Abstract
Background
Evidence from several Western studies has shown an alarmingly high and inappropriate rate of prescription of proton pump inhibitors (PPIs), which may be associated with increased healthcare costs and adverse outcomes. PPI prescribing patterns remain largely unknown in well-developed healthcare systems in Southeast Asia.
Aims
We aimed to determine the prevalence of inappropriate prescription of PPI among elderly patients without documentation of valid indications, in a tertiary teaching hospital in Singapore.
Method
We carried out a retrospective clinical records review of 150 elderly patients aged ≥65 years that had been admitted to two internal medicine wards between 25 May 2011 and 28 June 2011 to determine the appropriateness of indications for PPIs prescribed at hospital discharge. PPI indications were categorised as “valid”, “likely invalid”, and “probable” based on current clinical literature. Pre-admission and discharge prescriptions were reviewed to determine continuation of pre-admission and new PPI prescriptions at discharge. Data on clinical characteristics and concurrent use of ulcerogenic medications were collected.
Results
From a total of 150 patients, 80 (53 per cent) received prescriptions for PPIs. Of these, 65 (81.2 per cent) had no valid documented indications (i.e., the indication was classed as “likely invalid”); 10 (12.5 per cent) had valid indications; and in five cases (6.2 per cent) the indication was “probable”. The most common “likely invalid” indication was primary gastrointestinal bleeding prophylaxis (GIP) among low-dose aspirin users in 28 patients (43 per cent) of invalid PPI prescriptions.
Conclusion
Inappropriate prescribing of PPIs without documented valid indications was prevalent among elderly patients at our tertiary teaching hospital in Singapore, providing evidence that shows a similar trend to PPI prescribing to data from Western countries.
Keywords: Proton pump inhibitors, prescribing, elderly, valid indications
What this study adds:
-
What is known about this subject?
Despite extensive publication regarding the adverse outcomes from long-term PPI use, inappropriate PPI prescribing remains highly prevalent in Western healthcare systems.
-
What new information is offered in this study?
The most common undocumented invalid indication was PPI use for low-dose aspirin-related primary gastrointestinal bleeding prophylaxis among low-risk elderly patients. This points to a high incidence of non-evidence based practice.
-
What are the implications for research, policy, or practice?
Approaches to tackle this medication safety issue could include regular review of PPI indications at each patient contact. We further recommend pharmacist-led collaborative intervention aiming to improve evidence-based medication prescribing and patient outcomes by identifying and flagging medications without appropriate indications, and by providing evidence-based recommendations in electronic medication records.
Background
Proton Pump Inhibitors (PPIs) remain the leading evidence-based therapy for upper gastrointestinal disorders, including gastro-oesophageal reflux disease, dyspepsia, peptic ulcer disease, NSAID-induced ulcer, eradication of Helicobacter pylori, and hypersecretory disorders such as Zollinger-Ellison syndrome.1
The strong evidence supporting PPI efficacy and a favourable safety profile has led to overuse in multiple treatment arenas.1,2 Surprisingly, despite more than two decades of extensive literature addressing inappropriate PPI use, PPI overprescribing remains prevalent from primary to tertiary care centres in many countries in Europe and the United States (US).2 In the hospital setting, the prevalence of PPI overprescribing has been reported to be between 61 to 86 per cent in recent Western studies.3,4
Non-judicious PPI use is a matter of great concern in the elderly who often have multiple comorbidities, are taking multiple medications, and hence are at an increased risk of long-term PPI-related adverse outcomes. The most significant of the documented adverse effects of PPIs include: Clostridium difficile-associated diarrhea,5 pneumonia,6 hip fractures,7 malabsorption of iron, nutrients and vitamins,8 and acute interstitial nephritis.9 Therefore, inappropriate PPI use may lead to increasing healthcare costs, morbidity, and even mortality.5,6
The present study was designed to determine the prevalence of inappropriate PPI prescribing without documentation of valid indications among elderly patients, in a tertiary teaching hospital in Singapore. To our knowledge, PPI-prescribing patterns have been infrequently studied in well-developed healthcare systems in Southeast Asia.
Method
We performed a clinical record review of 150 consecutive patients aged ≥65 years who had been admitted to two internal medicine wards between 25 May 2011 and 28 June 2011 at a large tertiary care centre. Patient data regarding gender, number of comorbidities, functional status, pre-admission and discharge PPI prescriptions, and concomitant use of ulcerogenic medications was extracted.
To be eligible for the study, patients were to have a traceable PPI prescription in the National Healthcare Group (NHG) Cluster Shared Patient Record System (CPRS) to ensure accuracy of pre-admission PPI use and other medications. Discharge PPI prescriptions were obtained from physician discharge summaries. Pre-admission and discharge prescriptions were then reviewed to determine continuation of pre-admission PPIs and newly prescribed PPIs at discharge. Patients were excluded if they transferred to other wards, were taken over by other subspecialty, or died before hospital discharge.
Two senior clinicians reviewed case notes and physician discharge summaries to ascertain appropriateness of PPI prescriptions as per PPI-prescribing guidelines, consensus statements, and systematic reviews10–17 (`e 1).
Figure 1. Recommended proton pump inhibitors indications.
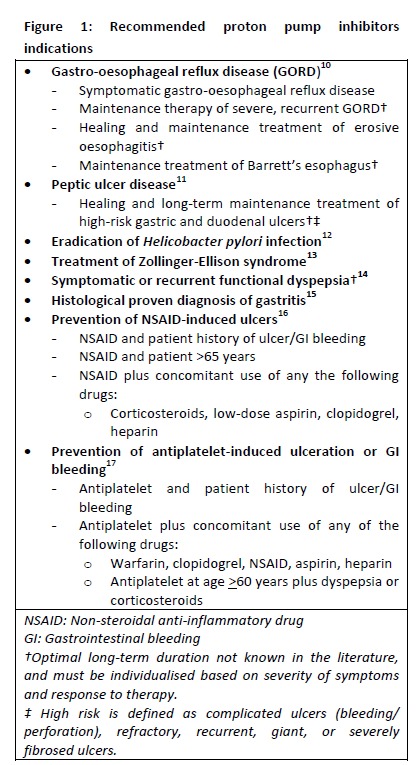
A PPI indication was considered “valid” if there was a documented indication for a prescription in the case notes or physician discharge summary consistent with one of the recommended PPI indications listed inFigure 1. When this indication was not explicitly documented, the two reviewers explored the clinical records, which included past medical history records, electronic and case notes review of past hospital admissions, outpatient clinic notes, gastrointestinal endoscopies, and histology findings. When the two reviewers were in disagreement about the classification of the PPI prescribing indication, a third senior clinician gave a final recommendation.
If an indication was determined to be valid it was classified as “undocumented valid”. Any PPI prescription without a documented recommended PPI indication or without justification of continued use was considered “likely invalid”. Indication was deemed “probable” when PPIs might have been indicated (e.g., “suspected gastrointestinal bleeding”) but either: 1) there was no clear evidence that bleeding actually occurred; 2) the absence of definitive investigations such as gastrointestinal endoscopies were refused by the families; or 3) the patient was deemed not suitable for further investigations to support PPI use.
Results
Of the 150 patients admitted during the study duration, 89 (59.3 per cent) were females and 61 (40.7 per cent) were males, with median interquartile range (IQR) age of 78 (72–83) years (Table 1). Eighty patients (54 per cent) had a prescription for PPI at discharge, of which 64 were pre-admission and 16 newly prescribed PPIs. Thirty-two patients (40 per cent) with PPI prescriptions were receiving low-dose aspirin.
Table 1. Characteristics of study patients (n=150).
| Female † | 89 (59.3) |
| Male | 61 (40.7) |
| Age (years)** | 78 (72–83) |
| Number of comorbidities* | 4 (2–5) |
| Number of pre-admission medications* | 5 (3–7) |
| Number of discharge medications* | 5 (3–7) |
| Number of PPI prescriptions at discharge† | 80 (54) |
| Pre-admission PPIs† ‡ | 66 (44) |
| New PPI prescriptions during hospitalization† | 16 (20) |
| PPI prescribed† | |
| Omeprazole | 70 (87.5) |
| Esomeprazole | 9 (11.2) |
| Lansoprazole | 1 (1.2) |
| Concurrent medications† | |
| Aspirin | 32 (40) |
| Aspirin plus clopidogrel | 4 (5) |
| Clopidogrel | 3 (3.7) |
| Corticosteroids | 3 (3.7) |
| Warfarin | 3 (3.7) |
| Heparin | 1 (1.2) |
Summaries reported as median (interquartile range).
Summaries reported as median (interquartile range).
Summaries reported as frequency (proportion).
Two pre-admission PPI prescriptions were discontinued during admission.
Figure 2 and Table 2 summarise the indications for PPI prescriptions. Of the 80 PPI prescriptions, 65 (81.2 per cent) did not have any documented valid indication, which we regarded as “likely invalid”; nine (11.2 per cent) had “documented valid” indications for a PPI prescription; and one had an “undocumented valid” indication. In five cases (6.2 per cent), the indication was “probable”.
Figure 2. Summary of proton pump inhibitor indications.
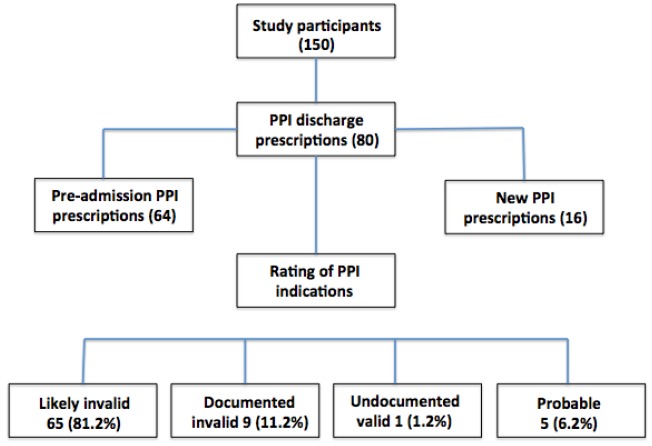
Table 2. Rating of PPI indications, results and categories (n=80).
| Rating of Indication | Reasons for continuous PPI prescription | Number (%) |
|---|---|---|
| Documented Valid Indications n=9 (11.2%) | GI prophylaxis (clopidogrel plus aspirin) | 4 (44.4) |
| Symptomatic GORD | 2 (22.2) | |
| Healing of complicated duodenal ulcer | 1 (11.1) | |
| Maintenance treatment of severe erosive oesophagitis | 1 (11.1) | |
| Histologically proven gastritis | 1 (11.1) | |
| Undocumented Valid Indications n=1 (1.2%) | History of recurrent GI ulceration | 1 (100) |
| Undocumented Likely Invalid Indications† n=65 (81.2 %) | Low-dose aspirin alone | 28 (43) |
| No documented history of GI problems | 20 (30.7) | |
| Antiplatelets§, anticoagulants or corticosteroids alone | 10 (15.3) | |
| Remote history of gastritis or peptic ulcer disease | 7 (10.7) | |
| Probable Indications n=5 (6.2%)† | Mild bloody vomiting | 2 (40) |
| Anemia in presence of aspirin | 2 (40) | |
| Drop in hemoglobin | 1 (20) |
GI: Gastrointestinal
GORD: Gastro-oesophageal reflux disease
Refer to methodology
Clopidogrel, Ticlid
The most common “likely invalid” indications were primary gastrointestinal bleeding prophylaxis (GIP) in 28 patients (43 per cent of invalid PPI prescriptions) taking low-dose aspirin, followed by 20 (30.7 per cent of invalid prescriptions) in the absence of any documented history of GI problems. Among the 10 (12.5 per cent) valid prescriptions, the major indications were GIP for dual antiplatelet therapy and symptomatic gastro-oesophageal reflux disease (GORD).
Discussion
Previous studies have mainly reported inappropriate PPI use on unselected cohorts,2,3 however, this study is among the few that have targeted PPI-prescribing patterns among the elderly and very elderly population at hospital discharge in Southeast Asia. In our study, 81 per cent of the patients had no documented indications for their PPI use, which is questionable and seems to indicate inappropriate prescribing. Our findings further support earlier Western studies reporting high inappropriate prescription of PPI across primary and tertiary care.3,4
A previous US study reported significantly high non-compliance to PPI guidelines among academic and non-academic hospitals (29 per cent vs. 59 per cent), even though non-compliance was significantly lower among academic hospitals.2 Reid et al. have recorded inappropriate PPI prescribing of 61 per cent and 73 per cent among two large university-affiliated health centres in the US, which has so far been described as the largest inpatient population evaluated for appropriateness of PPI prescriptions.3 Another primary care study in the United Kingdom (UK) reported 54 per cent inappropriate PPI use.18 Despite extensive publications addressing PPI-related adverse outcomes, physicians’ PPI-prescribing practice continues and does not seem to have changed significantly in recent years.
Moreover, 80 patients (54 per cent) in our study had a PPI prescription at discharge, which is higher than the reported rate of 41 per cent in a recent study in the UK among elderly patients where they reported inappropriate PPI use in 85 per cent of such prescriptions, which is a rate comparable to our findings.4 In our study, out of 80 PPI discharge prescriptions, 64 were pre-admission prescriptions that patients were taking for various reasons, such as previous history of upper GI symptoms (which had occurred from several years up to two decades earlier), peptic ulcer disease, GIP of low-dose aspirin, and non-specified reasons.
This questionable and likely inappropriate PPI use without documented evidence of clear indications is potentially due to the fact that many physicians routinely continue PPIs considering them safe, long-term medications, without assessing risks and benefits of long-term therapy.20 Other recent studies have shown that physicians do not review and document PPI indications in a large number of cases, which often results in their long-term or even indefinite continuation.1,19,20 This is of interest because even among the 16 patients in our study with newly prescribed PPI prescriptions at discharge, only two patients were discharged with any documented justification.
Another important and significant study finding relates to a high prevalence of PPI use amongst our elderly patients, prescribed for primary GIP. The guidance on primary preventive therapy for gastrointestinal bleeding risk of antiplatelet therapy comes from the consensus statement document in 2008,17 issued by the American College of Cardiology Foundation (ACCF), the American College of Gastroenterology (ACG), and the American Heart Association (AHA). The guideline recommends preventive therapy for older age (>60 years), plus any of the following: concomitant use of NSAIDS, corticosteroids, antithrombotic agents, or peptic ulcer disease. Most PPI prescriptions for primary GIP in our study were found to be inappropriate and not consistent with recommended guidelines.17 Even from our own personal experience, a large number of physicians commonly prescribe PPIs for primary GIP amongst elderly patients using antiplatelet therapy as “just in case” without stratifying the risk of antiplatelet-related GI complications and long-term PPI-related adverse outcomes.
Subsequent to the 2008 consensus statement document,17 the ACG 2009 guidelines16 recommended co-administration of a PPI among patients who require an NSAID according to risk for gastrointestinal toxicity. PPI prophylaxis may be considered in low-risk patients taking an NSAID (age >65: defined as one risk factor). However, it remains uncertain whether the same guideline recommendations also apply to the use of low-dose aspirin. There is evidence that low-dose aspirin significantly increases the risk of upper GI complications compared with placebo or non-use.21 In contrast to NSAID-related upper GI risk factors, risk factors for aspirin users are less well studied and it is not clear from the literature whether low-dose aspirin carries similar risk of gastrointestinal bleeding compared to other NSAIDs among older adults (age >65).22
The current study has several limitations. First, being retrospective in nature, it did not allow the assessors to confirm the decision-making process supporting PPI prescriptions, and indications had to be inferred from the list of PPI-prescribing guidelines when they were not documented. Second, even though reviewers had full access to patient handwritten case notes, electronic medical records including all past admissions, procedures, and investigations, we acknowledge that determining appropriateness or validity of an indication based on physician documentation and merely exploring clinical records alone might have led to overestimation of inappropriate PPI use in our study.
Because of the above limitations, we regarded all invalid PPI indications as “likely invalid” as it is possible that some of these prescriptions might have been justified by physicians during patient interviewing or assessment, but were not documented in medical records. Third, the small sample size might have limited the reliability or precision of results. However, all recent studies have reported a very high prevalence of inappropriate PPI prescribing and our result findings are consistent with these studies.2,3,4,18–20 Finally, as the guidelines recommend periodic assessment for the continued use of PPI, it may be challenging to assess symptoms among the very elderly, frail, and debilitated elderly with dementia and communication issues. This may have posed difficulties for the prescribing physicians in determining the need for a prescription.
Conclusion
PPI prescribing without documented valid indications is highly prevalent in our practice. Approaches to tackle this medication safety issue could include documented physician review of PPI indications at each patient contact. We further recommend interventions such as pharmacist advice being documented in electronic medication records, and flagging medications that lack appropriate indications.
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
Please cite this paper as: Akram F, Huang Y, Lim V, Huggan PJ, Merchant RA. Proton pump inhibitors: Are we still prescribing them without valid indications? AMJ 2014;7(11):465–470.http//dx.doi.org/10.4066/AMJ.2014.2093
References
- 1.Heidelbaugh J, Kim A, Chang R, Walker P. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv. Gastroenterol. 2012;5:219–32. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eid SM, Boueiz A, Paranji S, Mativo C, Landis R, Abougergi MS. Patterns and predictors of proton pump inhibitor overuse among academic and non-academic hospitalists. Intern Med. 2010;49(23):2561–68. doi: 10.2169/internalmedicine.49.4064. [DOI] [PubMed] [Google Scholar]
- 3.Reid M, Keniston A, Heller JC, Miller M, Medvedev S, Albert RK. Inappropriate Prescribing of Proton Pump Inhibitors in Hospitalized. J Hospital medicine. 2012;7(5):421–5. doi: 10.1002/jhm.1901. [DOI] [PubMed] [Google Scholar]
- 4.Jarchow-MacDonald AA, Mangoni AA. Prescribing patterns of proton pump inhibitors in older hospitalized patients in a Scottish health board. Geriatr Gerontol Int. 2013;13:1002–9. doi: 10.1111/ggi.12047. [DOI] [PubMed] [Google Scholar]
- 5.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile associated disease. JAMA. 2005;294(23) doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 6.Eom CS, Kulik JA, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183(3):310. doi: 10.1503/cmaj.092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947–2. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 8.McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol. 2009;(14Suppl 2):S5–9. doi: 10.1038/ajg.2009.45. [DOI] [PubMed] [Google Scholar]
- 9.Geevasinga N, Coleman PL, Webster AC, Roger SD. Proton pump inhibitors and acute interstitial nephritis. Clin Gastro-enterol Hepatol. 2006;4:597–604. doi: 10.1016/j.cgh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Institute technical review on the management of gastro-oesophageal reflux disease. Gastroenterology. 2008;135:1392–1413. doi: 10.1053/j.gastro.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Freston JW. Review article: role of proton pump inhibitors in non-H. Pylori-related ulcers. Aliment Pharmacol Ther. 2001;(15[Suppl 2]):2–5. doi: 10.1046/j.1365-2036.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- 12.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D. et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–81. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilcox CM, Hirschowitz BI. Treatment strategies for Zollinger-Ellison syndrome. Expert Opin Pharmacother. 2009;10:1145–1157. doi: 10.1517/14656560902887035. [DOI] [PubMed] [Google Scholar]
- 14.Wang WH, Huang JQ, Zheng GF, Xia HH, Wong WM, Liu XG. et al. Effects of proton-pump inhibitors on functional dyspepsia: a meta-analysis of randomized placebo-controlled trials. Clin Gastroenterol Hepatol. 2007;5(2):178. doi: 10.1016/j.cgh.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Redeen S, Petersson F, Jonsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946–950. doi: 10.1055/s-2003-43479. [DOI] [PubMed] [Google Scholar]
- 16.Lanza FL, Chan FK, Quigley EM. Guidelines for Prevention of NSAID-related ulcer complications. Am Gastroenterol. 2009;104:728–38. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD. et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52(18):1502. doi: 10.1016/j.jacc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Batuwitage BT, Kingham JG, Morgan NE, Bartlett RL. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J. 2007;83(975):66–8. doi: 10.1136/pgmj.2006.051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahrens D, Chenot JF, Behrens G, Grimmsmann T, Kochen MM. Appropriateness of treatment recommendations for PPI in hospital discharge letters. Eur J Clin Pharmacol. 2010;66:1265–71. doi: 10.1007/s00228-010-0871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haroon M, Yasin F, Gardezi KM, Adeeb F, Walker F. Inappropriate use of proton pump inhibitors among medical inpatients: a questionnaire-based observational study. JRSM Short Rep. 2013 Jun 25;4(8) doi: 10.1177/2042533313497183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanas A, Perez-Aisa MA, Feu F, Ponce J, Saperas E, Santolaria S. et al. Investigators of the Asociación Española de Gastroenterología (AEG).A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with non-steroidal anti-inflammatory drug use. Am J Gastroenterol. 2005;100:1685–93. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 22.Valkhoff VE, Sturkenboom MC, Kuipers EJ. Risk factors for gastrointestinal bleeding associated with low-dose aspirin. Best Pract Res Clin Gastroenterol. 2012 Apr;26(2):125–40. doi: 10.1016/j.bpg.2012.01.011. [DOI] [PubMed] [Google Scholar]


