Recombinant expression and nanobody-aided crystallization of a soluble BabA truncation (BabA25–460) corresponding to the predicted adhesin domain are reported.
Keywords: Helicobacter pylori, BabA, adhesin, nanobody
Abstract
Helicobacter pylori is a human pathogen that colonizes about 50% of the world’s population, causing chronic gastritis, duodenal ulcers and even gastric cancer. A steady emergence of multiple antibiotic resistant strains poses an important public health threat and there is an urgent requirement for alternative therapeutics. The blood group antigen-binding adhesin BabA mediates the intimate attachment to the host mucosa and forms a major candidate for novel vaccine and drug development. Here, the recombinant expression and crystallization of a soluble BabA truncation (BabA25–460) corresponding to the predicted extracellular adhesin domain of the protein are reported. X-ray diffraction data for nanobody-stabilized BabA25–460 were collected to 2.25 Å resolution from a crystal that belonged to space group P21, with unit-cell parameters a = 50.96, b = 131.41, c = 123.40 Å, α = 90.0, β = 94.8, γ = 90.0°, and which was predicted to contain two BabA25–460–nanobody complexes per asymmetric unit.
1. Introduction
Helicobacter pylori is a Gram-negative bacterium that colonizes the gastric mucosa of about 50% of the world’s population. Unless treated with antibiotics, H. pylori infections are usually persistent and are associated with chronic gastric inflammation, which in symptomatic carriers can develop into gastric and duodenal ulcers and even gastric malignancies such as mucosa-associated lymphoid tissue (MALT) lymphoma and gastric adenocarcinoma (Every, 2013 ▶).
H. pylori infection is dependent on a complex interplay between the bacterium and its human host. Apart from environmental factors and host genetic predisposition, a number of H. pylori virulence factors are associated with more severe clinical outcomes of infection (Delahay & Rugge, 2012 ▶; Backert & Clyne, 2011 ▶). Along with the secreted virulence factors CagA and VacA, another important virulence trait of H. pylori is the ability to strongly adhere to the gastric mucosa. The blood group antigen-binding adhesin (BabA) is a major adhesin that interacts with fucosylated Lewis b (Leb) and H type 1 blood group antigens on the surface of human gastric epithelial cells (Borén et al., 1993 ▶; Aspholm-Hurtig et al., 2004 ▶; Moore et al., 2011 ▶). The reference strain CCUG17875 possesses two babA genes: a silent babA1 locus and a babA2 gene that is expressed as functional BabA adhesin (Ilver et al., 1998 ▶). In conjunction with the cagA and vacA s1 alleles, a babA2-positive genotype is found to be associated with duodenal ulcer disease and gastric cancer (Gerhard et al., 1999 ▶).
In recent years, multidrug-resistant H. pylori strains have spread worldwide, creating a demand for new therapeutics that can both treat and prevent infections. In Gram-negative bacteria, secretion pathways play an important role in pathogenesis and recent studies have focused on chemotherapeutics for the directed intervention in secretion machineries such as bacterial pili that mediate host-cell adhesion (Lo et al., 2013 ▶).
Hence, in a quest for the rational design of structure-based anti-adhesives, we initiated structural characterization of the BabA–Leb interaction. BabA is one of the members of the Helicobacter outer membrane porin (HOP) family. Comparative genome analysis and predicted secondary-structure analysis of HOP adhesins highlight a conserved C-terminal domain predicted to be the corresponding membrane-anchoring β-barrel domain, which is preceded by a variable domain that is expected to contain the adhesive properties (Tomb et al., 1997 ▶; Alm et al., 2000 ▶). This architecture is reminiscent of autotransporter-like proteins, in which a C-terminal β-barrel guides the secretion of an N-terminally located passenger domain. Here, we report the expression and purification of a BabA truncation corresponding to the putative N-terminal adhesin domain and show that an N-terminal construct spanning amino-acid residues 25–460 can be expressed as a stable folded unit. We further report on the crystallization of the N-terminal soluble adhesin domain with the aid of nanobodies, which are camelid-derived single-domain antibodies that have successfully been used as crystallization chaperones, for example in the case of conformationally complex or aggregating proteins (Steyaert & Kobilka, 2011 ▶; Baranova et al., 2013 ▶).
2. Materials and methods
2.1. Cloning, expression and purification of BabA truncations
DNA coding sequences corresponding to the BabA fragments BabA1–460, BabA1–480, BabA1–496, BabA1–523 and BabA1–546 were PCR-amplified from H. pylori CCUG 17875 genomic DNA using primers (the forward primer BabA_IbaHR4-f and the reverse primers BabA_IbaHR4_460-r, BabA_IbaHR4_480-r, BabA_IbaHR4_523-r and BabA_IbaHR4_546-r) shown in Table 1 ▶. The amplicons were digested with BsaI and cloned into pASK-HR4, a derivative of the pASK-IBA32 vector (IBA GmbH) with the N-terminal ompA leader sequence replaced by the dsbA leader. DNA fragments corresponding to BabA25–460, BabA25–480, BabA25–523 and BabA25–546 were PCR-amplified from their respective pASK-HR4 parent constructs using primers (forward primer BabA_IbaHR3-f and reverse primer BabA_IbaHR3-r) as provided in Table 1 ▶, digested with BsaI and cloned into pASK-HR3, a pASK-IBA12-derived vector in which the ompA leader sequence was replaced by the dsbA leader. Thus, the resulting BabA fragments comprise an N-terminal DsbA signal peptide for periplasmic expression followed by a thrombin-cleavable Strep-tag II and a 6×His tag at their C-terminal end. Escherichia coli TOP10 cells were transformed with the pASK-HR3 or pASK-HR4 constructs, grown on lysogeny broth (LB) supplemented with 100 mg ml−1 ampicillin until an OD600 of 0.7 was reached and induced overnight at 293 K with 0.2 mg ml−1 anhydrotetracycline.
Table 1. Cloning of BabA truncations.
| Source organism | H. pylori |
| DNA source | H. pylori CCUG 17875 babA2 gene (accession No. AF033654.1) |
| Forward primer: BabA_IbaHR4-f | 5-ATGGTAGGTCTCAGGCCGAAGACGACGGCTTTTACACAAGCG-3 |
| Reverse primer: BabA_IbaHR4_460-r | 5-GTCGTAGGTCTCAGCGCTGAAATTCACTAGAGTGTCAGCGATGTTTTC-3 |
| Reverse primer: BabA_IbaHR4_480-r | 5-GTCGTAGGTCTCAGCGCTTTTGGAGAGCGCAGTAGTGATGCTG-3 |
| Reverse primer: BabA_IbaHR4_523-r | 5-GTCGTAGGTCTCAGCGCTTGATATCTAACTAAGCTTGACCTGTGAAGTG-3 |
| Reverse primer: BabA_IbaHR4_546-r | 5-GTCGTAGGTCTCAGCGCTCCCATTCATGGCACCATTGTTGGTTTGAGAAC-3 |
| Forward primer: BabA_IbaHR3-f | 5-ATGGTAGGTCTCACTCCGGCATCCAAGATCTTTCAGACAACTATG-3 |
| Reverse primer: BabA_IbaHR3-r | 5-GTCGTAGGTCTCATATCAGTGATGGTGATGGTGATGCGATCCTC-3 |
| Cloning vectors | pASK-HR3, pASK-HR4 |
For large-scale expression for functional and structural analysis, TOP10 cells grown and induced as described above were harvested by centrifugation at 6238g for 15 min at 277 K using a JA-8.1 rotor in an Avanti-J26 XP Beckman Centrifuge. Periplasmic extraction was performed by suspending the cells in 4 ml 20 mM Tris–HCl pH 8.8, 20%(w/v) sucrose (per 1 g of wet cell pellet) with the protease inhibitors AEBSF–HCl (0.4 mM) and leupeptin (1 mg ml−1) followed by treatment with 40 µl 0.5 M EDTA and 40 µl lysozyme (10 mg ml−1) and mixing gently on ice for 20 min. 80 µl 1 M MgCl2 was added and the periplasmic extract was cleared from spheroplasts by centrifugation at 17 418g for 20 min at 277 K in a JA-20 rotor. The periplasmic extract was further clarified by centrifugation at 48 384g for 30 min at 277 K in the same rotor and filtered through a 0.45 µm syringe filter.
BabA fragments were purified by consecutive nickel and Strep-Tactin affinity chromatography. Briefly, the periplasmic extract was loaded onto a 5 ml pre-packed Ni–NTA column (GE Healthcare) pre-equilibrated with buffer A (20 mM Tris–HCl pH 8.8, 250 mM NaCl, 5% glycerol), washed with five bed volumes of buffer A and the bound protein was eluted with 280 mM imidazole in buffer A. Eluted peak fractions were collected and loaded onto a 5 ml pre-packed Strep-Tactin column (IBA GmbH) equilibrated with buffer C (20 mM Tris–HCl pH 8.8, 150 mM NaCl, 5% glycerol), washed with five bed volumes of buffer C and the bound protein was eluted with 0.6 mg ml−1 d-desthiobiotin in buffer C. In addition to the crystallization trials for these BabA truncations, we opted for a nanobody-aided crystallization approach. For this purpose, we used Nb-ER19, a nanobody raised against native full-length BabA purified from the outer membrane of H. pylori strain CCUG 17875. Nb-ER19 was produced and purified as described elsewhere (Baranova et al., 2013 ▶; Pardon et al., 2014 ▶).
To form BabA–Nb-ER19 complexes, the periplasmic extracts of the BabA truncations (BabA25–460, BabA25–523 and BabA25–546) and Nb-ER19 were mixed in a 5:1(v:v) ratio on ice and nickel-affinity purification was performed as described above. Fractions containing the respective BabA fragments and Nb-ER19 were pooled and concentrated in a 10 kDa molecular-weight cutoff spin concentrator to a final volume of 500 µl. The concentrated sample was then loaded onto a Superdex S-200 16/60 column (GE Healthcare) pre-equilibrated with 20 mM sodium acetate pH 5.0, 150 mM NaCl, 5% glycerol. Eluted fractions containing the BabA–Nb-ER19 complex, with the affinity purification tags intact, were pooled and concentrated to a final concentration of 15 mg ml−1 using a 10 kDa molecular-weight cutoff spin concentrator prior to crystallization.
2.2. Crystallization
Crystallization of BabA1–460, BabA25–460 and its complex with Nb-ER19 was first screened at 293 K by the sitting-drop vapour-diffusion method with commercial sparse-matrix crystallization screening kits [SaltRx, SaltRx 2, Grid Screen Salt HT, PEGRx 2, Index HT (Hampton Research), JBScreen Basic, JBScreen Classic, JBScreen PEG/Salt (Jena Bioscience) and PACT premier HT-96, JCSG-plus HT-96 and ProPlex HT-96 (Molecular Dimensions)] in 96-well MRC plates. Each crystallization drop was prepared by mixing 400 nl protein solution and 400 nl reservoir solution (a 1:1 ratio) and equilibrating against 50 µl reservoir solution. Crystallization information is summarized in Table 2 ▶.
Table 2. Crystallization of BabA25460Nb-ER19.
| Method | Sitting-drop vapour diffusion |
| Plate type | MRC 96-well crystallization plate (Swissci) |
| Temperature (K) | 293 |
| Protein concentration (mgml1) | 15 |
| Buffer composition of protein solution | 20mM sodium acetate pH 5.0, 150mM NaCl, 5% glycerol |
| Composition of reservoir solution | 0.2M sodium nitrate; 0.1M bis-tris propane pH 6.5, 20%(w/v) PEG 3350 |
| Volume and ratio of drop | 0.4l, 1:1 |
| Volume of reservoir (l) | 50 |
2.3. Data collection and processing
A BabA25–460–Nb-ER19 crystal grown in 0.2 M sodium nitrate, 0.1 M bis-tris propane pH 6.5, 20%(w/v) PEG 3350 and supplemented with 10% glycerol was mounted in a SPINE-standard nylon loop and flash-cooled in liquid nitrogen. Diffraction data for the native protein were collected on beamline I04 at Diamond Light Source, Didcot, England equipped with a PILATUS 6M CCD detector. The diffraction data were processed with XDS and xia2 (Kabsch, 2010 ▶) and truncated to 2.25 Å resolution on the basis of an I/σ(I) cutoff of 2. The crystals belonged to the monoclinic space group P21, with unit-cell parameters a = 50.960, b = 131.410, c = 123.40 Å, α = 90.00, β = 94.80, γ = 90.00°, compatible with two to three BabA25–460–Nb-ER19 complexes per asymmetric unit, corresponding to a calculated V M of 3.3 and 2.2 Å3 Da−1 and a solvent content of 60 and 41%, respectively (Matthews, 1968 ▶). Crystallographic data statistics are presented in Table 3 ▶.
Table 3. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | I04, Diamond Light Source |
| Wavelength () | 0.97949 |
| Temperature (K) | 100 |
| Detector | PILATUS 6M CCD |
| Crystal-to-detector distance (mm) | 385.01 |
| Rotation range per image () | 0.2 |
| Total rotation range () | 200 |
| Exposure time per image (s) | 0.2 |
| Space group | P1211 |
| a, b, c () | 50.96, 131.41, 123.40 |
| , , () | 90.0, 94.8, 90.0 |
| Mosaicity () | 0.16 |
| Resolution range () | 65.712.25 (2.312.25) |
| Total No. of reflections | 339889 |
| No. of unique reflections | 76055 |
| Completeness (%) | 99.2 (100.0) |
| Multiplicity | 4.5 (4.5) |
| I/(I) | 14.5 (2.2) |
| R meas (%) | 6.3 (79.0) |
| Overall B factor from Wilson plot (2) | 43.6 |
3. Results and discussion
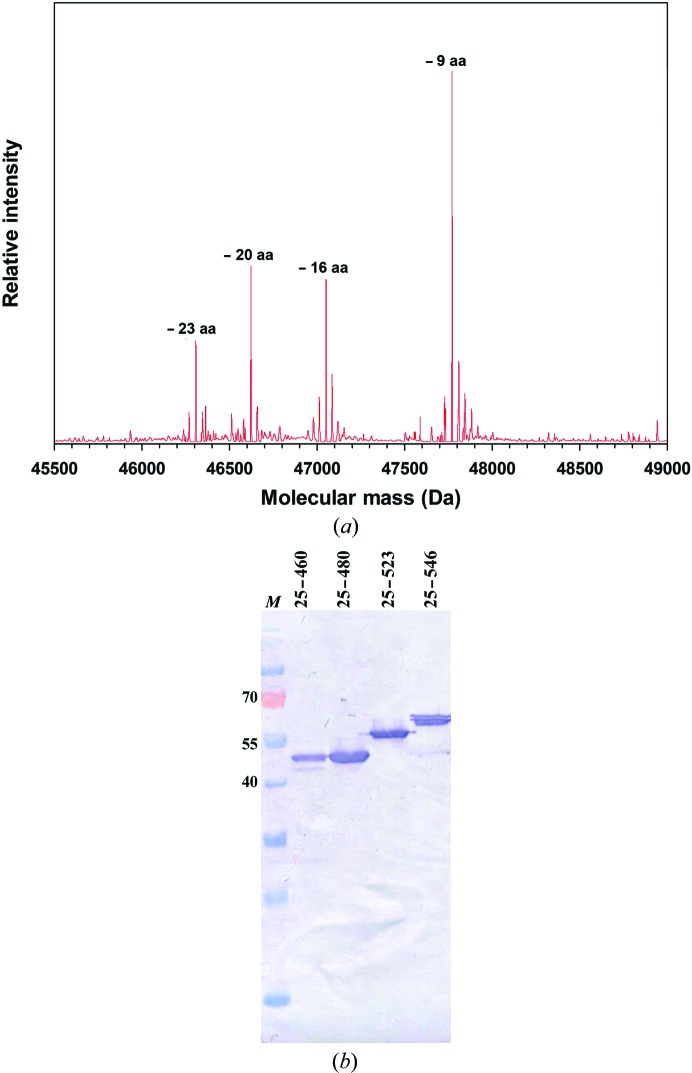
On the basis of comparative genome analysis and secondary-structure prediction of HOP adhesins, we made C-terminal truncation constructs that remove the predicted β-barrel domain. In this way, five constructs were generated with truncation sites falling in-between predicted secondary-structure elements upstream of the C-terminal β-strand region: BabA1–460, BabA1–480, BabA1–496, BabA1–523 and BabA1–546. Although these constructs were found to result in the expression of recombinant protein of the expected molecular weight, the expression products were mostly found in the insoluble fraction and the protein fractions purified from the periplasmic extracts were found to be prone to aggregation. Mass-spectrometric analysis showed the rapid proteolytic loss of the first 23 N-terminal residues (Fig. 1 ▶ a), indicating these were not part of a stably folded core in the BabA truncates. Based on this susceptibility to degradation, we modified the babA 1–460, babA 1–480, babA 1–523 and babA 1–546 constructs to remove the first 24 amino acids of the mature protein. With the exception of BabA25–480, the remaining N-terminal deletion constructs resulted in the periplasmic expression of soluble recombinant BabA fragments corresponding to the expected molecular weight (Fig. 1 ▶ b).
Figure 1.
(a) MS spectra of BabA1–460 reveal spontaneous proteolysis leading to the loss of up to 23 N-terminal amino acids. (b) Immunoblot detection (using α-His antibody) of BabA fragments in the respective periplasmic extracts. Lane M contains molecular-mass marker (labelled in kDa).
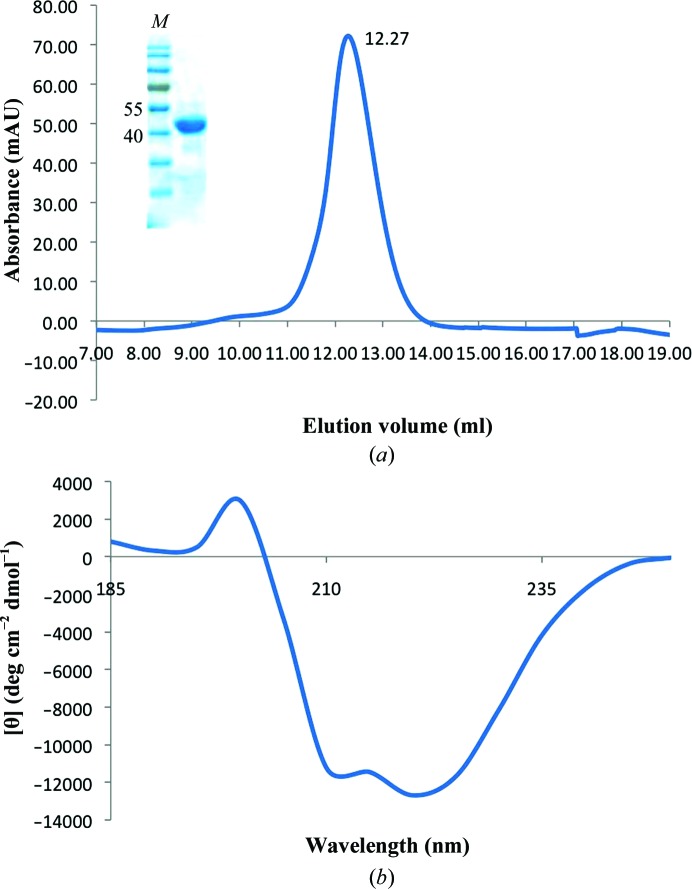
On analytical size exclusion (Superdex 75 10/300; Fig. 2 ▶ a), the minimal fragment, BabA25–460, eluted as a single peak corresponding to a calculated molecular mass of about 52 kDa, matching that expected for a BabA25–460 monomer with N-terminal and C-terminal tags (49 319 Da). When analysed by circular-dichroism (CD) spectroscopy, the BabA25–460 truncation showed a folded conformation with a high α-helical secondary-structure content (Fig. 2 ▶ b). A dominant α-helical secondary structure was also observed in the recently solved structure of the H. pylori HOP adhesin SabA (Pang et al., 2014 ▶). Similar to BabA25–460, expression of BabA25–52 3 and BabA25–546 resulted in folded recombinant BabA fragments (data not shown).
Figure 2.
(a) Size-exclusion chromatogram and SDS–PAGE analysis (inset) of BabA25–460 run on Superdex 75 10/300 (GE Healthcare). (b) CD spectrum of BabA25–460 in phosphate buffer (50 mM KH2PO4/K2HPO4 pH 8.0) reveals a predominantly α-helical secondary-structure content.
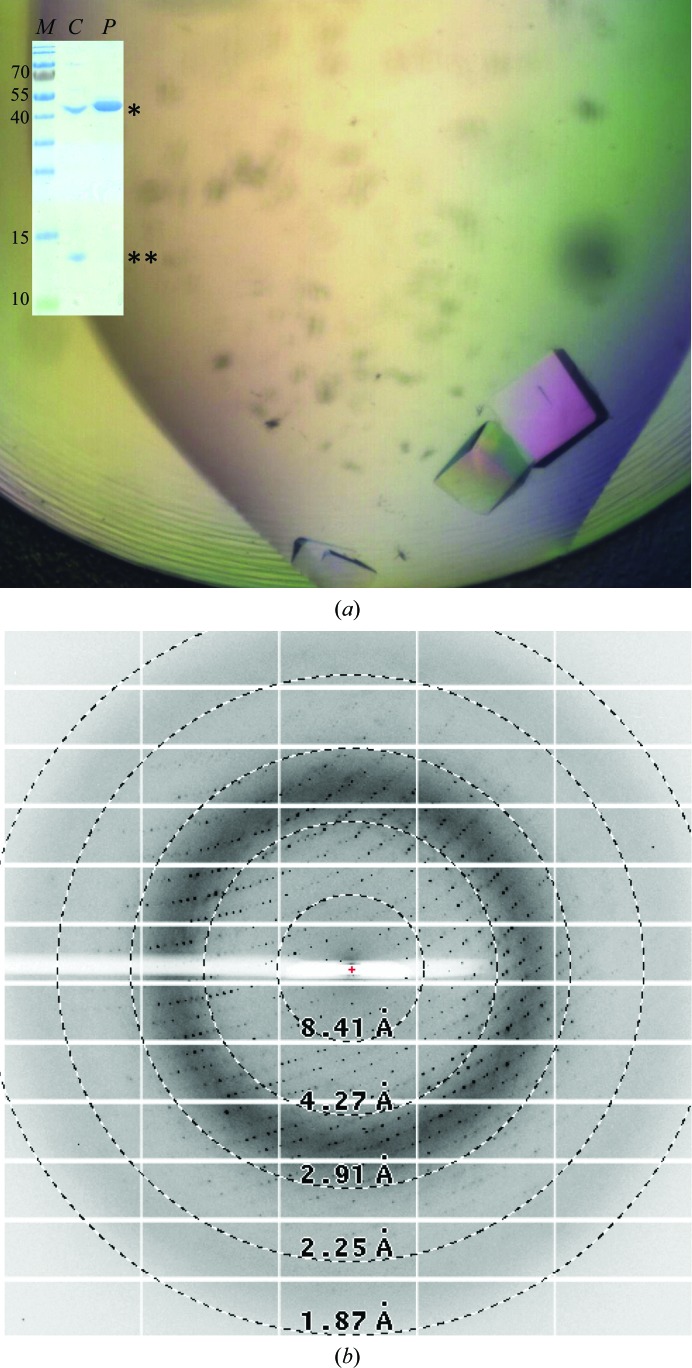
Recombinant proteins were concentrated to 10–15 mg ml−1 and subjected to crystallization trials using the commercially available sparse-matrix screens described above. Extensive screens for the BabA fragments did not result in the growth of single crystals in the conditions used. Hence, we opted for a nanobody-aided crystallization approach. Crystals of BabA25–460–Nb-ER19 were obtained in multiple different conditions of the PACT premier and JCSG-plus screens after 1–2 d incubation at 293 K. Optimization of PACT premier screen condition F5 with differing PEG 4000 concentrations (16–24%) also led to the reproducible growth of cube-shaped crystals of average dimensions of 0.06 × 0.06 × 0.06 mm (Fig. 3 ▶ a). SDS–PAGE analysis of washed and dissolved crystals confirmed that these contained both BabA25–460 and Nb-ER19 (Fig. 3 ▶ a, inset).
Figure 3.
(a) Image of BabA25–460–Nb-ER19 crystals and SDS–PAGE of dissolved crystals alongside purified BabA25–460 (inset, labelled C and P, respectively; *, BabA25–460; **, Nb-ER19). (b) Representative diffraction image (0.2° rotation, 0.2 s exposure at 20% transmission) from a crystal of BabA25–460 in complex with Nb-ER19 obtained by the sitting-drop vapour-diffusion method.
A crystal grown in 0.2 M sodium nitrate, 0.1 M bis-tris propane pH 6.5, 20%(w/v) PEG 3350 diffracted to at least 2.25 Å resolution (Fig. 3 ▶ b). The crystal belonged to the monoclinic space group P21, with unit-cell parameters compatible with a predicted two or three BabA25–460–Nb-ER19 complexes per asymmetric unit. Selenomethionine-derivatized protein is in production to allow experimental phase determination by a single anomalous dispersion experiment. The high-resolution structure of the BabA extracellular domain will be invaluable in understanding its ligand-binding properties and is anticipated to facilitate the development of anti-adhesive compounds that may serve as novel therapeutic and/or prophylactic eradication therapies against H. pylori.
Acknowledgments
We acknowledge the use of the synchrotron-radiation facility at Diamond Light Source, Didcot, England under proposal MX9426, and thank the staff of the Diamond MX group and beamline I04 for assistance during data collection. This work is supported by project grant PRJ9 from the Flanders Institute of Biotechnology (VIB), the Odysseus program of the Flanders Science Foundation (FWO) and equipment grant UABR/09/005 from the Hercules Foundation. SS is the recipient of an Erasmus Mundus PhD fellowship. TB is supported by grants from Vetenskapsrådet/VR and Cancerfonden.
References
- Alm, R. A., Bina, J., Andrews, B. M., Doig, P., Hancock, R. E. W. & Trust, T. J. (2000). Infect. Immun. 68, 4155–4168. [DOI] [PMC free article] [PubMed]
- Aspholm-Hurtig, M. et al. (2004). Science, 305, 519–522. [DOI] [PubMed]
- Backert, S. & Clyne, M. (2011). Helicobacter, 16, 19–25. [DOI] [PubMed]
- Baranova, E., Fronzes, R., Garcia-Pino, A., Van Gerven, N., Papapostolou, D., Péhau-Arnaudet, G., Pardon, E., Steyaert, J., Howorka, S. & Remaut, H. (2013). Nature (London), 487, 119–122. [DOI] [PubMed]
- Borén, T., Falk, P., Roth, K. A., Larson, G. & Normark, S. (1993). Science, 262, 1892–1895. [DOI] [PubMed]
- Delahay, R. M. & Rugge, M. (2012). Helicobacter, 17, 9–15. [DOI] [PubMed]
- Every, A. L. (2013). Trends Microbiol. 21, 253–259. [DOI] [PubMed]
- Gerhard, M., Lehn, N., Neumayer, N., Borén, T., Rad, R., Schepp, W., Miehlke, S., Classen, M. & Prinz, C. (1999). Proc. Natl Acad. Sci. USA, 96, 12778–12783. [DOI] [PMC free article] [PubMed]
- Ilver, D., Arnqvist, A., Ogren, J., Frick, I. M., Kersulyte, D., Incecik, E. T., Berg, D. E., Covacci, A., Engstrand, L. & Borén, T. (1998). Science, 279, 373–377. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst D66, 125–132. [DOI] [PMC free article] [PubMed]
- Lo, A. W. H., Moonens, K. & Remaut, H. (2013). Curr. Opin. Microbiol. 16, 85–92. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Moore, M. E., Borén, T. & Solnick, J. V. (2011). Gut Microbes, 2, 42–46. [DOI] [PMC free article] [PubMed]
- Pang, S. S., Nguyen, S. T. S., Perry, A. J., Day, C. J., Panjikar, S., Tiralongo, J., Whisstock, J. C. & Kwok, T. (2014). J. Biol. Chem. 289, 6332–6340. [DOI] [PMC free article] [PubMed]
- Pardon, E., Laeremans, T., Triest, S., Rasmussen, S. G. F., Wohlkönig, A., Ruf, A., Muyldermans, S., Hol, W. G. J., Kobilka, B. K. & Steyaert, J. (2014). Nature Protoc. 9, 674–693. [DOI] [PMC free article] [PubMed]
- Steyaert, J. & Kobilka, B. K. (2011). Curr. Opin. Struct. Biol. 21, 567–572. [DOI] [PMC free article] [PubMed]
- Tomb, J. F. et al. (1997). Nature (London), 388, 539–547.