To contribute to the molecular understanding of the function of a tandem-type universal stress protein, UspE from E. coli was overexpressed and crystals of the recombinant protein were obtained using sitting-drop vapour diffusion. A diffraction data set was collected to a resolution of 3.2 Å.
Keywords: tandem-type UspE, universal stress proteins, Usp domain
Abstract
Universal stress proteins (Usps) are among the most highly induced genes when bacteria are subjected to several stress conditions such as heat shock, nutrient starvation or the presence of oxidants or other stress agents. Escherichia coli has five small Usps and one tandem-type Usp. UspE (or YdaA) is the tandem-type Usp and consists of two Usp domains arranged in tandem. To date, the structure of UspE remains to be elucidated. To contribute to the molecular understanding of the function of the tandem-type UspE, UspE from E. coli was overexpressed and the recombinant protein was purified using Ni–NTA affinity, Q anion-exchange and gel-filtration chromatography. Crystals of UspE were obtained by sitting-drop vapour diffusion. A diffraction data set was collected to a resolution of 3.2 Å from flash-cooled crystals. The crystals belonged to the tetragonal space group I4122 or I4322, with unit-cell parameters a = b = 121.1, c = 241.7 Å.
1. Introduction
Universal stress proteins (Usps) are widespread proteins in the genomes of bacteria, archaea, fungi, flies and plants (Siegele, 2005 ▶; Gustavsson et al., 2002 ▶). The expression of Usps in bacteria is triggered in response to a large variety of adverse environmental factors such as heat and the presence of uncouplers, polymyxin, cycloserine, oxidants, metals, ethanol, nutrient starvation or DNA-damaging agents (Siegele, 2005 ▶; Tkaczuk et al., 2013 ▶). The overproduced Usps have been shown to play an important role in stress resistance and to aid survival through various mechanisms in several bacterial species (Nyström & Neidhardt, 1994 ▶). Usp-containing organisms are usually equipped with several usp genes (Kvint et al., 2003 ▶). The usp genes usually encode either small Usps (around 14–15 kDa) harbouring one Usp domain or a large version (around 30 kDa) consisting of two Usp domains (Pfam accession No. PF0582) in tandem. Usp domains are also found in combination with other various domains, such as transporters, permeases, channels and protein kinases, in different organisms (Kvint et al., 2003 ▶).
Escherichia coli has six Usp proteins. Five of them have one Usp domain: UspA, UspC (YecG), UspD (YiiT), UspF (YnaF) and UspG (YbdQ). UspE (YdaA) is a tandem-type Usp that contains two Usp domains (Sousa & McKay, 2001 ▶). Based on structural and amino-acid sequence analyses, E. coli Usps or Usp domains have been divided into four different classes. UspA, UspC and UspD belong to class I, whereas UspF and UspG belong to class II. The first Usp domain of UspE belongs to class III and the second domain to class IV (Zarembinski et al., 1998 ▶). The Usps have evolved different physiological functions. For example, the class I UspA and UspD are required for defence against superoxide-generating agents, while the class I UspC and the class II UspF and UspG are instead mainly related to cellular adhesion and motility. In addition, the role of the unique Usp protein of class III/IV, UspE, overlaps with the functions of all of the other classes (Nachin et al., 2005 ▶; Diez et al., 2000 ▶). These similarities and differences in expression patterns correlate with the biological roles defined by the analysis of usp mutants. However, the molecular function and the mechanism of action of the Usp proteins mostly remains unclear.
Many Usp protein structures have been solved using X-ray crystallography. However, the structure of UspE has not been published to date, although the crystal structure of UspE from Proteus mirabilis is available in the PDB. The structure of the UspE from E. coli is required to understand the function of this tandem-type Usp, as the Usp proteins from E. coli have been the most extensively studied. Here, we report the purification, crystallization and preliminary X-ray analysis of the intact form of UspE from E. coli.
2. Materials and methods
2.1. Construction of the UspE expression plasmid
To construct the plasmid expressing UspE, the DNA fragment encoding E. coli O157:H7 UspE (residues 1–316) was amplified from the genomic DNA of E. coli O157:H7 strain by PCR using standard methods and the following oligonucleotides: 5′-GGGCCATGGACATGGCTATGTATCAGAAC-3′ and 5′-GGGCTCGAGTAAATCGTCTTCTTCGTC-3′. The PCR product for the fragment was digested and ligated into the NcoI and XhoI sites of pPROEX-HTA (Invitrogen, USA) vector, which contains a hexahistidine tag-containing region (Met-Ser-Tyr-Tyr-His-His-His-His-His-His-Asp-Tyr-Asp-Ile-Pro-Thr-Thr) and a TEV protease cleavage site (Glu-Asn-Leu-Tyr-Phe-Gln) at the N-terminus. The resulting plasmid (pPROTEXHTA-UspE) expressed a hexahistidine tag and a TEV protease cleavage site at the N-terminus of UspE.
2.2. Expression and purification of the recombinant UspE protein
The recombinant plasmid pPROEXHTA-UspE was transformed into E. coli BL21(DE3) cells to produce the UspE protein. The cells were grown in 1.5 l LB broth with 50 µg ml−1 ampicillin at 310 K until an OD600 of 0.6–0.8 was reached. Protein expression was induced by adding 0.5 mM isopropyl β-d-1-thiogalactopyranoside to the culture at 303 K. Cells were harvested by centrifugation 6 h after induction and were then stored at 193 K until use. The harvested cells were suspended in a lysis buffer consisting of 20 mM Tris pH 8.0, 150 mM NaCl, 2 mM β-mercaptoethanol and were disrupted by sonication. The lysate was centrifuged at 45 000g for 30 min at 277 K. The resulting supernatant was mixed with Ni–NTA affinity resin (Qiagen, Netherlands) that had been pre-incubated with Tris buffer; the mixture was then stirred for 30 min at 4°C. After the slurry had been loaded into the column, unbound proteins were washed out with lysis buffer supplemented with 20 mM imidazole. Recombinant UspE with the hexahistidine tag was eluted with 30 ml Tris buffer supplemented with 200 mM imidazole. The eluted fractions were analyzed by SDS–PAGE. Fractions containing UspE were pooled and β-mercaptoethanol was added to a final concentration of 10 mM. This solution was incubated with recombinant TEV protease overnight at 298 K to remove the hexahistidine tag. The UspE was diluted threefold with 20 mM Tris pH 8.0 buffer and then loaded onto a HiTrap Q column (GE Healthcare, USA). The protein was eluted from the column using a linear gradient of 0–1 M NaCl in 20 mM Tris buffer pH 8.0. The collected fractions containing the UspE protein were concentrated using Centriprep (Millipore, USA) and separated on a HiLoad Superdex 200 gel-filtration column (GE Healthcare, USA) pre-equilibrated with lysis buffer. Total proteins were analyzed by SDS–PAGE on a 15% gel and stained with Coomassie Blue.
2.3. Crystallization and crystallographic data collection
Prior to crystallization, the pooled sample of purified UspE was concentrated to 10 mg ml−1 using a Vivaspin centrifugal concentrator fitted with a 10 kDa molecular-weight cutoff filter (Millipore, USA). Initial crystallization of UspE was performed with Crystal Screen HT, a high-throughput sparse-matrix screening kit (Hampton Research, USA), using the microbatch method at various temperatures (277, 279, 288 and 295 K). Crystals of the recombinant UspE protein were obtained by vapour diffusion, but only at 288 K. X-ray diffraction data were collected using an ADSC Q310 CCD detector on beamline 7A of Pohang Light Source (PLS), Republic of Korea.
3. Results and discussion
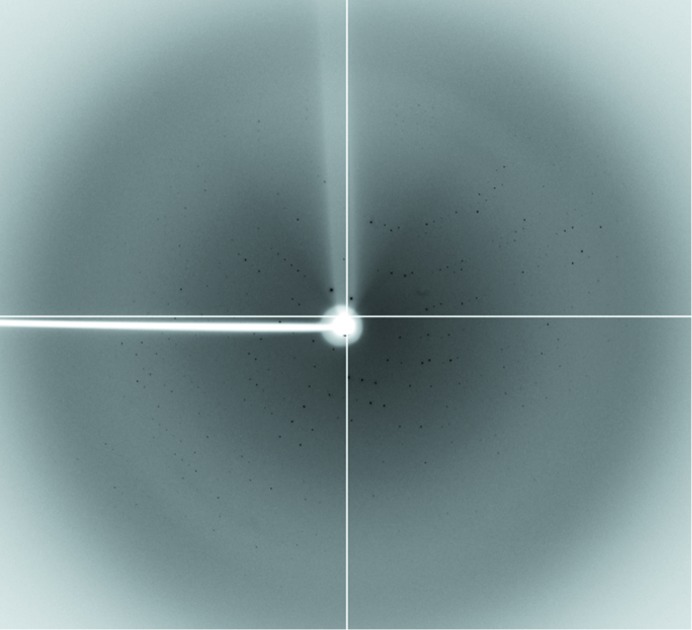
The recombinant full-length UspE protein was successfully expressed at 30°C in the cytosol of E. coli and the protein was purified to a sufficient level for crystallization using the protein-purification methods described in §2. The purity of the protein was determined by Coomassie-stained SDS–PAGE (Fig. 1 ▶). The protein sample successfully crystallized under several conditions in the initial crystallization screening trials using the sitting-drop vapour-diffusion method. The crystallization conditions were optimized to produce shiny single crystals (Fig. 2 ▶) in droplets consisting of 1 µl protein solution (10 mg ml−1 protein in 20 mM Tris buffer pH 8.0 containing 150 mM NaCl) and 1 µl precipitant solution consisting of 10% 1,4-dioxane, 1.6 M ammonium sulfate, 0.1 M MES pH 6.5. The droplets were equilibrated by the sitting-drop vapour-diffusion method against 1 ml precipitant solution at 288 K for 7 d. To collect the diffraction data set, the crystals were transferred into a cryoprotectant solution consisting of reservoir solution supplemented with 25%(v/v) glycerol. The best crystals diffracted to approximately 3.0 Å resolution (Fig. 3 ▶) and belonged to the tetragonal space group I4122 or I4322, with unit-cell parameters a = b = 121.1, c = 241.7 Å, and diffracted to a maximum resolution of 3.2 Å. In particular, analysis of the diffraction along the h, k, l axes clearly demonstrates that the c axis of the crystal is the 41 or 43 axis. The diffraction data set had a resolution range of 38–3.2 Å with 92.7% completeness and an R merge of 15.3 at the remote wavelength (Table 1 ▶). Complete data statistics are given in Table 1 ▶. In order to solve the structure, molecular replacement was attempted with MOLREP (Winn et al., 2011 ▶) using the structure of P. mirabilis PMl1202 (78.5% sequence identity; PDB entry 3olq; Midwest Center for Structural Genomics, unpublished work) as the search model. There were two molecules in the asymmetric unit, and the corresponding Matthews coefficient (Matthews, 1968 ▶) and solvent content are 3.10 Å3 Da−1 and 60.37%, respectively. Model building and structure refinement are under way.
Figure 1.
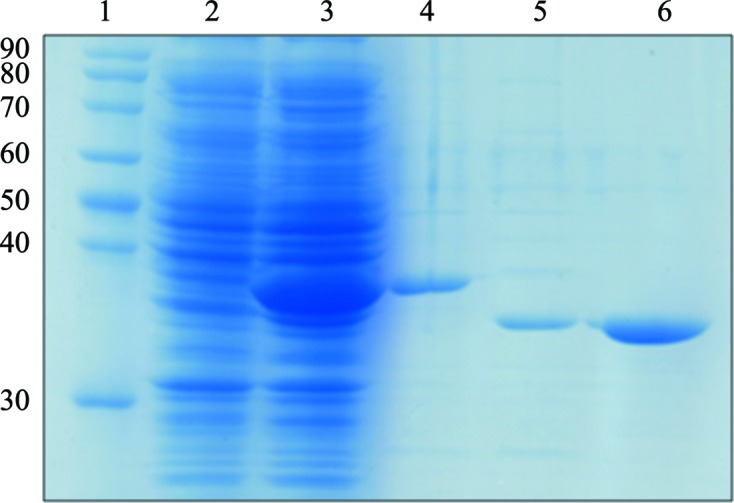
SDS–PAGE of purified UspE. Lane 1, molecular-weight markers (labelled in kDa); lane 2, uninduced cell supernatant; lane 3, 0.5 mM IPTG-induced cell supernatant; lane 4, pooled fractions eluted from a nickel-ion affinity column; lane 5, after treatment with TEV protease; lane 6, final sample.
Figure 2.
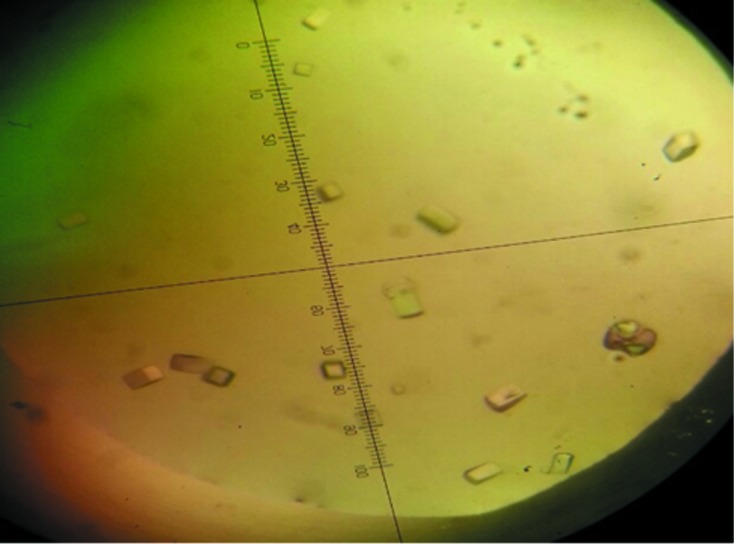
Crystals of E. coli UspE. The approximate dimensions of the crystals are 0.09 × 0.09 × 0.2 mm.
Figure 3.
Representative diffraction pattern from a crystal of UspE. The diffraction pattern was displayed using MOSFLM (Battye et al., 2011 ▶). The resolution at the edge of the image is 3.0 Å.
Table 1. Diffraction statistics.
| X-ray source | Beamline 7A, PLS |
| Space group | I4122 or I4322 |
| Wavelength () | 1.000 |
| Resolution () | 383.2 |
| Unit-cell parameters (, ) | a = b = 121.1, c = 241.7, = = = 90 |
| Completeness (%) | 92.7 (90.2) |
| R merge † (%) | 15.3 (33.4) |
| Multiplicity | 5.3 (3.6) |
| Average I/(I) | 9.2 (3.3) |
R
merge = 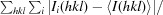
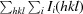 , where I
i(hkl) is the intensity of the ith measurement of reflection hkl,
, where I
i(hkl) is the intensity of the ith measurement of reflection hkl, 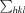 is the sum over all reflections and
is the sum over all reflections and 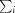 is the sum over i measurements of reflection hkl.
is the sum over i measurements of reflection hkl.
Acknowledgments
The authors acknowledge the use of beamline 7A at Pohang Light Source (PLS), Pohang, Republic of Korea. We are grateful to Jin-Sik Kim for help during data collection. This work was supported by the National Natural Science Foundation of China (Grant No. 31200556 to YX and Grant No. 21272031 to C-SQ) and the Fundamental Research Funds for the Central Universities (Grant No. DC13010308 to YX).
References
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Diez, A., Gustavsson, N. & Nyström, T. (2000). Mol. Microbiol. 36, 1494–1503. [DOI] [PubMed]
- Gustavsson, N., Diez, A. & Nystrom, T. (2002). Mol. Microbiol. 43, 107–117. [DOI] [PubMed]
- Kvint, K., Nachin, L., Diez, A. & Nyström, T. (2003). Curr. Opin. Microbiol. 6, 140–145. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 481–497.
- Nachin, L., Nannmark, U. & Nyström, T. (2005). J. Bacteriol. 187, 6265–6272. [DOI] [PMC free article] [PubMed]
- Nyström, T. & Neidhardt, F. C. (1994). Mol. Microbiol. 11, 537–544. [DOI] [PubMed]
- Siegele, D. A. (2005). J. Bacteriol. 187, 6253–6254. [DOI] [PMC free article] [PubMed]
- Sousa, M. C. & McKay, D. B. (2001). Structure, 9, 1135–1141. [DOI] [PubMed]
- Tkaczuk, K. L., Shumilin, I. A., Chruszcz, M., Evdokimova, E., Savchenko, A. & Minor, W. (2013). Evol. Appl. 6, 434–449. [DOI] [PMC free article] [PubMed]
- Zarembinski, T. I., Hung, L.-W., Mueller-Dieckmann, H.-J., Kim, K.-K., Yokota, H., Kim, R. & Kim, S.-H. (1998). Proc. Natl Acad. Sci. USA, 95, 15189–15193. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.