Abstract
Background & aims
Prior cross-sectional studies have found inconsistent relationships between body mass index (BMI) and disease progression in HIV-infected individuals.
Methods
Cross-sectional and longitudinal analyses were conducted on data from a sample of 864 HIV-infected men who have sex with men (MSM) obtained from a large, nationally-distributed HIV clinical cohort.
Results
Of the 864 HIV-infected MSM, 394 (46%) were normal weight, 363 (42%) were overweight, and 107 (12%) were obese at baseline. The baseline CD4 count was 493 (SE = 9), with viral load(log10) = 2.4 (SE = .04), and 561 (65%) were virologically suppressed. Over time, controlling for viral load, HAART adherence, age, and race/ethnicity, overweight and obese HIV-infected men possessed higher CD4 counts compared to normal weight HIV-infected men. Further, overweight and obese men possessed lower viral loads compared to normal weight HIV-infected men.
Conclusions
For HIV-infected MSM, in this longitudinal cohort study, possessing a heavier than normal BMI is longitudinally associated with improved immunological health.
Keywords: HIV/AIDS, Body Mass Index, Obesity, CD4, Viral Load
Introduction
Obesity has been linked with numerous deleterious health outcomes, including hypertension, diabetes, heart disease, myocardial infarction, and stroke.1,2 In addition, among the general population, there is a relationship between obesity and development of infections.3 However, the association between obesity and disease progression among HIV-infected populations remains unclear.4
Early research from the era before highly active antiretroviral therapy (HAART), revealed relationships between being overweight and obese and having higher CD4 cell counts,5–7 and lower viral load over time.7 More recently, one cross-sectional study found that higher body mass index (BMI) was associated with increased CD4 cell counts.8 However, longitudinal (and retrospective) data have revealed contradicting results, with some reports noting comparable immunological status between normal, overweight, and obese participants’ BMI categories,9 whereas others have noted the highest CD4 counts among an overweight (but not normal, or obese) group, while still others have found that overweight and obese HIV-infected individuals have higher CD4 counts than normal or underweight peers.10 More recently, a study suggested that obese individuals had significantly smaller increases in CD4 after HAART treatment compared to normal weight individuals.11
Of the limited number of studies conducted in the HAART era, only one11 used a prospective design with HAART-experienced patients (the remaining studies were either cross-sectional, and/or conducted with HAART-naïve patients); however, this study did not include viral load as an outcome variable. Given the conflicting data, the current study was designed to BODY MASS explore the prospective relationship between BMI and disease progression (CD4 count and viral load) among a HAART-experienced sample of HIV-infected men who have sex with men (MSM).
Methods
This cohort study was conducted among patients from the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS), a previously described large, nationally-distributed HIV clinical cohort.12 CNICS consists of 8 Center for AIDS Research (CFAR) sites across the United States, and includes more than 25,000 HIV-infected individuals, dating back to 1995. Approximately every two months, participating CNICS sites upload longitudinal patient data into the CNICS Data Repository. The repository integrates comprehensive clinical data from outpatient and inpatient encounters, such as standardized HIV-related information collected at enrollment (initial clinic visit), demographic, clinical, medication, laboratory, and socioeconomic data obtained from each site’s electronic health record and other institutional data sources. The CNICS protocol was approved by each collaborating site’s respective institutional review board.
For the current study, patients were eligible for inclusion if they were 1)A man who reported having sex with other men, and 2) had completed the CNICS clinical assessment13–15 of patient-reported measures (e.g., depression, as measured by the Patient Health Questionnaire-0 (PHQ-9))16; and self-reported HAART adherence, as measured by a z-score converted composite variable).
The clinical assessment was integrated into clinical care at four participating CNICS sites (the University of Washington, the University of Alabama at Birmingham 1917 HIV/AIDS Clinic, Fenway Health, and the University of California, San Diego). Patients completed the clinical assessment using web-based survey software developed specifically for patient-based measures. Clinical data (e.g., CD4 count, viral load, height and weight) were pulled to match the date as closely as possible (e.g., 6 months or less) to which the patients completed the self-reported measures. Data from September 2005 to July 2011 were included. Viral load values and CD4 counts were obtained in the course of routine clinical care at the respective CNICS sites. Given the highly skewed nature of the unadjusted raw viral load data, a log10 transformation was calculated for viral load analyses. The most commonly used viral load assays were HIV-1 RNA Roche Ultrasensitive, HIV-1 RNA Abbott m2000 real-time, and HIV-1 RNA Branched DNA version.
BMI was operationally defined as: (weight in pounds / (height in inches * height in inches)) *703, and the following categories were created based on guidelines from the National Institutes of Health: BMI < 18.5 (underweight), BMI 18.5 to 24.9 (normal weight), BMI 25 to 29.9 (overweight), and BMI > 30 (obese).17
Statistical Analyses
Associations of BMI categories (normal weight, overweight, and obese) to CD4 and viral load were tested via one-way analyses of covariance (ANCOVAs).
Associations of BMI as a continuous variable to viral load were tested via 2-step hierarchical multiple regressions. Accordingly, age, HAART adherence, and race/ethnicity were entered into Step 1 as covariates (see below for more detail on selection of covariates). For CD4 analyses, viral load was also entered in step 1. Step 2 then included BMI. These regressions were conducted separately for CD4 count and viral load as outcomes.
The primary and most robust set of analyses were of BMI as a longitudinal predictor of CD4 and viral load. These were conducted via generalized linear mixed-effects modeling in SPSS 20. Generalized linear mixed-effects models have the advantage over traditional data analytic approaches in that power is increased due to repeated measures, and no participant is dropped from the analysis if they miss a visit/data time point. Accordingly, for this data set, the longitudinal models allow modeling the effects of BMI, over time, to viral load and CD4. The time variable provided the structure to the model with three waves of data (corresponding to baseline, 11, and 14 months post-baseline), and was entered as both a fixed (unknown constant parameter) and random (unobserved random variable) effect. Random intercepts and slopes were also entered, which allow participants to have unique growth trajectories (the trajectory of the outcome variable may vary over time from one participant to another). Time variant values of BMI were entered into the model, as fixed effects, to predict changes in CD4 count and viral load over time. The autoregressive covariance structure was chosen based on the best goodness-of-fit (as evaluated by the Akaike Information Criterion—AIC), compared to competing covariance structures. The AIC was also used to evaluate the fit of linear vs. quadratic models, with linear models fitting the data better. The restricted maximum likelihood—REML—estimation method was chosen in lieu of maximum likelihood (ML) estimation, as the former approach tends to result in unbiased estimates of the variances and covariances.18
Results
Preliminary Analyses
The analyses included 864 eligible HIV-infected MSM in care in 4 U.S. cities, of whom 394 (46%) were normal weight, 363 (42%) were overweight, and 107 (12%) were obese. Only 21 (2%) participants fell into the underweight group; given the small n, underweight participants were excluded from all further analyses. The mean age of the sample was 44 years (SD = 10), and all participants were HAART-experienced (with those being on HAART for the previous 6 months or longer). The racial/ethnic make-up of the sample was: 80% White, 10.5% Black, 2.3% Asian, and 7.5% Other; and 28.4% identified as Hispanic. The mean CD4 count at baseline was 493 (SE = 9), with viral load(log10) = 2.4 (SE = .04), and 561 (65%) being virologically suppressed.
Demographic characteristics (along with depression symptoms and HAART adherence) were examined by BMI category. A trend (p = .06) indicated that age differed by BMI categories, with overweight men tending to be older than normal weight men. White participants were disproportionally represented (p = .04) in the overweight (84%) and normal weight (79%) categories compared to the obese category (73%). Although significant BMI category differences did not emerge on the HAART adherence composite, this variable was controlled for in all subsequent analyses (along with age and race/ethnicity), given that HAART adherence is associated with both CD4 counts and viral load. Further, given that viral load is significantly negatively correlated with CD4, in analyses in which CD4 was the outcome variable, viral load was statistically controlled.
Cross-sectional Analyses
BMI category and CD4
A one-way ANCOVA was conducted examining BMI category differences at baseline on CD4 count, controlling for age, race/ethnicity, adherence, and viral load. Results revealed a significant main effect for BMI category, F(2, 848) = 5.3, p = .005, with Fisher LSD posthoc tests indicating that obese (M = 545, SE = 24) and overweight (M = 506, SE = 13) men possessed higher CD4 counts than normal weight men (M = 463, SE = 13). Overweight and obese men did not significantly differ from each other.
Continuous BMI and CD4
BMI explained significant variance in CD4 counts, ΔR2 = .019, ΔF(1, 671) = 14.3, p < .0001, B = 8.7, t = 3.8, p < .0001, indicating that a 1 unit increase in BMI was associated with a 8.7 unit increase in CD4 count.
BMI group and viral load
A one-way ANCOVA was conducted examining BMI category differences at baseline on plasma viral load (see Table 2). Results revealed a non-significant main effect for BMI group, F(2, 652) = 2.0, p = .13.
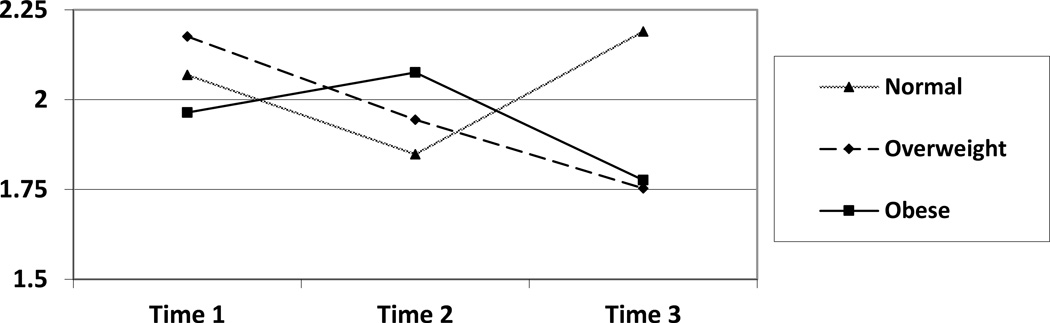
Table 2.
Means and Standard Errors of Viral Load by BMI Group and Time
| Time | BMI Group | Mean | Standard Error |
|---|---|---|---|
| Normal | 2.1 | .05 | |
| 1 | Overweight | 2.2 | .06 |
| Obese | 2.0 | .07 | |
| Normal | 1.8 | .06 | |
| 2 | Overweight | 2.0 | .09 |
| Obese | 2.1 | .17 | |
| Normal | 2.2 | .17 | |
| 3 | Overweight | 1.8 | .10 |
| Obese | 1.8 | .05 |
Note. Time 1 n = 885, Time 2 n = 225, Time 3 n = 48
Continuous BMI and viral load
BMI did not explain significant variance in viral load, (ΔR2 = .002, ΔF(1, 871) = 2.1, p = .15), indicating that BMI measured continuously was not significantly associated with viral load cross-sectionally.
Longitudinal Analyses
Viral load and CD4 over time
Initially, unconstrained models predicting viral load and CD4 count, respectively, over time were conducted. Results indicated that viral load significantly decreased over the course of the study (γ = −.25, SE = .06, 95% CI [−.37, −.14], t416 = −4.3, p < .0001). CD4 count significantly increased over the course of the study (γ = 31.1, SE = 8.1, 95% CI [15.1, 47.1], t171 = 3.8, p < .0001).
CD4
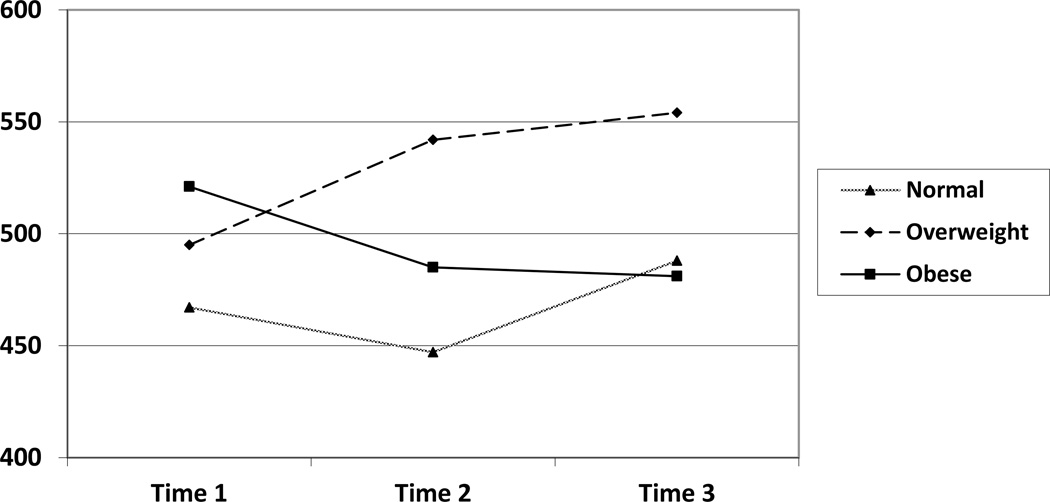
When analyzing CD4 count as the outcome variable, there was a significant main effect for BMI category, F(2, 318) = 3.7, p = .027 (see Table 1 and Figure 1). To follow-up this significant main effect, Fisher’s LSD analyses were conducted. Results revealed that overweight men (M = 514, SE = 18) possessed higher CD4 counts compared to normal weight men (M = 451, SE = 18), and neither group significantly differed from obese men (M = 476, SE = 39). Given that the interaction between BMI category and time was non-significant, F(4, 178) = 2.1, p = .09, this finding indicates that the BMI category differences did not vary as a function of time.
Table 1.
Means and Standard Errors of CD4 Count by BMI Group and Time
| Time | BMI Group | Mean | Standard Error |
|---|---|---|---|
| Normal | 467 | 14 | |
| 1 | Overweight | 495 | 15 |
| Obese | 521 | 26 | |
| Normal | 447 | 21 | |
| 2 | Overweight | 542 | 22 |
| Obese | 485 | 36 | |
| Normal | 488 | 36 | |
| 3 | Overweight | 554 | 42 |
| Obese | 481 | 54 |
Note. Time 1 n = 885, Time 2 n = 225, Time 3 n = 48
Figure 1.
CD4 Count by BMI Group Over Time
Note. Time 1 n = 885, Time 2 n = 225, Time 3 n = 48
These procedures were repeated with the continuous BMI variable as the predictor. Results revealed a significant effect for BMI, γ = 7.4, SE = 2.1, 95% CI = [3.2, 11.5], t(830) = 3.5, p < .0001, indicating that a one unit increase in BMI was associated with a +7.4 change in CD4 count.
Viral load
Analogous procedures were conducted with respect to plasma viral load as the outcome variable. No significant main effects for BMI category or time were revealed; however, a significant BMI category by time interaction emerged, F(4, 872) = 2.7, p = .03 (see Figure 2). To follow-up this significant interaction, Fisher LSD analyses were conducted. Results indicated a significant BMI category difference at time 3, with obese (M = 1.8, SE = .05), t(872) = −2.4, p = .02, and overweight men (M = 1.7, SE = .10), t(872) = −2.2, p = .03, possessing lower viral loads than normal weight men (M = 2.2, SE = .17).
Figure 2.
Viral Load by BMI Group Over Time
Note. Time 1 n = 885, Time 2 n = 225, Time 3 n = 48
The model was conducted again with a continuous BMI variable as the predictor. Results revealed a non-significant effect for BMI, γ = −.04, SE = .04, 95% CI = [.12, .05], t(613) = −.90, p = .39.
Discussion
In the general population, multiple negative effects of being overweight or obese have been well-documented.1–2 Prior studies have suggested that obese individuals have poorer immune functioning.3 However, the role of obesity (and BMI in general) among HIV-infected individuals remains unclear. This is a salient issue, as obesity among HIV-infected individuals has increased with trends largely mirroring those of the general population.10 Because HIV-infected individuals already possess some level of immunocompromise and chronic inflammation, understanding the relationship of weight to disease progression is of importance.
The main findings from the current study indicated that over time, overweight HIV-infected MSM possessed higher CD4 counts than normal weight HIV-infected MSM (neither category statistically differed from obese men in the longitudinal analysis). Among the handful of studies conducted in the HAART era, findings regarding obesity and CD4 count have been varied and contradictory.9,11,19 However, the current study is one of only two that assessed this relationship prospectively, over several time points.
The current study also assessed viral load, which is novel among the HAART era studies published on this topic. Prospectively, overweight and obese men possessed significantly lower viral loads compared to normal weight men. Given that men with elevated BMI possessed higher CD4 counts, perhaps it is not surprising that their viral loads were also relatively lower compared to normal weight men. Indeed, there is a strong negative association between CD4 and viral load.
Despite the novel findings from the current study, that heavier than normal HIV-infected patients have better control of their HIV infection, the behavioral and/or pathophysiological mechanism is not clear. It is plausible that leptin may be involved in the pathway of overweight and obesity to immune functioning among HIV-infected individuals. Leptin is an adipocyte-derived hormone that influences body weight (among other biological functions).20 Overweight and obese individuals have been found to have higher levels of serum leptin compared to normal weight individuals.20,21 Among some samples, increased levels of leptin have been found to be related to improved immunological health.22,23 Although obesity in the general population has been linked to worse immune functioning, among HIV-infected individuals, if there is actually a protective effect, it may be that higher concentrations of leptin provide a specific protective function by improving CD4 count, as leptin treatment has been associated with T-cell proliferation among mice23 and humans.22 However, some studies among HIV-infected individuals24,25 have failed to find a relationship between leptin treatment and CD4 count or viral load, although these studies were not designed to test intervention effects on disease progression, per se. Clearly, further studies evaluating the role of leptin and T-cell number and function in HIV-infected individuals are warranted to evaluate this hypothesis further.
Alternative explanations may be considered. Two variables that were not fully assessed in this study were the CD4 nadir when patients initiated HAART and their duration of treatment. If normal weight patients were more immunosuppressed when they initiated treatment, their ability to control viral replication and to restore immune function would be compromised, limiting their ability to achieve higher CD4 counts compared to patients who started treatment at higher CD4 counts. However, supplemental analyses revealed non-significant differences at baseline, and at each time point, in virological suppression as a function of BMI category.
Although the current study yielded novel findings, it is not without limitations. For instance, the nature of the sample may limit generalizability of the results, as the sample exclusively constituted MSM, and was primarily White. Thus, translating these findings to women, and non-White races should be done with caution. It is also quite possible that the patients in the CNICS cohort, by virtue of using an electronic medical record (from which the data were obtained from) have a higher degree of integration of care compared to that of the typical HIV-infected patient.
In summary, prospective analyses found that elevated BMI among HIV-infected MSM is associated with better CD4 counts and viral load compared to normal weight men. It is important to note that being overweight and obese is associated with a plethora of health problems; although these findings highlight the possibility that elevated BMI may have a specific protective factor for HIV-infected MSM, it does not suggest that overweight and obesity should be clinically ignored (or for that matter encouraged) in MSM living with HIV. However, future research is needed to understand the casual mechanisms of being overweight and obese and disease progression among this population, perhaps through the role of increased levels of serum leptin and other potential immune factors.
Acknowledgements
A.J.B. carried out data analysis and drafted the manuscript. K.H.M., S.A.S., and H.M.C. participated in the design of the study and provided editorial comments. C.G. provided and managed the data. All authors read and approved the final manuscript. Data collection and infrastructure support for this research is from the CFAR Network of Integrated Clinical Systems-CNICS, an NIH funded program (R24AI067039) that was made possible by the National Institute of Allergy and Infectious Diseases (NIAID). Some investigator time for analysis and authorship was supported by the National Institutes of Health [K24MH094214 to S.A.S.], [R01MH084759 to H.C.], [K23MH096647 to A.J.B.], and the Harvard University Center for AIDS Research/National Institutes of Health [5P30AI060354 to A.J.B.]. The study sponsors had no involvement in the design, collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
The authors have no known conflicts of interest.
References
- 1.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;6:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 2.Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obes Rev. 2001;2:131–140. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 4.Keithley JK, Duloy AM, Swanson B, Zeller JM. HIV infection and obesity: a review of the evidence. J Assoc Nurses AIDS Care. 2009;20:260–274. doi: 10.1016/j.jana.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Jones CY, Hogan JW, Snyder B, Klein RS, Rompalo A, Schuman P, Carpenter CC HIV Epidemiology Research Study Group. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis. 2003;37:S69–S80. doi: 10.1086/375889. [DOI] [PubMed] [Google Scholar]
- 6.Shor-Posner G, Campa A, Zhang G, Persaud N, Miguez-Burbano MJ, Quesada J, Fletcher MA, Page JB, Baum MK. When obesity is desirable: a longitudinal study of the Miami HIV-1-infected drug abusers (MIDAS) cohort. J Acquir Immune Defic Syndr. 2000;23:81–88. doi: 10.1097/00126334-200001010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Shuter J, Chang CJ, Klein RS. Prevalence and predictive value of overweight in an urban HIV care clinic. J Acquir Immune Defic Syndr. 2001;26:291–277. doi: 10.1097/00042560-200103010-00013. [DOI] [PubMed] [Google Scholar]
- 8.Quach LA, Wanke CA, Schmid CH, Gorbach SL, Mwamburi DM, Mayer KH, Spiegelman D, Tang AM. Drug use and other risk factors related to lower body mass index among HIV-infected individuals. Drug Alcohol Depend. 2008;95:30–36. doi: 10.1016/j.drugalcdep.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tedaldi EM, Brooks JT, Weidle PJ, Richardson JT, Baker RK, Buchacz K, Moorman AC, Wood KC, Holmberg SD The HOPS Investigators. Increased body mass index does not alter response to initial highly active antiretroviral therapy in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2006;43:35–41. doi: 10.1097/01.qai.0000234084.11291.d4. [DOI] [PubMed] [Google Scholar]
- 10.Crum-Cianflone N, Tejidor R, Medina S, Barahona I, Ganesan A. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS. 2008;22:925–930. doi: 10.1089/apc.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crum-Cianflone NF, Roediger M, Eberly LE, Ganesan A, Weintrob A, Johnson E, Agan BK Infectious Disease Clinical Research Program HIV Working Group. Impact of weight on immune cell counts among HIV-infected persons. Clin Vaccine Immunol. 2011;18:940–946. doi: 10.1128/CVI.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, Lober WB, Van Rompaey SE, Crane HM, Moore RD, Bertram M, Kahn JO, Saag MS. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37:948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredericksen R, Crane PK, Tufano J, Ralston J, Schmidt S, Brown T, Layman D, Harrington RD, Dhanireddy S, Stone T, Lober W, Kitahata MM, Crane HM. Integrating a web-based, patient-administered assessment into primary care for HIV-infected adults. J Acquir Immune Defic Dyndr. 2012;4:47–55. doi: 10.5897/jahr11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence ST, Willig JH, Crane HM, Ye J, Aban I, Lober W, Nevin CR, Batey DS, Mugavero MJ, McCullumsmith C, Wright C, Kitahata M, Raper JL, Saag MS, Schumacher JE. Routine, self-administered, touch-screen, computer-based suicidal ideation assessment linked to automated response team notification in an HIV primary care setting. Clin Infect Dis. 2010;50:1165–1173. doi: 10.1086/651420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crane HM, Lober W, Webster E, Harrington RD, Crane PK, Davis TE, Kitahata MM. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Curr HIV Res. 2007;5:109–118. doi: 10.2174/157016207779316369. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer R, Kroenke K, Williams J. Validation and utility of a self-report version of PRIME-MD: The PHQ Primary Care Study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 17.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162:2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 18.West BT. Analyzing longitudinal data with the linear mixed models procedure in SPSS. Eval Health Prof. 2009;32:207–228. doi: 10.1177/0163278709338554. [DOI] [PubMed] [Google Scholar]
- 19.Koethe JR, Jenkins CA, Shepherd BE, Stinnette SE, Sterling TR. An optimal body mass index range associated with improved immune reconstitution among HIV-infected adults initiating antiretroviral therapy. Clin Infect Dis. 2011;53:952–960. doi: 10.1093/cid/cir606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sørensen TI, Echwald S, Holm JC. Leptin in obesity. BMJ. 1996;313:953–954. doi: 10.1136/bmj.313.7063.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantzoros CS. The role of leptin in human obesity and disease: A review of current evidence. Ann Intern Med. 1999;130:671–680. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 22.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SY, Lim JH, Choi SW, Kim M, Kim ST, Kim MS, Cho YS, Chun E, Lee KY. Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem Biophys Res Commun. 2010;394:562–568. doi: 10.1016/j.bbrc.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Mulligan K, Khatami H, Schwarz JM, Sakkas GK, DePaoli AM, Tai VW, Wen MJ, Lee GA, Grunfeld C, Schambelan M. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypolepinemia. J Clin Endocrinol Metab. 2009;94:1137–1144. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improved insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91:2605–2611. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]