Abstract
This study examined the cardiovascular effects of GLP-1 (7–36) or (9–36) on myocardial oxygen consumption, function and systemic hemodynamics in vivo during normal perfusion and during acute, regional myocardial ischemia. Lean Ossabaw swine received systemic infusions of saline vehicle or GLP-1 (7–36 or 9–36) at 1.5, 3.0, and 10.0 pmol/kg/min in sequence for 30 min at each dose, followed by ligation of the left circumflex artery during continued infusion at 10.0 pmol/kg/min. Systemic GLP-1 (9–36) had no effect on coronary flow, blood pressure, heart rate or indices of cardiac function before or during regional myocardial ischemia. Systemic GLP-1 (7–36) exerted no cardiometabolic or hemodynamic effects prior to ischemia. During ischemia, GLP-1 (7–36) increased cardiac output by approximately 2 L/min relative to vehicle-controls (p=0.003). This response was not diminished by treatment with the non-depolarizing ganglionic blocker hexamethonium. Left ventricular pressure-volume loops measured during steady state conditions with graded occlusion of the inferior vena cava to assess load-independent contractility revealed that GLP-1 (7–36) produced marked increases in end diastolic volume (74 ± 1 to 92 ± 5 mL; p=0.03) and volume axis intercept (8 ± 2 to 26 ± 8; p=0.05), without any change in the slope of the end systolic pressure volume relationship vs. vehicle during regional ischemia. GLP-1 (9–36) produced no changes in any of these parameters compared to vehicle. These findings indicate that short-term systemic treatment with GLP-1 (7–36) but not GLP-1 (9–36) significantly augments cardiac output during regional myocardial ischemia, via increases in ventricular preload without changes in cardiac inotropy.
Keywords: Glucagon like peptide 1, ischemic injury, cardioprotection, ESPVR, contractility
INTRODUCTION
Full length GLP-1 (7–36), endogenously produced by intestinal L-cells, is generally considered to be the physiologically active form of GLP-1 [11]. Administration of GLP-1 (7–36) results in proportional increases in circulating GLP-1 (9–36) levels [21;40]. Data on cardiovascular effects of these peptides are mixed: Infusion of the (7–36) or (9–36) peptide have produced increased [3;31], decreased [24;26;36;41], or no change [19;28;35] in cardiac contractile function in normal hearts in rats, dogs and pigs. Effects of GLP-1 (7–36) or (9–36) to augment preload-dependent indices of cardiac function in ischemic and failing hearts [2;27–29;41] have been more clearly demonstrated, although again this effect is not consistently observed [30;38]. Importantly, no study to date has directly assessed preload-independent measures of cardiac contractility in either normal or ischemic hearts. Therefore, whether improvements in cardiac contractile function induced by GLP-1 are mediated by direct inotropic effects, increases in ventricular diastolic filling (i.e. Frank-Starling effects), and/or cardioprotective mitigation of ischemic injury has not been defined.
This set of studies was designed to evaluate the dose-dependent effects of GLP-1 (7–36) or (9–36) (1.5 – 10.0 pmol/kg/min, iv) on systemic hemodynamics, coronary flow, cardiac metabolism and preload-dependent and -independent measures of cardiac function in normal vs. ischemic hearts. Left ventricular pressure volume relations were assessed in lean Ossabaw swine with high-resolution admittance catheters before and during acute ligation of the left circumflex coronary artery. Inotropic status was directly evaluated by measuring the slope of the end-systolic pressure-volume relationship, using brief balloon occlusion of the inferior vena cava to produce graded reductions of ventricular preload. Our findings provide novel insight into the differential cardiovascular actions of GLP-1 isoforms and have important implications for the use of incretin-based therapies in circumstances of impaired cardiac function or ischemia.
METHODS
All protocols were approved by the Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85–23, Revised 1996) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Ossabaw Swine (n = 23) weighing between 66kg and 83kg were initially sedated with Telazol (tiletamin-zolazepam, 5mg/kg sc), xylazine (2.2mg/kg sc), and ketamine (3.0 mg/kg sc). Subsequent to endotracheal intubation and venous access, anesthesia was maintained with morphine (3.0mg/kg sc) and α-chloralose (100mg/kg, i.v.). Animals were mechanically ventilated (Harvard respirator) with O2 supplemented room air. Following completion of experimental protocols, hearts were fibrillated and excised in accordance with recommendation of the American Veterinary Medical Association Guide on Euthanasia (June 2007).
Surgical preparation
Acute in-vivo experiments were conducted in open chest, anesthetized pigs. Catheters were placed into the right femoral artery and vein for systemic hemodynamic measurements and administration of supplemental anesthesia, heparin and sodium bicarbonate respectively. A Fogarty balloon catheter (Edwards Lifesciences) was introduced into the left femoral vein and advanced into the inferior vena cava to allow for experimental reduction of venous return to the heart. Blood gas parameters were maintained within normal limits through periodic arterial blood gas analyses and appropriate adjustments to breathing rate and bicarbonate supplementation as necessary (arterial PO2 = 180 ± 63 mmHg; arterial PCO2 = 42 ± 1; pH = 7.4 ± 0.01; hematocrit = 35 ± 4). A left lateral thoracotomy was performed, allowing for access to the heart. The left circumflex artery (LCX) was then isolated and a suture placed loosely around it. At appropriate points in the study, this suture was used to ligate the LCX, thereby inducing regional myocardial ischemia. Next, the left anterior descending artery (LAD) was isolated and a perivascular flow transducer (Transonic Systems Inc.) was placed around the vessel. Following flow probe placement, a catheter was introduced into the coronary interventricular vein for coronary venous blood sampling. A pericardial cradle was then made to allow for adequate access to the heart apex and a purse string suture was placed at the apex through which an 18 gauge needle was passed into the LV cavity to allow for introduction and securing of a pressure volume admittance catheter (Transonic Systems). All data were collected using IOX acquisition software (EMKA Technologies, Falls Church VA. USA). Prior to any measurements, heparin was administered (bolus; 500 U/kg, iv) to prevent formation of blood clots during the protocol.
Experimental Protocol
Animals were randomly assigned to study infusions, with no differences in pre-treatment management, surgical preparation, or study procedures other than the study infusion. A total of n=23 animals were studied. This study employed four groups; vehicle treated (n=5), GLP-1 (7–36) treated (n=9), GLP-1 (9–36) treated (n=5), GLP-1 (7–36) treated with concurrent hexamethonium (5 mg/kg, iv) administration (n=3), and a single animal who was treated with epinephrine (n=1). Following a stabilization period of at least 20 min, animals received continuous intravenous infusions of vehicle (saline), or graded infusions of increasing concentrations of GLP-1 (7–36) or GLP-1 (9–36) at 1.5, 3.0, and 10.0 pmol/kg/min in sequence for 30 min at each dose. Following these infusions, the 10 pmol/kg/min was continued and the left coronary artery (LCX) was ligated to induce regional ischemia for an additional 30 min. In swine this ligation affects ~20% of the left ventricle [12]. The same animals that received graded vehicle or GLP-1 dosing also received coronary occlusion. However, only n=5 of the GLP-1 (7–36) treated animals were subjected to coronary ligation (i.e. 5 of the 9 GLP-1 (7–36) treated pigs received LCX occlusion).
Aortic pressure, left ventricular pressure, left ventricular volume, coronary blood flow (LAD) and ECG were measured throughout the entire protocol. The left ventricular end-systolic pressure volume relationship was assessed at each of the 30 minute time points by a brief inflation (< 5 sec) of the Fogarty balloon catheter to reduce venous return. Similar pressure volume measurements were performed in the animal treated with epinephrine (10µg/min) to demonstrate as a positive control the effects of a known inotrope on left ventricular pressure volume relationships measured using this methodology.
Metabolic Analysis
Arterial and coronary venous blood were collected simultaneously into untreated syringes, immediately sealed, and placed on ice. These samples were analyzed for pH, PCO2, PO2, O2 content, and hematocrit with an Instrumentation Laboratories automatic blood gas analyzer (GEM Premier 3000) and CO-oximeter (682) system. Myocardial oxygen consumption (µl O2/min/g) was calculated using the Fick principle as [coronary blood flow × (arterial O2 content – coronary venous O2 content)]. For these calculations, LAD perfusion territory was estimated to be 30% of total heart weight, as previously described by Feigl et al. [12]. Cardiac efficiency was calculated as the product of cardiac output (L/min) and mean arterial pressure (mmHg) divided by myocardial oxygen consumption (µl O2/min/g).
Statistical Analyses
Data are presented as mean ± SE. Statistical comparisons were made with two way (ANOVA) testing for differences between treatments in the dose response, and with one-way ANOVA comparing treatment groups under ischemia or comparing values within a treatment group before and after ischemia. For all comparisons, P ≤ 0.05 was considered statistically significant. When significance was found with ANOVA, a Student-Newman-Keuls multiple comparison test was performed to identify differences between treatment levels and/or GLP-1 (7–36) or (9–36) vs. saline infused time controls.
RESULTS
Hemodynamic and Metabolic Effects of Acute GLP-1 Administration
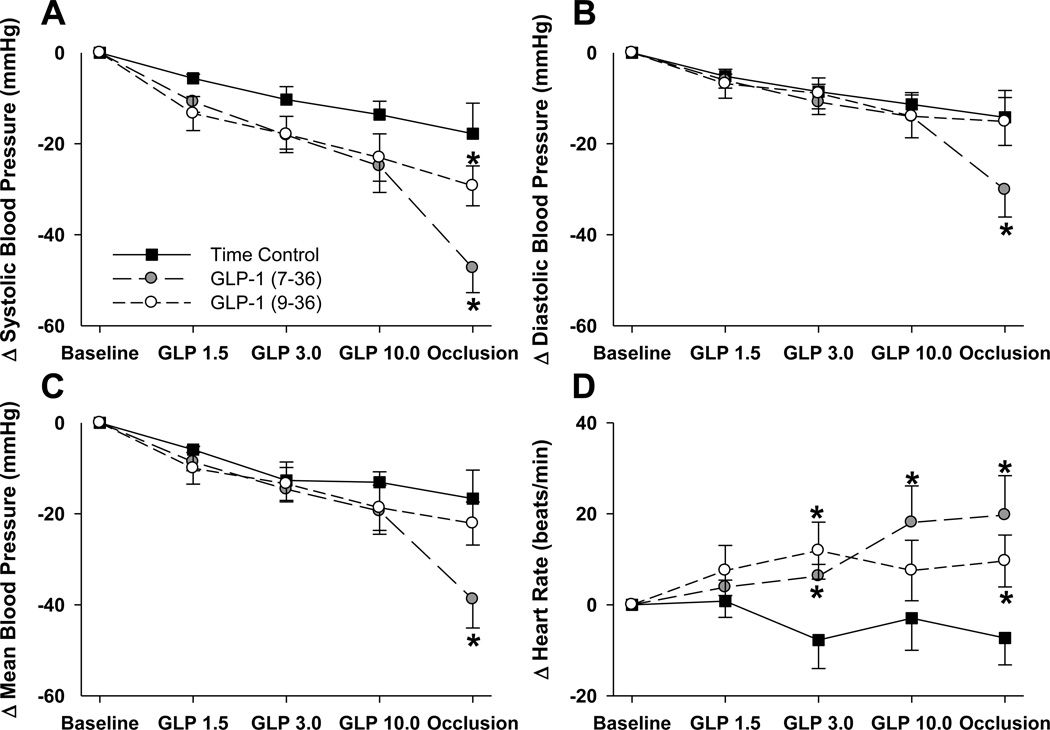
Effects of GLP-1 (7–36), GLP-1 (9–36) and time control saline infusions on systemic hemodynamic variables are listed in Table 1. Despite randomization to treatment conditions, modest (non-significant) differences in baseline blood pressure and heart rate were present between the treatment groups prior to GLP-1 administration. To avoid any bias resulting from this, we present results as absolute changes in these variables (Figure 1). We observed a time-dependent fall in blood pressure over the course of the experimental protocol that did not differ between groups (Figure 1). Acute coronary occlusion had little additional effect on mean blood pressure in control or GLP-1 (9–36) treated swine (Figure 1C). However, acute coronary occlusion was associated with a significant further decrease in mean blood pressure in GLP-1 (7–36) treated animals (P < 0.001). This decrease in arterial pressure was associated with a ~20 beat/min increase in heart rate in GLP-1 (7–36) treated swine relative to identically handled saline-infused time control animals (Figure 1D; P = 0.001).
Table 1.
Effects of GLP-1 (7–36) vs. (9–36) on systemic hemodynamics and metabolism
| Time-Control | GLP-1 (7–36) | GLP-1 (9–36) | |
|---|---|---|---|
| Systolic Blood Pressure (mmHg) | |||
| Baseline | 110±9 | 136± 4 | 127±9 |
| 1.5 pmol/kg/min | 104±9 | 125±5 | 114±12 |
| 3.0 pmol/kg/min | 99±9 | 118±6 | 109±12 |
| 10.0 pmol/kg/min | 97±9 | 114±8 | 104 ±13 |
| LCX Occlusion | 92± 8 | 92±10† | 98±12 |
| Diastolic Blood Pressure (mmHg) | |||
| Baseline | 82±7 | 93±3 | 86±6 |
| 1.5 pmol/kg/min | 76±6 | 87±4 | 80±6 |
| 3.0 pmol/kg/min | 73±5 | 82±4 | 77±7 |
| 10.0 pmol/kg/min | 71±6 | 80±6 | 72±8 |
| LCX Occlusion | 67±6 | 68±9† | 71±9 |
| Mean Blood Pressure (mmHg) | |||
| Baseline | 95±8 | 113± 4 | 105±6 |
| 1.5 pmol/kg/min | 89±8 | 105±4 | 95±8 |
| 3.0 pmol/kg/min | 82± 8 | 99±5 | 92±9 |
| 10.0 pmol/kg/min | 83±8 | 95±7 | 86±11 |
| LCX Occlusion | 78±7 | 78±10† | 83± 10 |
| Heart Rate (beats/min) | |||
| Baseline | 80±8 | 63± 4 | 67±10 |
| 1.5 pmol/kg/min | 81±11 | 68±5 | 75±10 |
| 3.0 pmol/kg/min | 73±7 | 69±5 | 79± 13 |
| 10.0 pmol/kg/min | 71±11 | 80±12 | 75±11 |
| LCX Occlusion | 72±9 | 87± 10 | 77± 11 |
| Coronary Blood Flow (ml/min/g) | |||
| Baseline | 0.47± 0.06 | 0.46 ± 0.03 | 0.43 ± 0.06 |
| 1.5 pmol/kg/min | 0.43± 0.05 | 0.39 ± 0.02 | 0.36 ± 0.06 |
| 3.0 pmol/kg/min | 0.35± 0.04 | 0.34 ± 0.02† | 0.33 ± 0.05 |
| 10.0 pmol/kg/min | 0.40± 0.03 | 0.33 ± 0.03† | 0.29 ± 0.06 |
| LCX Occlusion | 0.30± 0.05 | 0.30 ± 0.03† | 0.27 ± 0.07 |
| Myocardial O2 Consumption (µl O2/min/g) | |||
| Baseline | 50±6 | 54±3 | 48±7 |
| 1.5 pmol/kg/min | 50±4 | 48±2 | 41±6 |
| 3.0 pmol/kg/min | 43±3 | 42±3 | 39±6 |
| 10.0 pmol/kg/min | 41±1 | 40±3 | 36±7 |
| LCX Occlusion | 36±6 | 40±5 | 36±8 |
Values are mean ± SE for Time-Control (n = 5), GLP-1 (7–36) (n = 9; n = 5 for LCX occlusion) and GLP-1 (9–36) (n = 5).
P < 0.05 vs. baseline value (same treatment).
P < 0.05 vs. infusion rate matched, saline infused time controls.
Figure 1.
Data (mean±SEM) describing changes in SBP (Panel A), DBP (Panel B), MBP (Panel C) or HR (Panel D), presented as a change relative to within treatment group baseline. All data are presented for all animals receiving GLP-1 (7–36) infusion, GLP-1 (9–36) infusion or infusion rate matched saline-infused time controls. *p<0.05 vs. identically handled, time control.
Consistent with the changes in blood pressure, coronary flow and myocardial oxygen consumption in the non-ischemic LAD region tended to decrease in all treatment groups (Table 1). Reductions in coronary flow were statistically greater relative to within group baseline in GLP-1(7–36) treated swine at the 3.0 (P = 0.006) and 10.0 pmol/kg/min (P = 0.019) exposure as well as during regional myocardial ischemia (P = 0.013). However, absolute coronary blood flows were not different between time-control and GLP-1 treated swine at any time point before or during ischemia (Table 1). No differences in myocardial oxygen consumption were detected between groups at any treatment condition (Table 1).
Cardiac Effects of Acute GLP-1 Administration
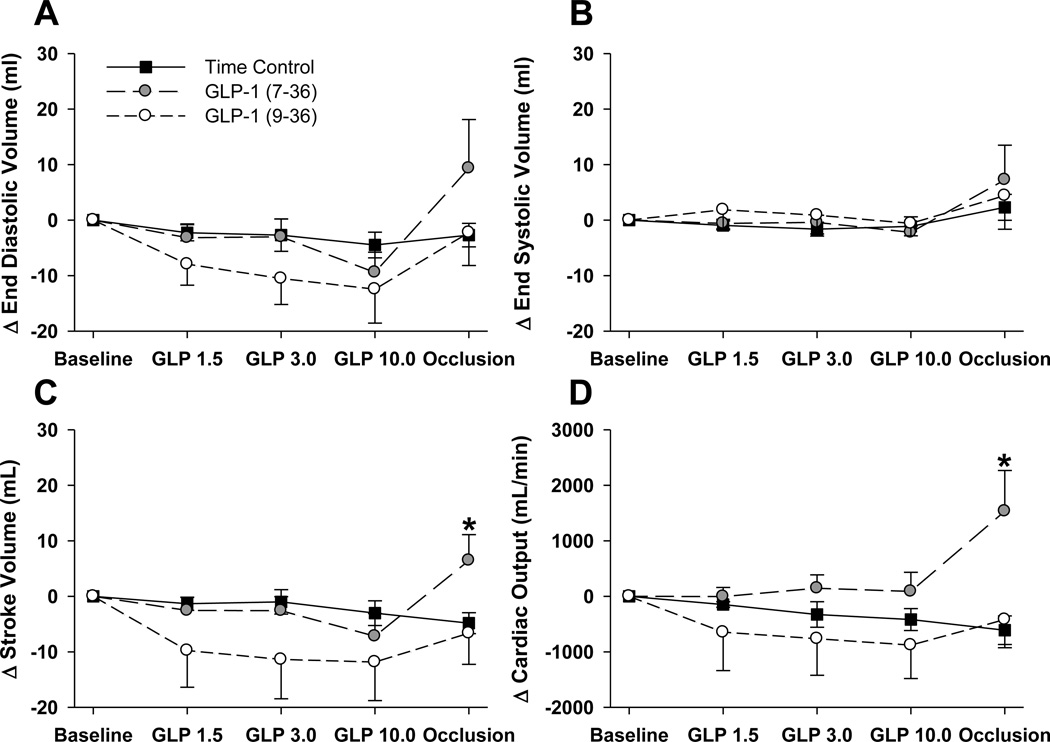
Administration of GLP-1 (7–36) or (9–36) had no effect on left ventricular diastolic filling, stroke volume, cardiac output or ejection fraction over the 1.0 to 10.0 pmol/kg/min dose range prior to the induction of myocardial ischemia (Table 2). However, during regional ischemia GLP-1 (7–36) treatment was significantly associated with increased left ventricular end diastolic volume (75 ± 1 vs. 92 ± 5 mL; P = 0.016), and stroke volume (32 ± 6 vs. 48 ± 6 mL; P = 0.040), without differences in end systolic volume or ejection fraction relative to time control animals (Table 2). These alterations in cardiac function were also evident when analyzed as absolute changes relative to their respective baseline values (Figure 2). One of the most striking findings of this study is the approximate 2 L/min increase in cardiac output (P = 0.015 within group; P < 0.001 relative to time control) observed in GLP-1 (7–36) treated swine during regional ischemia (Figure 2D). This effect was specific to the (7–36) peptide, as these variables were unaffected by the onset of myocardial ischemia in time-control or (9–36) treated swine.
Table 2.
Effects of GLP-1 (7–36) vs. (9–36) on cardiac contractile function
| Time-Control | GLP-1 (7–36) | GLP-1 (9–36) | |
|---|---|---|---|
| LV End Diastolic Volume (ml) | |||
| Baseline | 78± 1 | 82 ± 3 | 78 ± 1 |
| 1.5 pmol/kg/min | 75 ± 1 | 79 ± 4 | 71 ± 4 |
| 3.0 pmol/kg/min | 75±3 | 79 ± 4 | 68 ± 5 |
| 10.0 pmol/kg/min | 73±2 | 69 ± 4 | 66 ± 7 |
| LCX Occlusion | 75± 1 | 92 ± 5‡ | 76 ± 6 |
| LV End Systolic Volume (ml) | |||
| Baseline | 41±5 | 42±5 | 44±5 |
| 1.5 pmol/kg/min | 40±6 | 41±5 | 46±4 |
| 3.0 pmol/kg/min | 40±6 | 42±5 | 45±3 |
| 10.0 pmol/kg/min | 36±6 | 34±4 | 43±4 |
| LCX Occlusion | 43±6 | 49±6 | 48±3 |
| LV Stroke Volume (ml) | |||
| Baseline | 37±5 | 40±3 | 34 ±4 |
| 1.5 pmol/kg/min | 36±5 | 37±4 | 24±5 |
| 3.0 pmol/kg/min | 36±6 | 37±4 | 23±5 |
| 10.0 pmol/kg/min | 37±6 | 34 ±6 | 22±5 |
| LCX Occlusion | 32±6 | 48± 6‡ | 27±6 |
| Cardiac Output (ml/min) | |||
| Baseline | 2838±99 | 2531 ± 271 | 2324±529 |
| 1.5 pmol/kg/min | 2689±74 | 2524 ± 331 | 1675±262 |
| 3.0 pmol/kg/min | 2510±280 | 2676 ± 429 | 1558±201 |
| 10.0 pmol/kg/min | 2474 ±255 | 2576 ± 575 | 1442±213 |
| LCX Occlusion | 2227±315 | 4319 ± 908†‡ | 1902±329 |
| LV Ejection Fraction (%) | |||
| Baseline | 48± 6 | 49 ± 5 | 44±6 |
| 1.5 pmol/kg/min | 48±7 | 48 ± 5 | 34± 6 |
| 3.0 pmol/kg/min | 48±8 | 48 ± 5 | 33± 6 |
| 10.0 pmol/kg/min | 51±9 | 49 ± 6 | 33±6 |
| LCX Occlusion | 43±8 | 50 ± 6 | 35±6 |
| End Systolic Pressure Volume Relationship (mmHg/ml) | |||
| Baseline | 11±3 | 11±4 | 15±4 |
| 1.5 pmol/kg/min | 16± 5 | 17±6 | 15±6 |
| 3.0 pmol/kg/min | 17±5 | 17±4 | 14±3 |
| 10.0 pmol/kg/min | 16±7 | 20 ±5 | 20±9 |
| LCX Occlusion | 12±4 | 13±3 | 13± 0 |
| Volume Axis Intercept (ml) | |||
| Baseline | 7 ± 2 | 9 ± 2 | 4 ± 4 |
| 1.5 pmol/kg/min | 7± 2 | 9 ± 6 | 5 ± 3 |
| 3.0 pmol/kg/min | 8 ± 2 | 19 ± 2‡ | 11 ± 5 |
| 10.0 pmol/kg/min | 8±2 | 24 ± 4‡ | 7 ± 1 |
| LCX Occlusion | 9± 2 | 26 ± 8‡ | 9 ± 0 |
| Tau ½ | |||
| Baseline | 43±7 | 42 ± 3 | 48 ± 13 |
| 1.5 pmol/kg/min | 48±9 | 43 ± 4 | 52 ± 11 |
| 3.0 pmol/kg/min | 44±8 | 44 ± 5 | 55 ± 14 |
| 10.0 pmol/kg/min | 64± 13 | 45 ± 8 | 63 ± 17 |
| LCX Occlusion | 60± 14 | 32 ± 3 | 83 ± 27 |
Values are mean ± SE for Time-Control (n = 5), GLP-1 (7–36) (n = 5) and GLP-1 (9–36) (n = 5).
P < 0.05 vs. baseline value (same treatment).
P < 0.05 vs. infusion rate matched, saline infused time controls.
Figure 2.
Data (mean±SEM) describing changes in EDV (Panel A), ESV (Panel B), SV (Panel C) or CO (Panel D), presented as a change relative to within treatment group baseline. All data are presented for all animals receiving GLP-1 (7–36) infusion, GLP-1 (9–36) infusion or infusion rate matched saline-infused time controls. *p<0.05 vs. identically handled, time control.
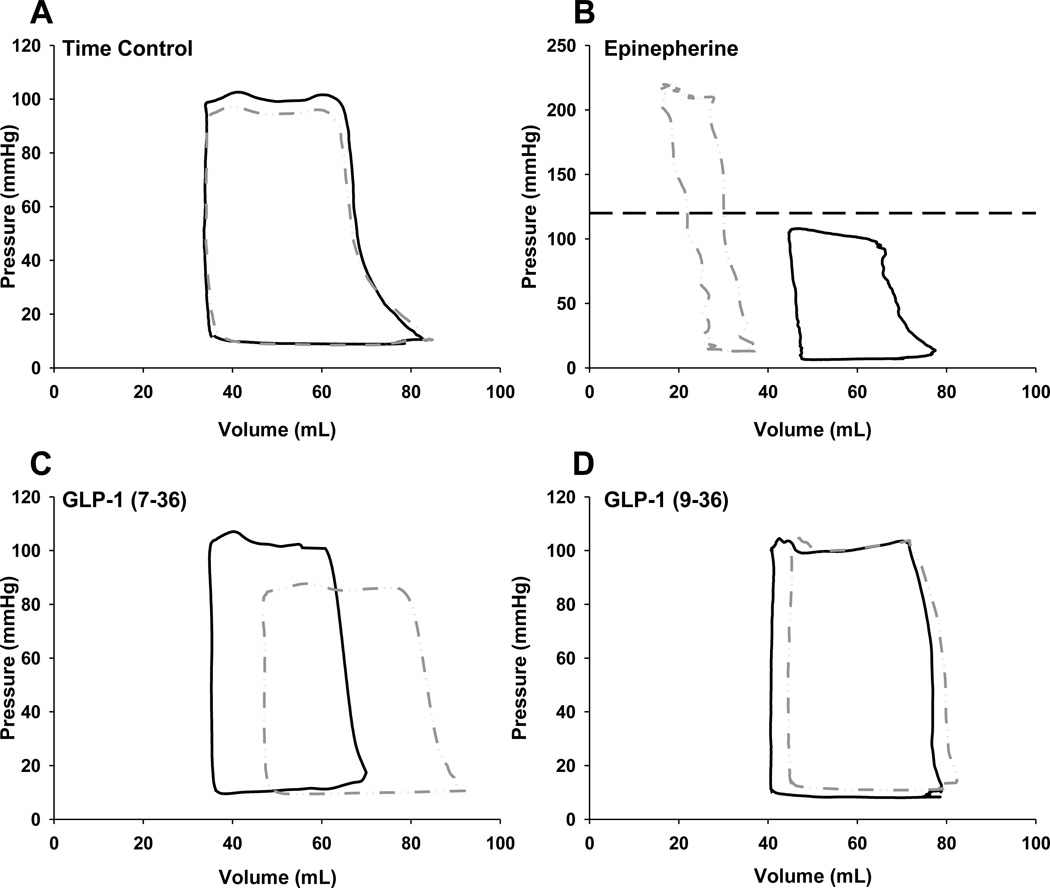
Steady state pressure-volume loops demonstrating the effect of GLP-1 administration at the 10 pmol/kg/min dose before and during regional ischemia are presented in Figure 3. These loops, presenting averaged pressure-volume data from representative animals, demonstrate the lack of an effect of regional ischemia on the left ventricular pressure volume relationship under control conditions (Figure 3A). To demonstrate the effects of a classic positive inotropic (contractility) and chronotropic (heart rate) response in our preparation, left ventricular pressure-volume loops were determined during the administration of intravenous epinephrine (10µg/min) in one animal under normal perfusion conditions (Figure 3B). Epinephrine produced a marked upward-leftward shift in the pressure-volume loop as a result of substantial increases in ventricular systolic pressure (~200 mmHg) and decreases in end-systolic and end-diastolic volumes (consequence of heart rates >200 beats/min). Note the change in scale of Figure 3B; the dashed line represents the maximal Y scale value of other panels. Consistent with previous data [28], GLP-1 (9–36) had no effect on pressure volume parameters in normal or ischemic hearts (Figure 3D). In contrast, infusion of GLP-1 (7–36) (10 pmol/kg/min) during regional myocardial ischemia resulted in diminished left ventricular pressure generation and a notable right shift of the pressure-volume relationship; i.e. increased left ventricular end-diastolic volume (preload) (Figure 3C).
Figure 3.
Representative pressure-volume loops from saline infused time controls (Panel A), GLP-1 (7–36) (Panel C) and GLP-1 (9–36) (Panel D) treated animals at the highest infusion rate (10 pmol/kg/min) during normal perfusion (solid line; black) and subsequent to induction of regional myocardial ischemia (interrupted line; gray). Each representative loop is the result of averaging 3 consecutive loops from 3 separate animals during plateau of responses during the relevant, presented conditions. Panel B provides representative data from a single animal (3 averaged loops per condition) demonstrating effect of epinephrine on pressure volume relationships (interrupted gray line) during normal myocardial perfusion. Note the change in scale of Panel B; the dashed line represents the maximal Y scale value of other panels.
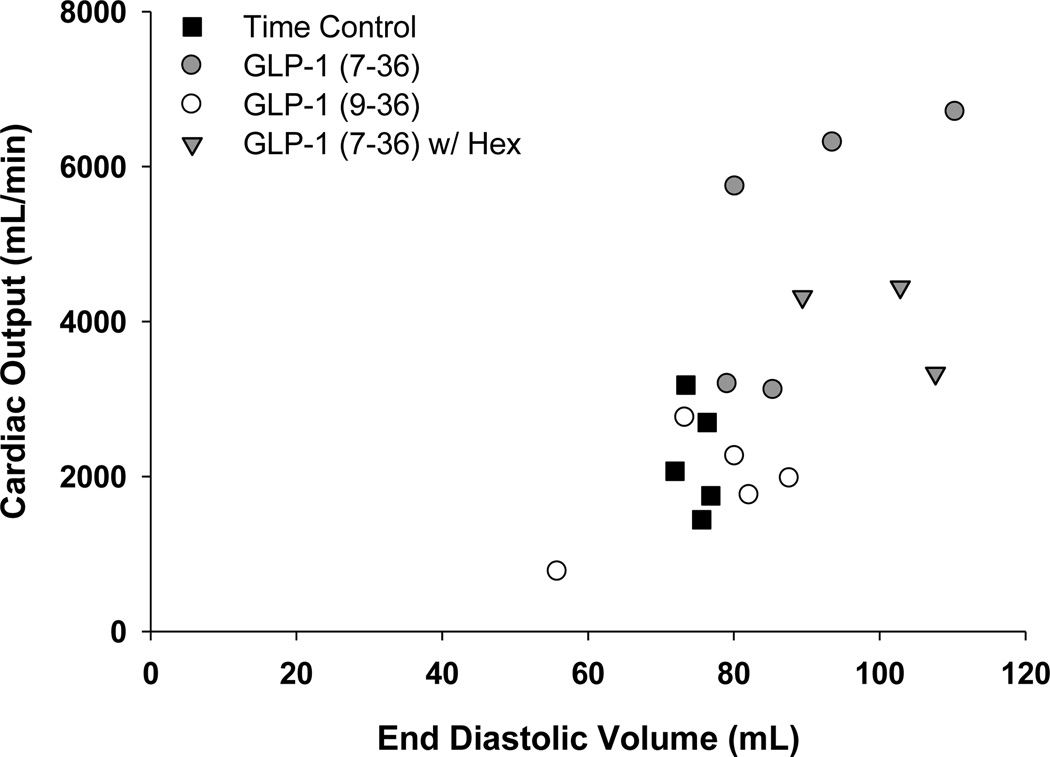
Effects of GLP-1 peptides on Ees and V0 are found in Table 2. Neither GLP-1 (7–36) nor (9–36) influenced Ees at any dose, regardless of experimental condition. Despite the lack of change in slope of ESPVR, GLP-1 (7–36) significantly increased V0 at the 10.0 pmol/kg/min dose, both before and during regional myocardial ischemia (Table 2). V0 was unchanged in time-control and GLP-1 (9–36) treated swine. Plotting the relationship between cardiac output and end-diastolic volume (Frank-Starling relationship) during regional ischemia demonstrates that the increase in cardiac output in GLP-1 (7–36) treated swine is directly related to increases in end-diastolic volume (preload) (Figure 4), without any apparent contribution from direct effects on contractility. Examination of the time constant of ventricular relaxation (Tau ½) suggests that administration of GLP-1(7–36) improved diastolic function during LCX occlusion (Table 2). In contrast, Tau ½ tended to worsen with LCX occlusion in time control and GLP-1 (9–36) treated animals.
Figure 4.
Summary plot for the relationships between end-diastolic volume (EDV) and cardiac output (CO) during regional myocardial ischemia and exposure to study treatments.
To assess whether the effects of GLP-1 (7–36) are mediated centrally, additional studies were performed in the presence of the non-depolarizing ganglionic blocker hexamethonium (n=3). Results of these studies are included in Figure 4 and demonstrate that both cardiac output and end diastolic volume are elevated in the ischemic heart with GLP-1 (7–36) treatment regardless of hexamethonium administration.
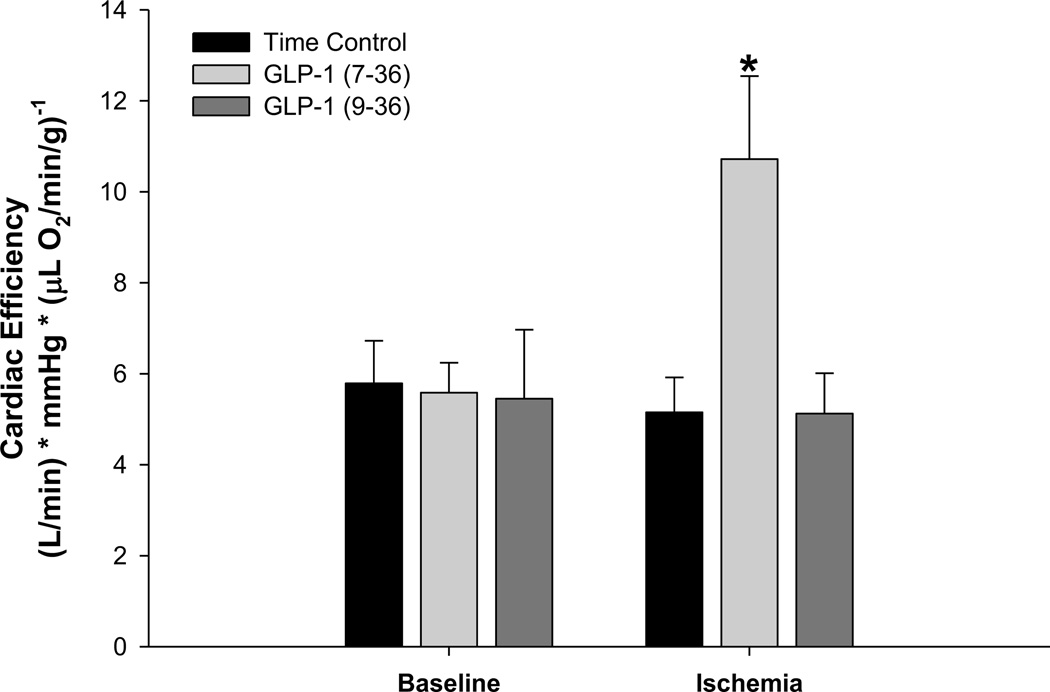
Additional examination of the effects of GLP-1 on cardiac efficiency (cardiac output (L/min) × mean arterial pressure (mmHg)) / myocardial oxygen consumption (µl O2 min/g) demonstrated no effect of vehicle, GLP-1 (7–36) or (9–36) administration under baseline-control conditions (Figure 5). However, cardiac efficiency was significantly augmented by GLP-1 (7–36) administration during regional myocardial ischemia (Figure 5). GLP-1 (9–36) had no effect on cardiac efficiency during ischemia.
Figure 5.
Effects of GLP-1 therapies on cardiac efficiency (cardiac output per unit oxygen consumption) at baseline and during ischemia. Data are presented as mean±SEM *p<0.05 GLP-1 (7–36) vs. saline and vs GLP-1 (9–36).
DISCUSSION
This investigation examined the effects of systemically infused GLP-1 (7–36) and (9–36) on systemic hemodynamics, coronary flow and preload-dependent and -independent measures of cardiac function in normal and ischemic hearts. Neither GLP-1 isoform had any effect on systemic pressure, coronary blood flow or cardiac function in normally perfused hearts relative to saline infused time-controls. Moreover, GLP-1 (9–36) had no effect on any measured cardiovascular parameter in normal or ischemic hearts. In contrast, the induction of regional ischemia during GLP-1 (7–36) administration produced significant reductions in systemic blood pressure ~20mmHg and increased cardiac output and efficiency. Pressure volume analyses revealed that this (7–36)-mediated increase in cardiac performance was not associated with any change in myocardial contractility (as assessed by ESPVR) but was accompanied by a significant increase in left ventricular end diastolic volume and an increase in volume axis intercept (V0). Together these observations demonstrate that acute administration of GLP-1 (7–36) significantly augments cardiac output during regional myocardial ischemia via increases in ventricular preload, independent of changes in cardiac inotropy (i.e. Frank-Starling mechanism). The findings further indicate that GLP-1 (9–36) is unlikely to significantly contribute to improvements in cardiovascular function produced by GLP-1(7–36) in ischemic hearts.
Hemodynamic effects of GLP-1 (7–36)
Consistent with previous studies from our laboratory and others [23;24;28], we found that short-term treatment with GLP-1 (7–36) had no effect on coronary flow or myocardial oxygen consumption under any experimental condition (Table 1). Therefore, improvements in cardiac function observed in response to GLP-1 (7–36) cannot be the result of differences in myocardial perfusion. GLP-1 (7–36) also had no effect on systolic, diastolic or mean blood pressure relative to identically handled time control animals in non-ischemic hearts. This lack of a pressor effect in otherwise healthy hearts is consistent with previous investigations in human subjects [7;24;32;33;35], but contrasts findings in rodent models which largely report hypertensive effects of GLP-1 [4–6;8;15;19;39]. However, we did observe significant reductions in blood pressure in GLP-1 (7–36) treated swine during regional myocardial ischemia (Figure 1). This hypotensive effect has also been reported by other labs in the setting of various pathologic conditions such as ischemia-reperfusion injury, myocardial infarction and heart failure [27;28;35].
Inotropic effects of GLP-1 (7–36)
Prior evidence supports the ability of GLP-1 based therapies to augment preload-dependent indices of cardiac function (e.g. dP/dt, developed pressure, cardiac output) in ischemic and failing hearts [3;27;28;32;41]. This effect is also evident in the current studies by the marked increase in cardiac output in GLP-1 (7–36) treated swine following the induction of regional myocardial ischemia (Figure 2D). However, no prior study has directly assessed preload-independent measures of cardiac contractility in response to GLP-1 to distinguish true inotropic effects from Frank-Starling effects. This distinction has important implications for the clinical circumstances where these effects can be used to advantage, or importantly where the true nature of the effects might imply adverse outcomes. In order to examine this key issue, we employed high sensitivity pressure volume catheters to obtain end systolic pressure volume relationships (ESPVR), the “gold-standard” measure of cardiac inotropy [9;34]. ESPVR is experimentally measured by progressive reductions in ventricular preload via transient balloon occlusion of the inferior vena cava, such that increases in contractility (inotropy) augment pressure at a given ventricular volume resulting in an elevation in the slope of ESPVR (see effect of 10µg/min epinephrine in Figure 3B). In the current study, GLP-1 (7–36) did not affect the slope of ESPVR at any dose in normal hearts, or following acute ligation of the left circumflex coronary artery. These findings indicate that GLP-1 mediated increases in cardiac output observed following the onset of acute, regional ischemia are independent of changes in myocardial contractility. However, our data do not address the potential inotropic effects of longer term GLP-1 administration, an avenue meriting future studies.
Starling effects of GLP-1 (7–36)
Preload-independent assessments of cardiac contractility include two key measurements; slope of ESPVR and the volume axis intercept (V0 – ventricular volume at zero pressure). Under controlled conditions, a shift in V0 indicates a volume dependent action on contractile force making V0 a suitable index of the Frank-Starling mechanism. We found that 10.0 pmol/kg/min GLP-1 (7–36) administration significantly increased V0 in both normal and ischemic hearts (Table 2). Additionally, GLP-1 (7–36) treatment significantly increased left ventricular end diastolic volume (Figure 2A) with no significant change in end systolic volume (Figure 2B) during regional ischemia. This phenomenon is readily apparent by examination of averaged left ventricular pressure-volume relationships at the 10.0 pmol/kg/min dose (vehicle or GLP-1 analogues) during normal perfusion and ischemia (Figure 3) and by examination of the relationship between end-diastolic volume and cardiac output in Figure 4. This effect was maintained in the presence of the non-depolarizing ganglionic blocker hexamethonium (Figure 4). Taken together, these findings indicate that acute administration of GLP-1 (7–36) significantly increases in cardiac output mediated through a preload-dependent, Frank-Starling effect via effects on the heart itself as opposed to via centrally mediated processes.
Since the initial description of load-dependent changes in cardiac contractile force [20], numerous molecular phenomena have been implicated as the mechanism of cardiac heteromeric autoregulation (i.e. Frank-Starling Responses) [1;9;10;12–14;16;17;32]. While the precise mechanisms responsible for this effect of GLP-1 (7–36) remain unclear it is important to point out that the increases in cardiac output occurred without concomitant increase in myocardial oxygen consumption; i.e. GLP-1 (7–36) augmented cardiac efficiency during ischemia (Figure 5). We postulate this effect is related at least in part to optimization of the myocellular contractile apparatus (i.e. Starling effect) and/or energetically favorable alterations in myocardial substrate metabolism (i.e. augmented glucose uptake) [18;22;23;25;37]. Understanding of these mechanisms could have significant therapeutic relevance for development of energetically favorable therapies for heart failure.
CONCLUSION
Short-term systemic exposure to GLP-1 (7–36) augments cardiac output under conditions of ischemia through increases in preload (Frank-Starling mechanism) without direct effects on contractility or other centrally mediated phenomena. This “Starling” response was facilitated by enhanced cardiac relaxation as indicated by elevations in V0. Neither GLP-1 (7–36) nor (9–36) affected coronary flow or systemic pressure regulation, and in contrast to intact GLP-1 (7–36), the GLP-1 (9–36) fragment did not exert any effects on cardiac output during ischemia. Taken together, these results support a role for GLP-1 (7–36) in enhancing cardiac output under conditions of regional myocardial ischemia. This enhancement is energetically favorable as the process is a passive response resulting from facilitation of diastolic filling as opposed to an active inotropic mechanism.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge financial support from multiple agencies. This work was supported by a National Institutes of Health grant, HL117620 (J. Tune and K. Mather, PI). Dr. Goodwill was supported by National Institutes of Health T32HL079995 (K. March, PI) and American Heart Association 13POST1681001813 (A. Goodwill, PI). Mr. Conteh was supported by National Institutes of Health HL117620-S1 (J. Tune and K. Mather, PI). Mr. Sassoon was supported by grant number TL1 TR000162 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Reference List
- 1.Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, Hayden MR, Johnson MS, Salam M, Whaley-Connell A, Demarco VG. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology. 2013;154:2501–2513. doi: 10.1210/en.2013-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 4.Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277:E784–E791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- 5.Barragan JM, Rodriguez RE, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7–36) amide in rats. Am J Physiol. 1994;266:E459–E466. doi: 10.1152/ajpendo.1994.266.3.E459. [DOI] [PubMed] [Google Scholar]
- 6.Barragan JM, Rodriguez RE, Eng J, Blazquez E. Interactions of exendin-(9–39) with the effects of glucagon-like peptide-1-(7–36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept. 1996;67:63–68. doi: 10.1016/s0167-0115(96)00113-9. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, Low PA. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R874–R880. doi: 10.1152/ajpregu.00153.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bojanowska E, Stempniak B. Effects of centrally or systemically injected glucagon-like peptide-1 (7–36) amide on release of neurohypophysial hormones and blood pressure in the rat. Regul Pept. 2000;91:75–81. doi: 10.1016/s0167-0115(00)00119-1. [DOI] [PubMed] [Google Scholar]
- 9.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–H512. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 10.Calaghan S, White E. Activation of Na+-H+ exchange and stretch-activated channels underlies the slow inotropic response to stretch in myocytes and muscle from the rat heart. J Physiol. 2004;559:205–214. doi: 10.1113/jphysiol.2004.069021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deacon CF. Therapeutic strategies based on glucagon-like peptide 1. Diabetes. 2004;53:2181–2189. doi: 10.2337/diabetes.53.9.2181. [DOI] [PubMed] [Google Scholar]
- 12.Feigl EO, Neat GW, Huang AH. Interrelations between coronary artery pressure, myocardial metabolism and coronary blood flow. J Mol Cell Cardiol. 1990;22:375–390. doi: 10.1016/0022-2828(90)91474-l. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, DeStefano F. Risk factors for mortality from all causes and from coronary heart disease among persons with diabetes. Findings from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Am J Epidemiol. 1991;133:1220–1230. doi: 10.1093/oxfordjournals.aje.a115834. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs F, Wang YP. Sarcomere length versus interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol. 1996;28:1375–1383. doi: 10.1006/jmcc.1996.0129. [DOI] [PubMed] [Google Scholar]
- 15.Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther. 2006;316:852–859. doi: 10.1124/jpet.105.093104. [DOI] [PubMed] [Google Scholar]
- 16.Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon AM, Ridgway EB. Cross-bridges affect both TnC structure and calcium affinity in muscle fibers. Adv Exp Med Biol. 1993;332:183–192. doi: 10.1007/978-1-4615-2872-2_17. [DOI] [PubMed] [Google Scholar]
- 18.Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isbil-Buyukcoskun N, Gulec G. Effects of intracerebroventricularly injected glucagon-like peptide-1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul Pept. 2004;118:33–38. doi: 10.1016/j.regpep.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Katz AM. Ernest Henry Starling, his predecessors, and the "Law of the Heart". Circulation. 2002;106:2986–2992. doi: 10.1161/01.cir.0000040594.96123.55. [DOI] [PubMed] [Google Scholar]
- 21.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. doi: http://dx.doi.org/10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 22.Knaapen P, Germans T, Knuuti J, Paulus WJ, Dijkmans PA, Allaart CP, Lammertsma AA, Visser FC. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation. 2007;115:918–927. doi: 10.1161/CIRCULATIONAHA.106.660639. [DOI] [PubMed] [Google Scholar]
- 23.Moberly SP, Berwick ZC, Kohr M, Svendsen M, Mather KJ, Tune JD. Intracoronary glucagon-like peptide 1 preferentially augments glucose uptake in ischemic myocardium independent of changes in coronary flow. Exp Biol Med (Maywood) 2012;237:334–342. doi: 10.1258/ebm.2011.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moberly SP, Mather KJ, Berwick ZC, Owen MK, Goodwill AG, Casalini ED, Hutchins GD, Green MA, Ng Y, Considine RV, Perry KM, Chisholm RL, Tune JD. Impaired cardiometabolic responses to glucagon-like peptide 1 in obesity and type 2 diabetes mellitus. Basic Res Cardiol. 2013;108:365. doi: 10.1007/s00395-013-0365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow DA, Givertz MM. Modulation of myocardial energetics: emerging evidence for a therapeutic target in cardiovascular disease. Circulation. 2005;112:3218–3221. doi: 10.1161/CIRCULATIONAHA.105.581819. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen TD, Shingu Y, Amorim PA, Schwarzer M, Doenst T. Glucagon-like peptide-1 reduces contractile function and fails to boost glucose utilization in normal hearts in the presence of fatty acids. Int J Cardiol. 2013;168:4085–4092. doi: 10.1016/j.ijcard.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 28.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 29.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 30.Ossum A, van DU, Engstrom T, Jensen JS, Treiman M. The cardioprotective and inotropic components of the postconditioning effects of GLP-1 and GLP-1(9–36)a in an isolated rat heart. Pharmacol Res. 2009;60:411–417. doi: 10.1016/j.phrs.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Penna C, Pasqua T, Perrelli MG, Pagliaro P, Cerra MC, Angelone T. Postconditioning with glucagon like peptide-2 reduces ischemia/reperfusion injury in isolated rat hearts: role of survival kinases and mitochondrial KATP channels. Basic Res Cardiol. 2012;107:272. doi: 10.1007/s00395-012-0272-6. [DOI] [PubMed] [Google Scholar]
- 32.Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ, Jr, Maher TD, Dean DA, Bailey SH, Marrone G, Benckart DH, Elahi D, Shannon RP. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 34.Suga H. Ventricular energetics. Physiol Rev. 1990;70:247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- 35.Thrainsdottir I, Malmberg K, Olsson A, Gutniak M, Ryden L. Initial experience with GLP-1 treatment on metabolic control and myocardial function in patients with type 2 diabetes mellitus and heart failure. Diab Vasc Dis Res. 2004;1:40–43. doi: 10.3132/dvdr.2004.005. [DOI] [PubMed] [Google Scholar]
- 36.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 37.Tune JD, Mallet RT, Downey HF. Insulin improves cardiac contractile function and oxygen utilization efficiency during moderate ischemia without compromising myocardial energetics. J Mol Cell Cardiol. 1998;30:2025–2035. doi: 10.1006/jmcc.1998.0763. [DOI] [PubMed] [Google Scholar]
- 38.Vila Petroff MG, Egan JM, Wang X, Sollott SJ. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res. 2001;89:445–452. doi: 10.1161/hh1701.095716. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zander M, Madsbad S, Deacon CF, Holst JJ. The metabolite generated by dipeptidyl-peptidase 4 metabolism of glucagon-like peptide-1 has no influence on plasma glucose levels in patients with type 2 diabetes. Diabetologia. 2006;49:369–374. doi: 10.1007/s00125-005-0098-y. [DOI] [PubMed] [Google Scholar]
- 41.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]