Abstract
Background
The cytokine IL-9 has been implicated in allergic reactions, including food allergy, but its contribution to parenteral versus oral antigen–induced anaphylaxis remains unclear.
Objectives
We sought to delineate the contribution of the IL-9/IL-9 receptor a-chain (IL-9R) pathway to parenteral and oral antigen–induced anaphylaxis.
Methods
Wild-type, IL-9–deficient (Il92/2), and IL-9R–deficient (Il9R2/2) mice were subjected to passive and active parenteral and oral antigen (ovalbumin [OVA])-induced anaphylaxis. Severity of systemic anaphylaxis was gauged by decreased body temperature; intestinal anaphylaxis was assessed based on secretory diarrhea, intestinal mastocytosis, and serum murine mast cell protease 1 level. Specific immunoglobulin isotypes or immunoglobulin receptor–blocking antibodies were administered before challenge to define the role of the IgE and IgG pathways.
Results
Repeated oral antigen challenge of OVA-sensitized wild-type mice induced anaphylaxis with both systemic and intestinal involvement; both were IgE dependent and attenuated in Il92/2 and Il9R2/2 mice. In contrast, parenteral OVA challenge of OVA-sensitized wild-type mice induced systemic anaphylaxis, which was independent of the IL-9/IL-9R pathway. Strikingly, the IL-9/IL-9R pathway had no role in either the IgG or IgE component of parenteral antigen–induced or anti-IgE and anti-FcgRII/III mAb–induced systemic anaphylaxis.
Conclusions
Parenteral antigen–induced murine systemic anaphylaxis is mediated by both IgG- and IgE-dependent pathways, and both can occur independently of IL-9/IL-9R signaling. In contrast, oral antigen–induced intestinal and systemic anaphylaxis is strictly IgE mediated and requires IL-9/IL-9R signaling. These studies indicate differential involvement of the IL-9/IL-9R pathway in systemic and oral antigen–induced anaphylaxis.
Keywords: Cytokine, anaphylaxis, mast cells, IgE
Anaphylaxis is a rapid, life-threatening allergic reaction often triggered by food, drugs, insect venoms, latex, and allergen immunotherapy.1–5 Early clinical and experimental evidence suggested that systemic anaphylaxis was mediated by IgE/mast cell degranulation and the rapid release of secondary mediators, including histamine, proteoglycans, platelet-activating factor (PAF), serotonin, tryptase, chymase, and lipid-derived mediators (prostaglandin D2 and leukotriene [LT] C4, LTD4, and LTE4).6–10 These mediators are thought to act on target cells to induce vasodilation, increased vascular permeability and hypotension, bronchospasm, and, as a result, shock. Although substantial evidence supports a central role for the classical IgE/mast cell pathway in human anaphylaxis, circumstantial evidence supports the possibility that human anaphylaxis can also be mediated by an IgG-dependent alternate pathway that has been well defined in mice.6 Notably, anaphylactic reactions have been described in patients in the absence of detectable allergen-specific serum IgE antibody and no detectable evidence of mast cell degranulation (ie, no increase in serum tryptase levels).12–14
Experimental studies in rodent models of systemic anaphylaxis have provided evidence that the “classical pathway” involves cross-linking of IgE-bound FceRI on mast cells, leading to the degranulation and release of PAF and histamine.6 Mice deficient in components of mast cell and IgE function, including IL-4/IL-4 receptor a, mast cells, FceRI, and IgE, are protected against IgE-mediated anaphylaxis.7 The alternate pathway involves IgG, FcgRIII, macrophages and basophils, and PAF.6,8 Blockade of FcgRIII, macrophages, or basophils or antagonism of PAF has been shown to suppress this pathway.7
TH2 cytokines (IL-4, IL-5, IL-9, and IL-13) can augment anaphylaxis. Indeed, peripheral blood and intestinal tissue from patients with food allergy contain increased numbers of activated T cells, which correlate with increased levels of TH2 cytokines and the degree of gastrointestinal inflammation and dysfunction.9,10 Furthermore, in vitro allergen-stimulated T cells and T-cell clones generated from patients with food allergy produce TH2 cytokines.11 IL-9 is a CD41 TH2-derived pleiotropic cytokine that has recently been implicated in the regulation of allergic-inflammatory reactions.12 IL-9 signals through the IL-9 receptor, which is composed of the IL-9–specific a-chain (Il9R) and the common g-chain (gc) that is also a component of the IL-2, IL-4, IL-7, and IL-15 receptors. Transgenic airway expression of IL-9 promotes TH2-mediated allergic pulmonary disease characterized by increased TH2 cytokine levels, mucus hypersecretion, and bronchial hyperresponsiveness.13,14 Further-more, IL-9 enhances IL-4–induced IgE production,15–17 airway epithelial cell–derived CC chemokine expression, and mast cell maturation, survival, recruitment, and effector function.12
Recently, there has been a controversy with respect to the involvement of IL-9 in anaphylaxis.18,19 We have previously demonstrated that anaphylaxis after antigen (ovalbumin [OVA]) sensitization and subsequent oral gavage challenge was dependent on IL-9.18 In contrast, IL-9 was shown to promote but not be necessary for anaphylaxis induced by means of intravenous administration of OVA to OVA-sensitized mice.19 To date, no possible explanation for this apparent discrepancy has been put forth; however, one important difference between these analyses was the route of antigen challenge. In the present study we have used Il92/2 and Il9R2/2 mice and models of parenteral antigen–induced and oral antigen–induced systemic anaphylaxis to test the hypothesis that IL-9 is important for ingested antigen-and not parenteral antigen–induced anaphylaxis. We show that oral antigen–induced intestinal and systemic anaphylaxis is strictly IgE and mast cell mediated and is critically dependent on IL-9/IL-9 receptor a-chain (IL-9R) signaling. In contrast, parenteral antigen–induced anaphylaxis is mediated through both IgG- and IgE-dependent pathways, and both pathways can induce anaphylaxis independently of IL-9/IL-9R signaling. These observations identify distinct roles for the IL-9/IL-9R pathway in parenteral and oral antigen–induced systemic anaphylaxis.
METHODS
Mice
Four- to 8-week-old Il92/2 mice (N10 BALB/c)20 were a gift from Andrew McKenzie (MRC, Laboratory of Molecular Biology, Cambridge, United Kingdom). Il9R2/2 mice (N10 BALB/c) were obtained from Jean-Christophe Renauld (Ludwig Institute for Cancer Research, Brussels, Belgium).19 Wild-type (WT) BALB/c mice were obtained from the National Cancer Institute (Bethesda, Md). All mice were maintained in a barrier facility, and animals were handled under International Animal Care and Use Committee–approved protocols from the Cincinnati Children’s Hospital Medical Center.
Reagents
Hybridomas were obtained from the following sources: 2.4G2 (rat IgG2b anti-mouse FcgRII/III mAb) from ATTC (Rockville, Md),21 EM-95 (rat IgG2a anti-mouse IgE mAb)22 from Zelig Eshhar (Rehoveth, Israel), and rat IgG2a (GL117) and rat IgG2b (J1.2) control mAbs from Dr John Abrams (DNAX Research Institute, Calif). The anti-IgE antibody (clone EM95) recognizes a domain of the e-chain of IgE that is not blocked by the binding of IgE to FceRI.23 The anti-FcgRII/III antibody (clone 2.4G2) is specific for the extra-cellular domains of murine FcgRIIB and FcgRIII.21 The mAbs were purified from ascites produced by the hybridomas in pristane-primed athymic nude mice by means of ammonium sulfate precipitation followed by DEAE (diethylaminoethyl) cellulose ion exchange chromatography, as previously described.24 All antibodies were diluted in normal saline to a final volume of 200 mL per mouse, unless otherwise stated.
Passive anaphylaxis model
Mice were injected intravenously with 10 mg of anti-FceRI (EM95) or 500 mg of anti-FcgRII/III (2.4G2) or control immunoglobulin (GL117: 10 mg/200 mL of saline or J1.2: 500 mg/200 mL of saline), and the severity of shock was assessed by means of rectal temperature with a rectal probe (Physitemp Model BAT-12; Physitemp Instruments, Inc, Clifton, NJ).25,26
Active systemic antigen-induced anaphylaxis
WT, Il92/2, and Il9R2/2 mice were immunized intraperitoneally with OVA (Grade V; Sigma Aldrich, St Louis, Mo; 9.3 ng of endotoxin/mg OVA)/alum (50 mg OVA/1 mg alum/200 mL) or alum alone on day 0. On day 14, mice were intravenously challenged with 100 mg of OVA in 200 mL of saline. Rectal temperatures were measured prior to and 5, 10, 15, 30, 45, and 60 minutes following OVA challenge. As controls, naive WT mice were intravenously administered anti-IgE or isotype control or OVA (100 mg/200 mL of saline). The active anaphylactic model is characterized by a pronounced shock response, as observed by the rapid and significant temperature decrease. All experiments were performed out to 60 minutes; however, in some instances (approximately 20%) mice died or were sacrificed prior to the 60-minute time point because of hypovolemia and loss of consciousness. We observed no differences among the WT, Il92/2, and Il9R2/2 genotypes. Thus we only included the temperature loss values up to 45 minutes because all mice were conscious at this time point.
Experimental oral antigen–induced intestinal anaphylaxis
Four- to 8-week-old mice were sensitized twice, 2 weeks apart, with 50 mg of OVA/1 mg of alum in sterile saline by means of intraperitoneal. injection. Two weeks later, mice were held in the supine position 3 times a week (every other day) and orally gavaged with 250 mL of OVA (50 mg) in saline. Before each intragastric challenge, mice were deprived of food for 3 to 4 hours. Challenges were performed with intragastric feeding needles (01-290-2B; Fisher Scientific Co, Pittsburgh, Pa). Rectal temperatures were measured before and 60 minutes after OVA challenge. Diarrhea was assessed by visually monitoring mice for up to 60 minutes after intragastric challenge. Mice demonstrating profuse liquid stool were recorded as diarrhea positive.
Blockade of IgG- and IgE-mediated anaphylaxis
Mice were injected intravenously with 10 mg of anti-IgE or 500 mg of anti-FcgRII/III mAb in saline 24 hours before challenge experiments to deplete IgE and desensitize mast cells or block FcgRII/III.21,23 Concentrations of anti-IgE and anti-FcgRII/III mAb were determined in preliminary dose-response experiments and also as we have previously demonstrated.7,25 These treatments induce a mild form of anaphylaxis from which mice rapidly recover while depleting serum and cell-bound IgE or blocking FcgRII and FcgRIII for several days.21,23
ELISA measurements
Mcpt-1 serum levels were measured by means of ELISA, according to the manufacturer’s instructions (Moredun Scientific, Midlothian, United Kingdom, and Beckman Coulter, Fullerton, Calif). Serum total and OVA-specific IgE and IgG1 levels were determined by means of ELISA. Plates were coated with 100 mL of OVA (100 mg/mL) and anti-IgE antibody (EM-95; 10 mg/mL; BD PharMingen, San Jose, Calif) or rat anti-mouse IgG1 (A85-1; BD Biosciences PharMingen, San Jose, Calif) and blocked with 200 mL of 10% FBS before adding serial dilutions of plasma samples (100 mL per well). After overnight incubation, plates were washed and incubated with either horseradish peroxidase–conjugated anti-mouse IgG1 (X56; 0.5 mg/mL; BD Biosciences PharMingen, San Jose, Calif), biotinylated OVA (2.5 mg/mL, 100 mL per well), or biotin-conjugated anti-mouse IgE (0.5 mg/mL, BD Biosciences PharMingen, San Jose, Calif). After 1 hour of incubation, streptavidin–horseradish peroxidase (1 mg/mL; Biosource, Camarillo, Calif) was added for the total IgE assay. Before the initiation of each step, plates were washed with 0.05% Tween-20 in PBS. Finally, after a 1-hour incubation, 100 mL of substrate (TMB substrate reagent set; BD OptEIA, San Diego, Calif) was added. Colorimetric reaction was stopped with 1 mol/L H2SO4 and was quantified by measuring optical density with an ELISA plate reader at 450 nm.
Intestinal mast cell quantification
The tongue, ear, and jejunum (7–10 cm distal to the stomach) were fixed in 10% formalin and processed by using standard histologic techniques. The 5-mm tissue sections were stained for mucosal mast cells with chloroacetate esterase activity27 and lightly counterstained with hematoxylin. At least 4 random sections per mouse were analyzed. Quantification of stained cells was performed by counting the number of chloroacetate-positive cells from 25 to 50 fields of view (magnification 340).
Statistical analysis
Data are expressed as means 6 SEMs, unless otherwise stated. Statistical significance comparing different sets of mice was determined by using the 1-way ANOVA nonparametric and a Tukey posttest. A P value of less than .05 was considered significant. All analyses were performed with Prism 4.0 software.
RESULTS
Importance of IgE and IL-9/IL-9R in oral antigen–induced intestinal and systemic anaphylaxis
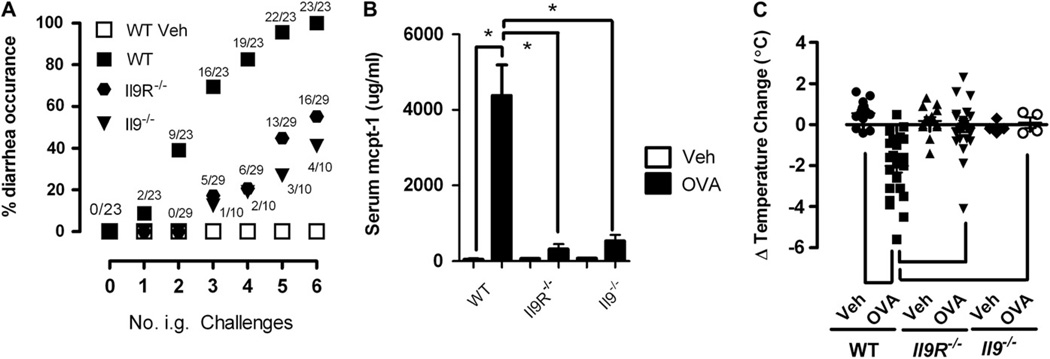
Mice were primed intraperitoneally with OVA/alum and subsequently challenged by means of oral gavage with OVA to assess the role of IL-9/IL-9R in anaphylaxis induction by ingested antigen. Consistent with our previous investigations, repeated oral gavage of OVA induced diarrhea in WT mice. Notably, the incidence of allergic diarrhea was significantly attenuated in Il92/2 and Il9R2/2 mice compared with that seen in WT mice after repeated intragastric OVA challenge (Fig 1, A). Mitigation of anaphylaxis in Il92/2 or Il9R2/2 mice was associated with reduced intestinal mastocytosis and mast cell degranulation (Fig 1, B, and see Fig E1, A, in this article’s Online Repository at www.jacionline.org) but not with reductions in either OVA-specific IgE or IgG1 levels (see Fig E1, B, and results not shown). Notably, repeated administration of OVA by means of oral gavage induced anaphylaxis with systemic involvement (hypothermia) in WT mice (Fig 1, C). Notably, WT, but not Il92/2 or Il9R2/2, mice were hypothermic 1 hour after oral gavage, indicating that oral antigen–induced intestinal and systemic involvement depends on IL-9/IL-9R signaling (Fig 1, A and C). Previous investigations have demonstrated that anaphylaxis in mice can be mediated through either an IgE pathway involving mast cells or, alternatively, a pathway involving IgG, FcgRIII, macrophages and basophils, and PAF.6,8 Mice were sensitized intraperitoneally with OVA/alum and oral gavage challenged 6 times with OVA to induce anaphylaxis (diarrhea and hypothermia) to define the role of IgE in oral antigen–induced intestinal and systemic anaphylaxis. After the sixth intragastric challenge, mice were treated with either control immunoglobulin or anti-IgE mAb to block subsequent IgE-mediated reactions.21,23 Oral antigen–induced intestinal and systemic anaphylaxis was completely IgE dependent; that is, anti-IgE treatment between intragastric OVA challenge 6 and challenge 7 totally abrogated the induction of both allergic diarrhea and hypothermia by the seventh intragastric challenge; Table I). In contrast, blocking with anti-FcgRII/RIII mAb had no effect on either allergic diarrhea or hypothermia (Table I). Thus the IL-9/IL-9R pathway is important for oral gavage–induced intestinal (diarrhea) and systemic (hypothermia) anaphylaxis.
FIG 1.
Critical role for the IL-9/IL-9R pathway in anaphylaxis induced by oral antigen challenge. A, Diarrhea occurrence in vehicle (Veh)– or OVA-sensitized intragastric (i.g.) OVA-challenged WT, Il9R2/2, and Il92/2 mice. A and B, Serum mouse mcpt-1 levels (Fig 1, B) and temperature change (Fig 1, C) from 0 to 60 minutes after the sixth intragastric vehicle or OVA challenge in OVA-sensitized WT, Il9R2/2, and Il92/2 mice. Data in Fig 1, A, are represented as a percentage of diarrhea occurrence after a number of OVA challenges. The fraction indicates the number of mice with diarrhea out of the total number of mice in that group. Data in Fig 1, B and C, are representative after the sixth intragastric vehicle or OVA challenge in OVA-sensitized WT, Il9R2/2, and Il92/2 mice. Fig 1, B, Four to 8 mice per group from n = 3 experiments. *P < .05. Fig 1, C, Individual symbols represent 1 mouse. *P < .001.
TABLE I.
Intragastric antigen–induced anaphylaxis is IgE dependent
| Oral antigen |
Challenge no. 6 | Antibody treatment (24 h before challenge no. 7) |
Challenge no. 7 |
|---|---|---|---|
| Diarrhea occurrence |
Diarrhea occurrence |
||
| Vehicle | 0/6 | 0/6 | |
| OVA | 8/8 | Ig control (10 mg) | 8/8 |
| OVA | 6/6 | Anti-IgE | 0/6* |
| OVA | 4/4 | Ig control (500 mg) | 4/4 |
| OVA | 6/6 | Anti-FcgRII/III | 6/6 |
| n Temperature (8C) | n Temperature (8C) | ||
| Vehicle | 0.700 6 0.38 | 0.70 6 0.40 | |
| OVA | 23.45 6 0.73 | Ig control (10 mg) | 23.52 6 0.73 |
| OVA | 23.58 6 0.38 | Anti-IgE | 1.12 6 0.45* |
| OVA | 23.27 6 0.62 | Ig control (500 mg) | 23.05 6 0.88 |
| OVA | 23.81 6 1.34 | Anti-FcgRII/III | 22.5 6 1.32 |
P < .0001 compared with day 6 treatment by using the Wilcoxon paired T test. OVA-sensitized mice received 6 intragastric OVA challenges. Diarrhea occurrence and change in temperature at 60 minutes were determined after the sixth intragastric challenge. Following the sixth challenge, 24 hours prior to the seventh challenge, mice were treated with control immunoglobulin (GL117 or J1.2), anti-IgE, or anti-FcgRII/III blocking antibody. Twenty four hours later the mice subsequently received the seventh intragastric OVA challenge, and diarrhea occurrence and change in temperature at 60 minutes were determined.
Assessment of IL-9/IL-9R pathway involvement in passive systemic IgE- or IgG-mediated anaphylaxis
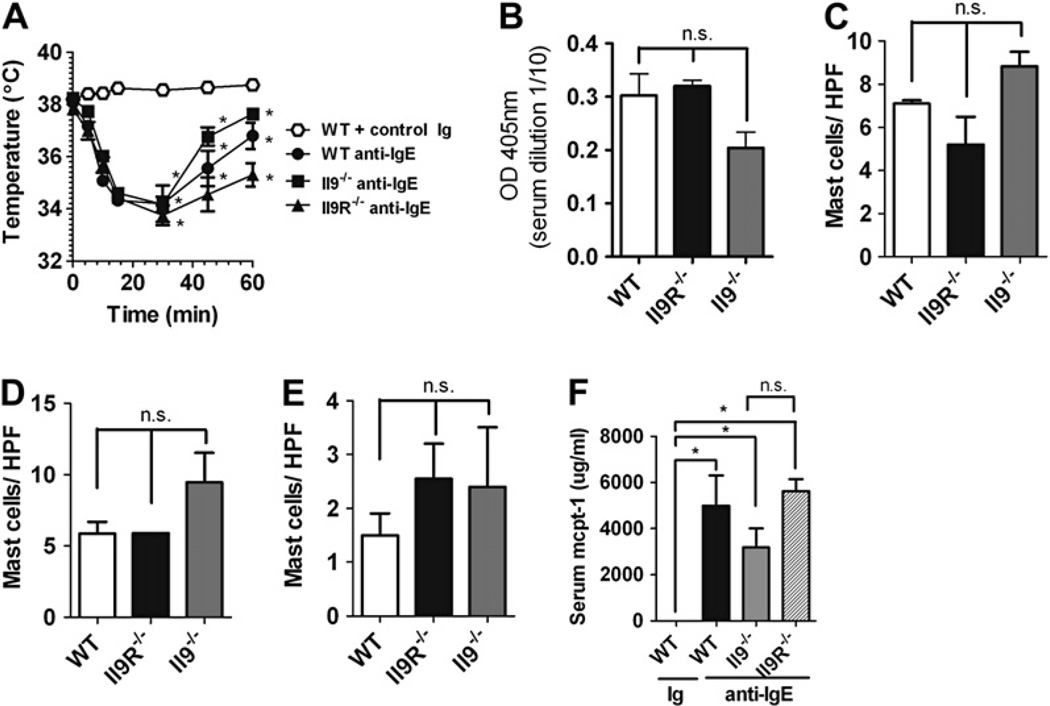
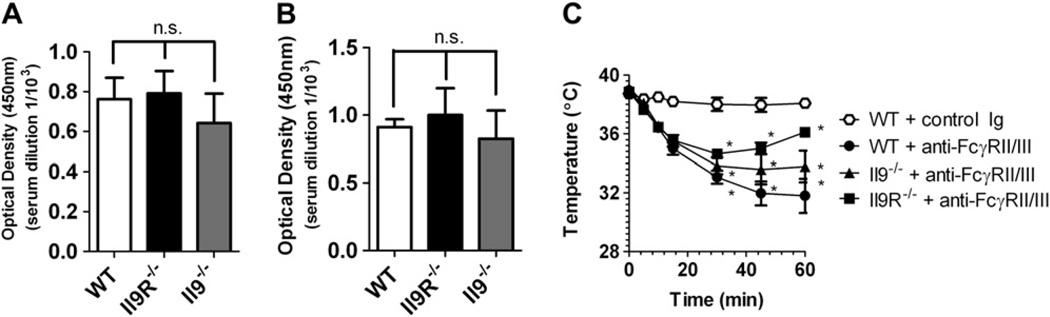
We next assessed the role of the IL-9/IL-9R pathway in parenteral antigen–induced anaphylaxis. First, we examined the role of the IL-9/IL-9R pathway in passive IgE-mediated anaphylaxis. WT, Il92/2, and Il9R2/2 mice were injected intravenously with 10 mg of anti-IgE, and development of shock was assessed by means of rectal thermometry. Surprisingly, equivalent hypothermia developed in WT, Il92/2, and Il9R2/2 mice (Fig 2, A), demonstrating that the IL-9/IL-9R signaling pathway is not essential for passive IgE-mediated systemic anaphylaxis. Furthermore, these studies suggest that IL-9/IL-9R signaling was not critical for homeostatic IgE production, the development and maintenance of basal tissue mast cells, or mast cell activation in this system, as shown by similar basal serum IgE levels; skin, tongue, and intestinal mast cell numbers; and serum murine mast cell protease 1 (mcpt-1) responses to anti-IgE challenge in WT, Il92/2, and Il9R2/2 mice (Fig 2, B-F). We also found no differences in total serum IgG1 or IgG2a concentrations in WT, Il92/2, and Il9R2/2 mice (Fig 3, A and B), as well as similar development of hypothermia in response to intravenous anti-FcgRII/III mAb injection in these 3 strains (Fig 3, C, and data not shown). Thus the IL-9/IL-9R pathway does not contribute to either passive IgE- or IgG-mediated systemic anaphylaxis.
FIG 2.
No role for the IL-9/IL-9R pathway in anti-IgE–mediated passive systemic anaphylaxis. A, Rectal temperature in control immunoglobulin (Ig)– and anti-IgE–treated WT, Il92/2, and Il9R2/2 mice. Total IgE levels (B) and mast cells per high-power field (HPF) in the tongue (C), ear skin (D), and small bowel (E) in control Ig– and anti-IgE–treated WT, Il92/2 and Il9R2/2 mice. F, Serum mcpt-1 concentrations in control Ig and anti-IgE mAb–challenged WT, Il92/2, and Il9R2/2 mice. Fig 2, A and F, n = 3 to 7 mice per group. *P < .05 compared with WT control Ig–treated mice. Fig 2, C through E, n = 4 mice per group. Fig 2, B, n = 4–6 mice per group from duplicate experiments. n.s., Not significant. OD; optical density.
FIG 3.
No role for the IL-9/IL-9R pathway in anti-FcgRII/III–mediated passive systemic anaphylaxis. A and B, Total IgG1 and IgG2a levels in WT, Il92/2, and Il9R2/2 mice. C, Rectal temperature response of WT, Il92/2, and Il9R2/2 mice to intravenous injection of anti-FcgRII/III mAb or control immunoglobulin (control Ig; J1.2). Fig 3, A and B, n = 4 mice per group from duplicate experiments. Fig 3, C, n = 5–11 mice per group. *P < .05 compared with WT control mice.
Assessment of IL-9/IL-9R pathway involvement in parenteral antigen–induced anaphylaxis
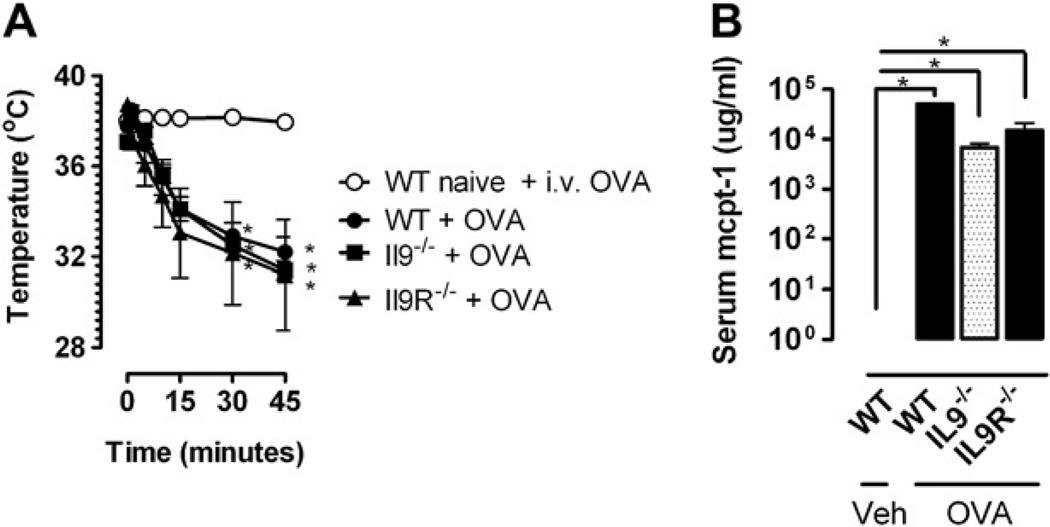
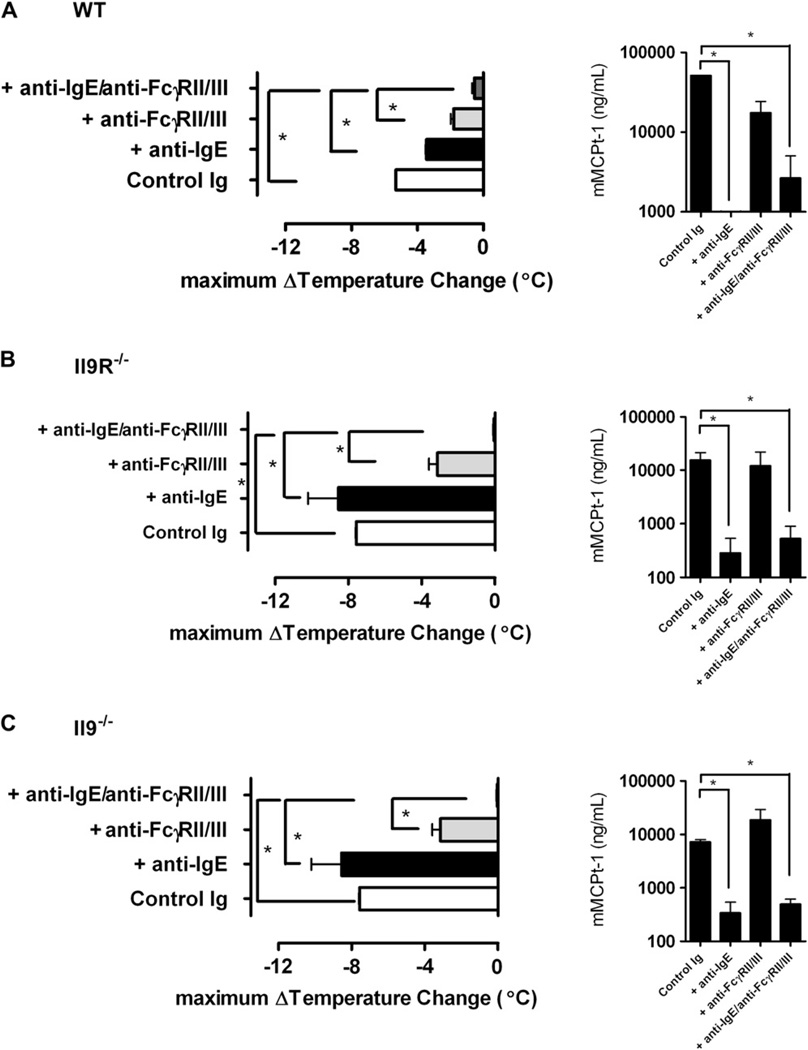
We next assessed the role of the IL-9/IL-9R pathway in parenteral antigen–induced systemic anaphylaxis by priming WT, Il92/2, and Il9R2/2 mice with a single intraperitoneal injection of 50 mg of OVA/alum and challenging them intravenously 14 days later with 100 mg of OVA. IL-9/IL-9R deficiency had no effect on parenteral OVA–induced hypothermia (Fig 4, A). Furthermore, all strains showed similar increases in serum mcpt-1 concentrations (Fig 4, B), suggesting that the IgE/mast cell component of parenteral antigen–induced systemic anaphylaxis occurred independently of IL-9/IL-9R signaling. To definitively assess the contribution of the IL-9/IL-9R pathway to IgE- and the IgG-mediated parenteral OVA–induced systemic anaphylaxis, we also examined parenteral antigen–induced anaphylaxis in WT, Il92/2, and Il9R2/2 mice after blockade of the IgE and IgG pathways. WT, Il92/2, and Il9R2/2 mice were primed with a single intraperitoneal immunization with 50 mg of OVA/alum and then injected intravenously 14 days later with anti-IgE, anti-FcgRII/III, or both mAbs to block the IgE, IgG, or both pathways, respectively. Twenty-four hours later, mice were challenged intravenously with 100 mg of OVA. Consistent with our observations in Fig 4, parenteral antigen–induced systemic anaphylaxis was comparable among WT, Il92/2, and Il9R2/2 mice treated with control immunoglobulin (Fig 5). Furthermore, these experiments demonstrate roles for both anaphylaxis pathways in this model, although the IgG pathway appears to dominate (Fig 5, A). Parenteral OVA challenge of antibody-treated Il92/2 and Il9R2/2 mice yielded similar results, although the dominance of the IgG pathway might have been greater in these mice than in WT mice (Fig 5, B and C, which show no inhibitory effect of blocking the IgE pathway with anti-IgE mAb and less of an mcpt-1 response in unblocked Il92/2 and Il9R2/2 mice than in WT mice). Despite this, equivalent hypothermia developed in anti-FcgRII/III mAb–treated Il92/2, Il9R2/2, and WT mice, indicating comparable involvement of the IgE pathway. Importantly, hypothermia was only totally abrogated in all 3 strains by blockade of both the IgE and IgG pathways. Collectively, these studies demonstrate that IL-9/IL-9R signaling is not required for either the IgE or IgG component of parenteral antigen–induced systemic anaphylaxis.
FIG 4.
No role for the IL-9/IL-9R pathway in parenteral antigen–induced anaphylaxis. Rectal temperatures (A) and serum mcpt-1 concentrations (B) in OVA-sensitized WT, Il92/2 , and Il9R2/2 mice injected intravenously with OVA or vehicle (Veh). Fig 4, A and B, n = 4 mice per group from duplicate experiments. *P < .05 compared with WT control.
FIG 5.
No role for the IL-9/IL-9R pathway in IgE- or IgG-mediated parenteral OVA-induced systemic anaphylaxis. Rectal maximum temperature changes and serum mcpt-1 concentrations in OVA-sensitized mice desensitized with immunoglobulin control (control Ig; GL117 1 J1.2), anti-IgE mAb, or anti-FcgRII/III mAb and challenged intravenously with OVA are shown. A, WT mice. B, Il92/2 mice. C, Il9R2/2 mice. Data represent means 6 SEMs; n = 3–6 mice per group from duplicate experiments. *P < .05 compared with control Ig.
DISCUSSION
Our observations demonstrate that both intestinal and systemic involvement in anaphylaxis induced by systemic immunization followed by intragastric challenge with the same antigen is mediated solely by IgE and is predominantly IL-9/IL-9R dependent. In contrast, systemic anaphylaxis induced by systemic immunization and intravenous challenge with OVA is mediated by both the IgE and IgG pathways, and neither pathway induced by systemic challenge requires IL-9 or IL-9R. These studies demonstrate contrasting roles for IL-9/IL-9R in the induction of anaphylaxis by systemic versus intragastric antigen challenge.
Anaphylaxis induced by systemic immunization and challenge is mediated in mice by both IgG/macrophage/basophil- and IgE/mast cell–mediated pathways.6,8 Our analyses demonstrate that although both pathways contribute to the anaphylactic reaction, the IgG/macrophage/basophil pathway dominates in our systemic challenge model. Notably, we show that the IgE/mast cell–mediated component is IL-9 independent. This is consistent with the normal IgE and mast cell responses to systemic antigen immunization that have been shown to develop in the absence of IL-9/IL-9R.20 In contrast, the IgE-mediated intestinal and systemic anaphylaxis induced by systemic antigen priming followed by repeated oral antigen gavage is predominantly IL-9 and IL-9R dependent. IgE-mediated anaphylaxis is mast cell dependent, regardless of whether it is triggered by ingested or injected antigen.7 The differential role for IL-9/IL-9R in IgE-mediated oral and parenteral antigen–induced anaphylaxis might be explained by the requirement for a tissue mastocytosis in oral gavage and not the parenteral-induced anaphylaxis. Moreover, IL-9 drives intestinal mastocytosis, an essential requirement for oral anti-gen–induced IgE-mediated anaphylaxis but not parenteral anti-gen–induced IgE anaphylaxis (see Fig E1, A, and Fig 2, C-F). IL-9 is obviously not required for mast cell development or degranulation, but it potently enhances mastocytosis that is induced by other stimuli, such as stem cell factor, IL-3, and IL-4.28–30 In light of these data and our observation that IL-9/IL-9R promotes intestinal mastocytosis after oral gavage but not systemic immunization, it is likely that stem cell factor, IL-3, and/or IL-4 are more limiting or IL-9 is more abundant after oral immunization. It is relevant that TGF-b, which is present at high levels in the intestine, can shift T-cell cytokine production toward a CD41 (IL-9–producing) TH9 pathway.31 Consequently, it seems likely that increased IL-9 levels produced in the intestine as a result of repeated oral antigen administration allow development of sufficient intestinal mastocytosis to increase intestinal permeability to the point that enough ingested antigen is absorbed to trigger systemic IgE-dependent mast cell degranulation and shock. In support of this, we have previously demonstrated that a transgene-mediated increase in intestinal IL-9 production increases intestinal mastocytosis and mast cell–dependent intestinal permeability sufficiently to allow ingested antigen to induce allergic diarrhea even when mice are not initially primed systemically with antigen plus adjuvant.18
Although the molecular mechanisms for the differences between anaphylaxis induced by antigen exposure through oral and parenteral routes are not completely known, our results and previous studies indicate that there are key molecular differences. For example, oral antigen–induced anaphylaxis is IgE dependent, whereas parenteral antigen–induced anaphylaxis is mediated by both the IgG and IgE pathways. The involvement of the IgE or IgG pathways in anaphylaxis depends on the concentration of antigen that enters the systemic circulation after oral or parenteral antigen challenge and the rate with which it accesses IgG-bearing macrophages/basophils or IgE-bearing mast cells. In the absence of blocking antibody, approximately 100-fold less antigen is required to induce IgE-mediated than IgG-mediated anaphylaxis.25 Ingested antigen is digested, excreted, or both, and thus relatively small quantities are absorbed. Furthermore, the ingested antigen is absorbed over several minutes, unlike parenteral antigen administration, which happens rapidly. Thus it is likely that antigen ingestion only provides sufficient antigen to induce the IgE-dependent pathway. Consistent with this, blockade of the IgE and not the IgG pathway abrogated both systemic and intestinal anaphylaxis induced by oral antigen.
IgG has also been shown to inhibit IgE-mediated anaphylaxis by means of both allergen interception and FcgRIIb-dependent inhibition.25 The inhibitory activity of IgG on IgE-mediated anaphylactic reactions only occurs at low antigen concentrations, concentrations less than that required to trigger IgG-mediated anaphylaxis. We demonstrated that blocking with anti-FcgRII/RIII mAb had no effect on either allergic diarrhea or hypothermia (Table I), suggesting that IgG did not inhibit IgE-mediated anaphylaxis by means of FcgRIIb-dependent inhibition. To assess whether IgG inhibited IgE-mediated anaphylaxis through allergen interception, we examined systemic and intestinal anaphylaxis induced by repeated oral gavage with OVA in IgG12/2 mice. IgG1 deficiency had no effect on intragastric OVA-induced hypothermia or allergic diarrhea (see Table E1 in this article’s Online Repository at www.jacionline.org). Collectively, these data indicate that IgG1 does not inhibit oral antigen–induced IgE-mediated anaphylaxis. The lack of a stimulatory or inhibitory effect of IgG on systemic and intestinal anaphylaxis induced by oral antigen suggests that there might be very little systemic absorption of ingested antigen. Moreover, absorbed ingested antigen might primarily activate the IgE/mast cell pathway within the intestine before it contacts blood with its high IgG concentrations, and it is the intestinal IgE/mast cell response driving the systemic and intestinal anaphylaxis mediated by oral antigen. Consistent with this, IgG levels are low in the gastrointestinal tract,25 and mice with increased intestinal mast cell numbers have increased oral antigen–induced anaphylaxis.
In contrast to this study, Brandt et al27 have previously demonstrated that OVA/alum immunization and subsequent multiple oral gavage OVA challenges of BALB/c WT mice induced diarrhea with no evidence of systemic shock. The observed differences in systemic involvement could be explained by (1) increased absorption of ingested OVA in our system, (2) increased or higher-affinity antigen-specific IgE in our system, (3) the increased number of intestinal mast cells in our system, and (4) decreased systemic antigen-specific IgG levels (which might intercept absorbed OVA before it could bind to OVA-specific, mast cell–associated IgE) in our system. Comparison of the protocols used in these 2 studies reveal differences in the immunization protocol. Brandt et al27 performed 2 intra-peritoneal sensitization steps with OVA/alum. Furthermore, OVA used in the present study contained 7-fold less LPS than that used by Brandt et al (68.8 ng of LPS/mg of OVA vs 9.3 ng of LPS/mg of OVA). Because the 2 models have never been directly compared, we are currently unable to determine which, if any, of these possibilities account for the difference. However, it is important to note that experimental studies with murine models of allergic inflammatory responses have previously demonstrated that varying levels of LPS contamination can alter antigen-specific TH2 cytokine levels and IgE/IgG ratios.32
These observations also resolve an apparent discrepancy between studies reporting that anaphylaxis was or was not IL-9 dependent,18,19 inasmuch as the studies that demonstrated IL-9 dependency used oral antigen challenges, whereas those that failed to demonstrate dependency challenged mice parenterally.19
Although additional studies are required to test these hypotheses, our results firmly establish a dichotomy in the requirement for IL-9 for anaphylaxis induction by ingested versus injected antigen. As such, they suggest that IL-9 and IL-9R antagonists might be useful for the treatment of allergy associated with antigen ingestion, such as food allergy, but not allergy induced by antigens, such as insect venoms or injectable drugs, that enter the body through the parenteral route.
Supplementary Material
Clinical implications.
Our findings might provide a basis for anti–IL-9 therapeutic approaches for the clinical management of IgE-mediated food-induced anaphylaxis.
Acknowledgments
Supported in part by the American Heart Association Midwest Affiliate (S.P.H), the American Academy of Allergy, Asthma and Immunology Interest Section Award 2007 (S.P.H.), Food Allergy and Anaphylaxis Network and National Institutes of Health grant R01AI073553-01 (S.P.H).
We thank Drs Marc E. Rothenberg, Pablo Abonia, Nives Zimmermann, and Ariel Munitz for helpful discussions.
Abbreviations used
- gc
Common g-chain
- IL-9R
IL-9 receptor a-chain
- LT
Leukotriene
- mcpt-1
Murine mast cell protease 1
- OVA
Ovalbumin
- PAF
Platelet-activating factor
- WT
Wild-type
Footnotes
Disclosure of potential conflict of interest: F. D. Finkelman is a consultant for Abbott and Associate Editor for the Journal of Allergy and Clinical Immunology, has received research support from Abbott, and is treasurer-elect for the American Association of Immunologists. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Simons FE, Frew AJ, Ansotegui IJ, Bochner BS, Golden DB, Finkelman FD, et al. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;120(suppl):S2–S4. doi: 10.1016/j.jaci.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hyper-sensitivity reactions to foods, drugs, and insects. J Allergy Clin Immunol. 2005;116:153–163. doi: 10.1016/j.jaci.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Greenberger PA. Drug Allergy. J Allergy Clin Immunol. 2006;117(suppl):S464–S470. doi: 10.1016/j.jaci.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Sampson HA. Food anaphylaxis. Clin Exp Allergy. 2007;37:651–660. doi: 10.1111/j.1365-2222.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 6.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 8.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Eigenmann PA. T lymphocytes in food allergy: overview of an intricate network of circulating and organ-resident cells. Pediatr Allergy Immunol. 2002;13:162–171. doi: 10.1034/j.1399-3038.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 10.Eigenmann PA, Huang SK, Ho DG, Sampson HA. Human T cell clones and cell lines specific to ovomucoid recognize different domains and consistently express IL-5. Adv Exp Med Biol. 1996;409:217. doi: 10.1007/978-1-4615-5855-2_29. [DOI] [PubMed] [Google Scholar]
- 11.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–1072. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 13.Temann UA, Ray P, Flavell RA. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J Clin Invest. 2002;109:29–39. doi: 10.1172/JCI13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLane MP, Haczku A, van de Rijn M, Weiss C, Ferrante V, MacDonald D, et al. Interleukin-9 promotes allergen-induced eosinophilic inflammation and airway hyperresponsiveness in transgenic mice. Am J Respir Cell Mol Biol. 1998;19:713–720. doi: 10.1165/ajrcmb.19.5.3457. [DOI] [PubMed] [Google Scholar]
- 16.Vink A, Warnier G, Brombacher F, Renauld JC. Interleukin 9-induced in vivo expansion of the B-1 lymphocyte population. J Exp Med. 1999;189:1413–1423. doi: 10.1084/jem.189.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dugas B, Renauld JC, Pene J, Bonnefoy JY, Peti-Frere C, Braquet P, et al. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993;23:1687–1692. doi: 10.1002/eji.1830230743. [DOI] [PubMed] [Google Scholar]
- 18.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoops L, Louahed J, Van Snick J, Renauld JC. IL-9 promotes but is not necessary for systemic anaphylaxis. J Immunol. 2005;175:335–341. doi: 10.4049/jimmunol.175.1.335. [DOI] [PubMed] [Google Scholar]
- 20.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 21.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, et al. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baniyash M, Eshhar Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur J Immunol. 1984;14:799–807. doi: 10.1002/eji.1830140907. [DOI] [PubMed] [Google Scholar]
- 23.Baniyash M, Eshhar Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur J Immunol. 1984;14:799–807. doi: 10.1002/eji.1830140907. [DOI] [PubMed] [Google Scholar]
- 24.Finkelman F, Kessler SW, Mushinski JF, Potter M. IgD-secreting murine plasmacytomas: identification and partial characterization of two IgD myeloma proteins. J Immunol. 1981;126:680–687. [PubMed] [Google Scholar]
- 25.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strait RT, Morris SC, Smiley K, Urban JF, Jr, Finkelman FD. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–3842. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 27.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godfraind C, Louahed J, Faulkner H, Vink A, Warnier G, Grencis R, et al. Intra-epithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut trachea, and kidneys of IL-9 transgenic mice. J Immunol. 1998;160:3989–3996. [PubMed] [Google Scholar]
- 29.Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol. 2003;170:3461–3467. doi: 10.4049/jimmunol.170.7.3461. [DOI] [PubMed] [Google Scholar]
- 30.Madden KB, Urban JF, Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147:1387–1391. [PubMed] [Google Scholar]
- 31.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3 1 T cells and together with TGF-beta, generates IL-9 1 IL-10 1 Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.