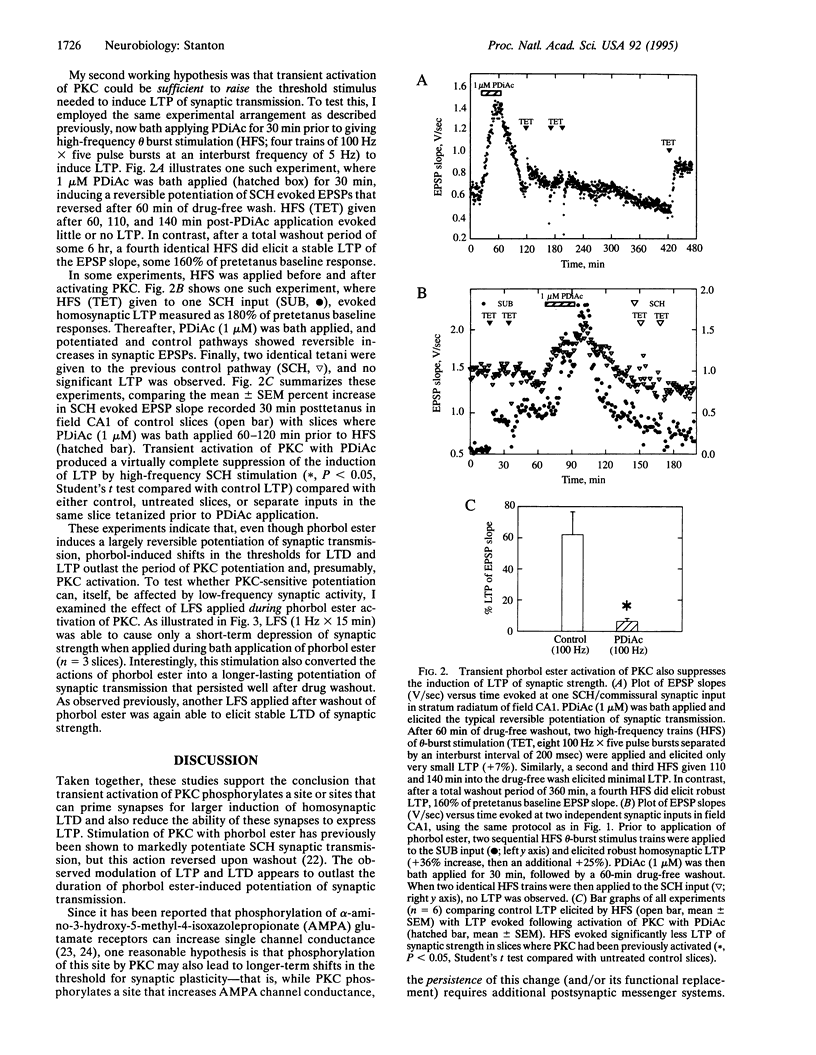
Activity-dependent long-lasting plasticity in hippocampus and neocortex includes long-term potentiation (LTP) and long-term depression (LTD) of synaptic strength. Recent studies have confirmed theoretical predictions that the sensitivity of LTP- and LTD-inducing mechanisms is dynamically regulated by previous synaptic history. In particular, prior induction of either repeated short-term potentiations or LTP lowers the threshold for induction of LTD and raises the threshold for LTP. In the current study, transient activation of protein kinase C with phorbol 12,13-diacetate was able to substitute for synaptic activity in priming synapses to exhibit enhanced homosynaptic LTD and to suppress the induction of LTP at Schaffer collateral synapses in area CA1 of hippocampal slices. This priming lasted 30 min, but not 3 hr, following phorbol 12,13-diacetate bath application. These data suggest that a protein kinase C-sensitive phosphorylation site may be an activity-sensitive target mediating the rapid expression of LTP and LTD.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artola A., Bröcher S., Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990 Sep 6;347(6288):69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Bienenstock E. L., Cooper L. N., Munro P. W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982 Jan;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie B. R., Abraham W. C. Priming of associative long-term depression in the dentate gyrus by theta frequency synaptic activity. Neuron. 1992 Jul;9(1):79–84. doi: 10.1016/0896-6273(92)90222-y. [DOI] [PubMed] [Google Scholar]
- Dudek S. M., Bear M. F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Saito K., Miyakawa H., Ito K., Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991 Jul 26;555(1):112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H., Abraham W. C., Huang Y. Y. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987 Mar;7(3):774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. L., Schaerer B., Vosmansky M. A simple perfusion chamber for the study of nervous tissue slices in vitro. J Neurosci Methods. 1979 Dec;1(4):323–325. doi: 10.1016/0165-0270(79)90021-9. [DOI] [PubMed] [Google Scholar]
- Huang Y. Y., Colino A., Selig D. K., Malenka R. C. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992 Feb 7;255(5045):730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- Kimura F., Tsumoto T., Nishigori A., Yoshimura Y. Long-term depression but not potentiation is induced in Ca(2+)-chelated visual cortex neurons. Neuroreport. 1990 Sep;1(1):65–68. doi: 10.1097/00001756-199009000-00018. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Lovinger D. M., Wong K. L., Murakami K., Routtenberg A. Protein kinase C inhibitors eliminate hippocampal long-term potentiation. Brain Res. 1987 Dec 8;436(1):177–183. doi: 10.1016/0006-8993(87)91573-3. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., Waxham M. N. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989 Aug 17;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Herron C. E., Malenka R. C. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993 Aug 20;261(5124):1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Malenka R. C. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992 Nov;9(5):967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Muller D., Buchs P. A., Dunant Y., Lynch G. Protein kinase C activity is not responsible for the expression of long-term potentiation in hippocampus. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4073–4077. doi: 10.1073/pnas.87.11.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993 Feb 18;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Mody I., Heinemann U. A role for N-methyl-D-aspartate receptors in norepinephrine-induced long-lasting potentiation in the dentate gyrus. Exp Brain Res. 1989;77(3):517–530. doi: 10.1007/BF00249605. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sejnowski T. J. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature. 1989 May 18;339(6221):215–218. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- Staubli U., Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1-5 Hz stimulation. Brain Res. 1990 Apr 9;513(1):113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Taverna F. A., Huang X. P., MacDonald J. F., Hampson D. R. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993 Feb 19;259(5098):1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- Wexler E. M., Stanton P. K. Priming of homosynaptic long-term depression in hippocampus by previous synaptic activity. Neuroreport. 1993 May;4(5):591–594. doi: 10.1097/00001756-199305000-00034. [DOI] [PubMed] [Google Scholar]



