Abstract
Background
Abuse of alcohol during adolescence continues to be a problem, and it has been shown that earlier onset of drinking predicts increased alcohol abuse problems later in life. High levels of impulsivity have been demonstrated to be characteristic of alcoholics, and impulsivity has also been shown to predict later alcohol use in teenage subjects, showing that impulsivity may precede the development of alcohol use disorders. These experiments examined adolescent drinking in a high-drinking, relatively impulsive mouse population, and assessed its effects on adult drinking and adult impulsivity.
Methods
Experiment 1: Selectively bred High-Alcohol Preferring (HAPII) mice were given either alcohol (free choice access) or water only for two weeks during middle adolescence or adulthood. All mice were given free choice access to alcohol 30 days later, in adulthood. Experiment 2: Adolescent HAPII mice drank alcohol and water, or water alone, for two weeks, and were then trained to perform a delay discounting task as adults to measure impulsivity. In each experiment, effects of volitional ethanol consumption on later behavior were assessed. We expected adolescent alcohol exposure to increase subsequent drinking and impulsivity.
Results
Mice consumed significant quantities of ethanol, reaching average blood ethanol concentrations (BECs) of 142 mg/dl (adolescent) or 154 mg/dl (adult) in Experiment 1. Adolescent mice in experiment 2 reached an average of 108 mg/dl. Mice exposed to alcohol in either adolescence or adulthood showed a transient increase in ethanol consumption, but we observed no differences in impulsivity in adult mice as a function of whether mice drank alcohol during adolescence.
Conclusions
These findings indicate that HAPII mice drink intoxicating levels of alcohol during both adolescence and adulthood, and that this volitional intake has long-term effects on subsequent drinking behavior. Nonetheless, this profound exposure to alcohol during adolescence does not increase impulsivity in adulthood, indicating that long-term changes in drinking are mediated by mechanisms other than impulsivity.
Key words/phrases: alcoholism, impulsivity, adolescence, ethanol consumption, alcoholism animal model
Introduction
Alcohol abuse among adolescents is a problem in the U.S. Earlier onset of drinking is correlated with increased alcohol abuse problems later in life; for example, one study showed that among high school students, those who drank alcohol before age 13 were 7 times more likely to binge drink 6 or more times per month than students who did not drink prior to high school (Grunbaum et al., 2004). Additionally, it is estimated that 45% of subjects that drink alcohol before age 14 develop alcohol abuse disorders, compared with 10% who start drinking after age 21 (Grant and Dawson, 1997). Adolescent heavy drinkers tend to drink less frequently than adults with alcohol use disorders (AUDs), but drinking episodes are marked by excessive binge-like consumption (Schulenberg et al., 1995). Risk factors for binge drinking in adolescents include attention-deficit/hyperactivity disorder and conduct disorder, each of which is characterized by the underlying factor of high levels of impulsive behavior, or impulsivity (4th ed., DSM-IV, APA, 2000). Furthermore, strong risk factors for AUDs in adulthood are anti-social personality disorder and bipolar disorder, which also share the symptom of impulsivity (Grant et al., 2005).
A suggested mechanism for the correlation between an onset of drinking in adolescence and continued alcohol use problems is that adolescence represents a critical period of cortical development (Crews et al., 2007). Adolescents tend to be highly active and seek sensation and novelty, traits that manifest themselves differently across individuals (Spear, 2000). It has been suggested that activities engaged in during adolescence become habits in adulthood, partially due to the shaping of the brain during this period (Crews et al., 2007). Importantly, the prefrontal cortex (PFC) and limbic system undergo many developmental changes during adolescence which may be stunted by substance use (Crews and Boettiger, 2009). However, genetics may be a more crucial factor in alcoholism (Knopik et al., 2009). Endophenotypes are heritable biological processes and trait behaviors that may underlie, and are genetically related to, dysfunction including addiction and comorbid disorders (Gottesman and Gould, 2003). Evidence from many human and animal studies supports impulsivity as a candidate endophenotype for alcohol-related disorders (Ernst et al., 2006; Mitchell et al., 2006; Oberlin and Grahame, 2009; Petry, 2001). However, the possibility that adolescent alcohol use increases impulsivity beyond heritable levels is difficult to study in humans.
Impulsivity has been categorized into components, one of which is cognitive impulsivity, or impulsive choice. This is generally represented by difficulties in waiting for a reward or in declining an immediate reward associated with later punishment (Arce and Santisteban 2006). Most studies of impulsivity and alcoholism in humans have concentrated on cognitive impulsivity as measured by the Iowa gambling task (IGT) or delay discounting (DD) tasks (Bechara et al., 1994; Mazur, 1987). DD operationally defines impulsivity as preference for smaller, immediate rewards over larger, delayed rewards (Rachlin and Green, 1972), and has revealed a positive relationship between alcohol preference and impulsivity, in naïve animals and alcohol-naïve or abstinent human subjects with a family history of alcoholism (Bjork et al., 2004; Ernst et al., 2006; Mitchell et al., 2006; Oberlin and Grahame, 2009; Petry, 2001). Additionally, earlier onset of alcohol abuse during adolescence correlates with increased impulsivity in abstinent adult subjects (Bjork et al., 2004). The question remains whether heightened impulsivity in these early drinkers causes or stems from the alcohol drinking experience. By manipulating voluntary alcohol consumption prior to impulsivity testing, we sought to assess the ability of past alcohol consumption to influence both impulsivity and subsequent drinking behavior.
Lines of high-alcohol preferring (HAP) and low-alcohol preferring (LAP) mice were bidirectionally selected for high and low voluntary ethanol consumption (Grahame et al., 1999; Oberlin et al., 2011). HAPII mice are impulsive relative to LAPII mice as measured by DD, making them a valid animal model of an impulsive, at-risk human population (Oberlin and Grahame, 2009). It is unknown if HAPII mice are useful developmental model for studying the ontogeny of alcohol-drinking behaviors. Adolescent rodents differ from adults on measures of novelty-seeking, impulsivity, and stress-responsivity, modeling behaviors observed in teenage humans (Hefner and Holmes, 2007; Spear, 2000). The adaptations in neural circuitry, neurobehavioral characteristics, and rapid body growth that are most comparable to the teenage years in humans occur during early and middle adolescence, comprising postnatal days (P) 28–42 (Spear, 2000). However, even high-drinking rodent populations do not drink sufficient alcohol during 24-h, free-choice access to induce overt intoxication, making it difficult to study pharmacologically relevant drinking behavior in adolescents (Bell et al., 2006; Crabbe et al., 2010).
Studies have examined adolescent and adult drinking concurrently using rats (i.e. Bell et al., 2006; Siegmund et al., 2005; Vetter et al., 2007), and assessed behavioral changes in adulthood following adolescent ethanol exposure (i.e. Fullgrabe et al., 2007; Salimov et al., 1996; Siegmund et al., 2005), but relatively few studies have performed similar assessments of adolescent and adult drinking using mice. One study that did compare adolescents and adults under conditions that led to pharmacologically relevant intakes showed that adolescent C57BL/6J mice, a commonly used high-drinking inbred strain, consume more ethanol than their adult counterparts during 2-h daily access periods during which water is unavailable (Moore et al., 2010). However, adolescents and adults did not differ in blood ethanol concentrations (BECs) from adult mice under these conditions. HAPII mice freely drink far greater amounts of ethanol than C57 mice during free-choice access (Matson and Grahame, 2011; Oberlin et al., 2011; Yoneyama et al., 2008), suggesting that free-choice drinking in HAPII mice may be an effective model for studying adolescent volitional alcohol intake and its potential effects.
To examine the adolescent free-choice drinking patterns of HAPII mice and test the effects of exposure to ethanol on later drinking, a two-bottle choice drinking study was conducted using adolescent and adult animals. This experiment featured concurrent characterization alcohol drinking patterns during the dark-cycle (when the vast majority of intake occurs) in adults alongside adolescents, to assess whether the patterns were consistent with similar levels of pharmacologically relevant alcohol exposure. After a period of abstinence, drinking was re-examined when both populations were adults. We hypothesized that adolescent exposure would increase ethanol intake in adulthood, and that adolescent animals would drink more than adult animals. Additionally, we assessed the effects of free-choice adolescent drinking on adult impulsivity in HAPII mice, using the DD task to assess differences in impulsivity in adulthood. We hypothesized that mice that freely drink ethanol during adolescence would demonstrate greater adult impulsivity, and that increases in impulsivity within that group would depend on the amount of ethanol consumed during adolescence.
Materials and Methods
Experiment 1 – Drinking in Adolescence and Adulthood
Animals
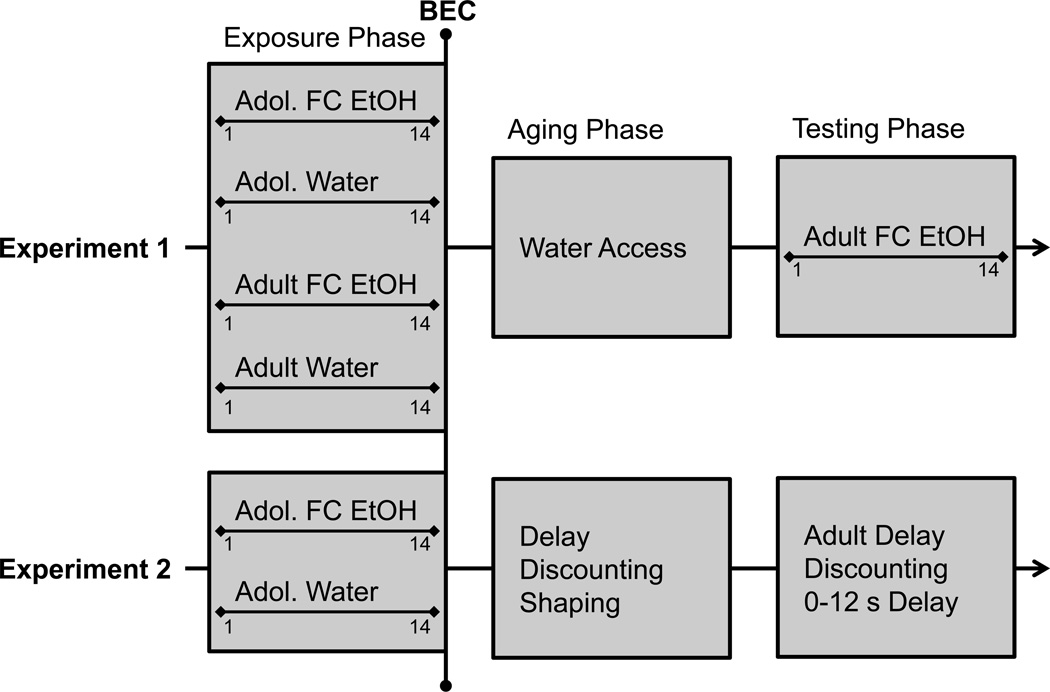
60 mice were bred on-site from HAPII progenitors, and represented the 37th (adolescent) and 38th (adult) generation of HAPIIs. Eight litters of adolescent mice and 8 litters of adult mice were used, each litter yielding 1–2 animals of each sex. Mice were counterbalanced across sex and litter within each age group into a water and alcohol group such that 7 of 8 litters were represented in each treatment group. Mice were individually housed in polycarbonate cages (27.9×9.5×12.7 cm) with Cellsorb bedding at an ambient temperature of 21 ± 1° C starting one week prior to the Exposure Phase. A reverse light cycle was used, with lights on from 20:00 to 08:00 hours. Mice had ad lib access to food and water during all phases. Animals in the adolescent group were 28 ± 2 days old at the beginning of the Exposure Phase, while animals in the adult group were 60 ± 2 days old. These two age groups were run concurrently. A timeline for this experiment is displayed in Figure 1.
Figure 1.
Timeline schematic of Experiment 1 and Experiment 2. Exposure (alcohol drinking), Aging (deprivation or shaping), and Testing (drinking or delay discounting) phases are shown for each experiment.
Exposure Phase
Procedure
During the Exposure Phase, adolescent and adult Alcohol Group mice drank from two 25ml graduated cylinders containing either water or 10% EtOH solution. Food was placed on the floor. Water group adolescent and adult mice also drank from two 25ml graduated cylinders, but both contained water. Volume readings were taken daily at the same time for 14 days using red illumination, with the exception of the last 2 days, when readings were taken every 2 hours from 08:00 hours to 20:00 hours. On these 2 days only, mice drank from 10ml serological pipets. Bottle sides were alternated every third day to eliminate side preference. Body weights were also taken at this time.
Retro-orbital Bloods
Based on the results of multiple dark-cycle bottle readings, we determined that retro-orbital blood samples taken at 16:00 hours would provide a good estimation of peak blood ethanol concentrations (BECs). On the 15th day of the Exposure Phase, mice were taken to another room for sample collection via the retro-orbital sinus within 5 min of removing alcohol bottles, using a light-shielded transporter. Bloods from water group mice were discarded, while alcohol group bloods were centrifuged, and a 1µL sample of the plasma was analyzed for ethanol concentration using gas chromatography against a 0–250mg/dl standard. Mice were then returned to the colony room and water bottles were restored.
Data Analysis
Intake data from each Phase was organized into SPSS and repeated-measures analyses of variance (ANOVAs), with group, age, and sex as between-subjects factors and day as a within-subjects factor, were performed. The final 2 days of the experiment were separately analyzed to assess the pattern of daily intakes. ANOVAs were used to assess water intakes in ml/kg, weights of animals, and bihourly alcohol intake data. Individual ANOVAs were used to assess day-by-day differences of ethanol drinking and preference. A p-value of 0.05 was set as the significance threshold for statistical tests throughout experiments.
Testing Phase – Adult Drinking
The Testing Phase began 30 days after the completion of the Exposure Phase and was followed a similar protocol, but all animals had alcohol and water access, and bihourly intakes were not measured. Data from this phase were analyzed in the same manner as the Exposure Phase.
Experiment 2 – Adolescent Drinking and Adult Impulsivity
Animals
48 HAPII, S37 mice were bred on-site. Six litters of adolescent mice were used, each litter yielding 2–6 animals of each sex. Mice were counterbalanced across sex, litter, and DD run order into a water and alcohol group. Given the presence of multiple littermates in like treatment conditions, data from these animals were averaged and treated as an n of 1 in statistical analysis. Mice were housed identically to Experiment 1. Animals were transported into the experimental room on P22/P23 and single-housed on P25/P26. Mice had ad lib access to lab food during all phases. During the Exposure Phase mice had 24-hour access to water, while in the Testing Phase mice had restricted water access for 2 hours a day after testing. Mice were 28/29 days old at the beginning of the Exposure Phase, 46/47 days old at the beginning of Testing Phase shaping, and 105 ± 15 days old during the collection of target DD data. A timeline for this experiment is displayed in Figure 1.
Exposure Phase
All data collection procedures were modeled after those described for Experiment 1 Exposure Phase. A repeated-measures ANOVA with group and sex as between-subjects factors and day as a within-subjects factor was performed.
Testing Phase – Adult Impulsivity
Procedure
DD shaping began at P46/47. Mice were moved to the testing room using a light-shielded transporter unit at 10:00 hours, and were tested 5 days a week. Operant boxes were wiped with a wet sponge prior to each session. At the end of daily testing, all mice received 2-hour water access in their home cage. Additionally, mice were weighed and had their ear tags checked each Monday. During weekends, they had ad lib access to water, and were deprived at 5 PM on Sundays.
Apparatus and Behavioral Assessment
Twelve identical operant boxes were configured according to protocol of Oberlin and Grahame (2009). These boxes featured a nosepoke light, two levers with lights, and descending sipper tube to deliver saccharin (0.032%) reinforcement. Boxes were controlled using MedPC IV software on a PC. Mice underwent 5 stages of shaping with the final stage serving as 0-second delay testing, according to procedure of Oberlin and Grahame (2009). If mice failed to complete 20 trials during Stages 1–4 of shaping or to achieve adjusted amounts of 1.6 seconds or higher on 3 consecutive days during Stage 5, they were excluded from testing. Delays of 1, 2, 4, 8, and 12 seconds were introduced, and each delay received 4 days of testing.
Data Analysis
Adjusted amounts, saccharin intake, and trials completed were organized using Microsoft Excel spreadsheets. K values, overall constants representing impulsivity for each subject, were generated as described by Oberlin and Grahame (2009). A multivariate ANOVA examined for effects of group and sex, as well as any interaction effects, on adjusted amount data, while a univariate ANOVA assessed effects on k values. Individual ANOVAs were also performed on each delay. Paired-samples t-tests tested for overall differences between each consecutive delay. Additionally, k values were correlated with both the first day of drinking in adolescence to assess effects of novelty on impulsivity as well as all averaged days of drinking to assess high consumption’s effect on impulsivity.
Results
Experiment 1
Exposure Phase
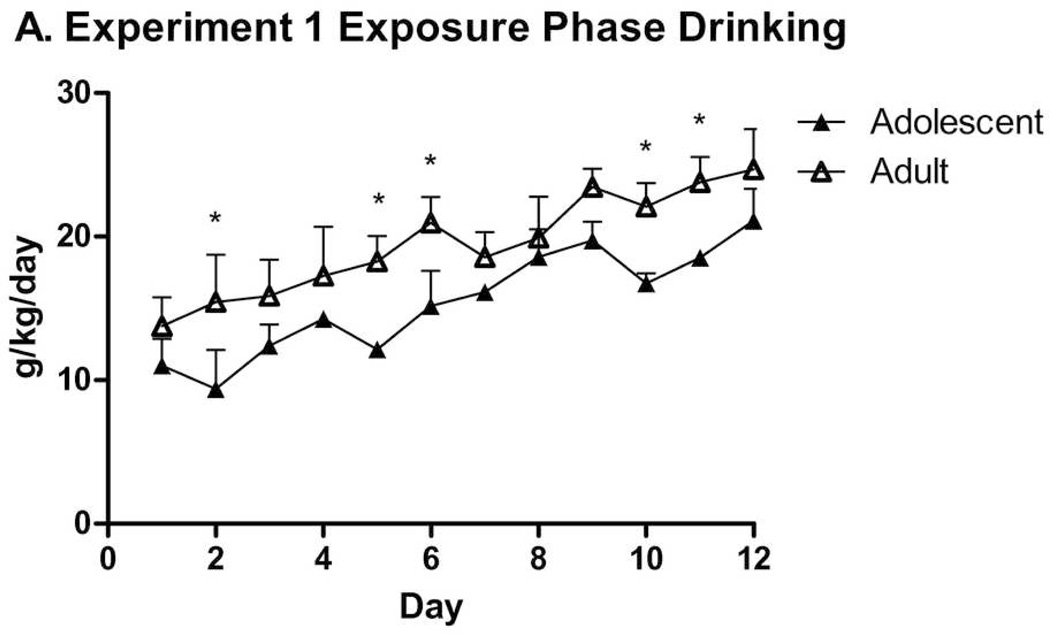
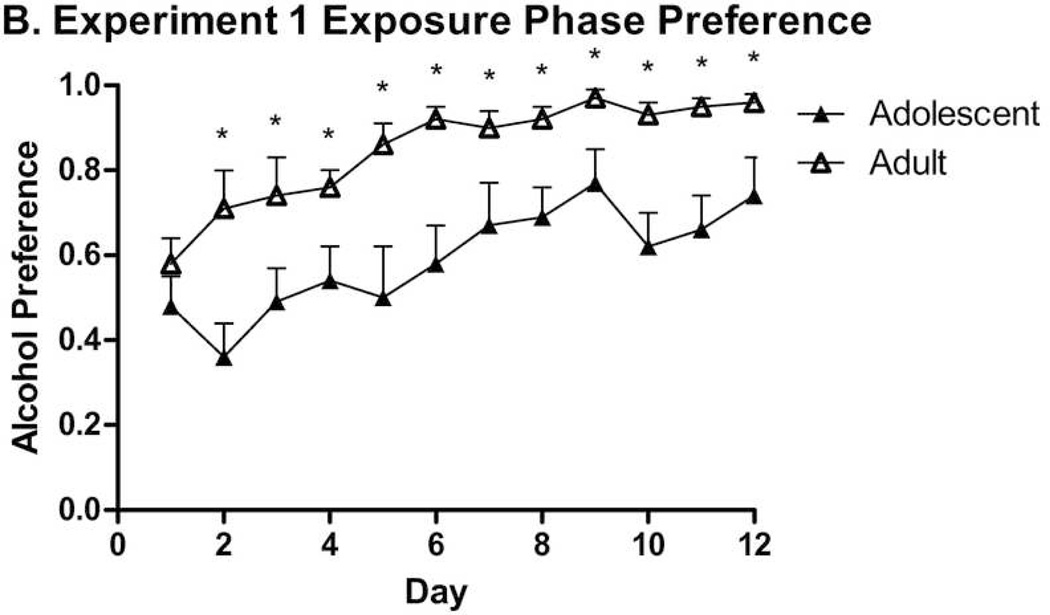
All subjects used in Experiment 1 proceeded through to its completion. Mice increased g/kg alcohol intake over the course of the first 12 days (Fig. 2-A). A repeated-measures ANOVA revealed significant effects of Day (F(11,286) = 24.52, p < .001) and Sex (F(1,26) = 6.75, p = .015), but no significant Day × Sex or Day × Age interaction effects. Contrary to our hypothesis, adult animals drank more than adolescent animals (F(1,26) = 10.45, p = .003; Madult = 19.35 ± 0.42 g/kg/day, Madolescent = 15.50 ± 0.51 g/kg/day). While a Sex × Age interaction was not seen, a second ANOVA on adolescent data only revealed no effect, whereas an ANOVA considering adult data only showed a significant sex difference (F(1,13) = 12.70, p = .003). Overall means and SEMs (n = 7 or 8): adult female: 21.75 ± 0.46; adult male: 17.25 ± 0.59; adolescent female: 16.46 ± 0.74; adolescent male: 14.41 ± 0.67. Day-by-day intake stratified by sex is seen in Table 1. Adult consumptions were consistent with prior HAPII data (Oberlin et al., 2011). Alcohol preference data demonstrated an Age difference consistent with reduced g/kg ethanol consumption and greater water consumption by adolescents (F(1,26) = 29.29, p < .001; Fig. 2-B). An ANOVA on ml/kg water drinking data, stratified by Group, revealed similar effects of Age (Fs(1,26) ≥ 15.84, ps < .001) and Day (Fs(11,286) ≥ 4.94, p < .001) in each group. A Day × Sex interaction was in the water group (F(11,286) = 4.067, p < .001), but not the ethanol group (Table 2).
Figure 2.
A. Experiment 1 Exposure Phase overall drinking in adolescent (n = 15) and adult (n = 15) animals, collapsed across sex, over 12 days, from P28–P39 or P60–P71. Adult animals consumed significantly more ethanol over this time period, and significant differences on individual days are marked by *’s (p < .05). B. Experiment 1 Exposure Phase alcohol preference in adolescent and adult groups over 12 days. Adult animals demonstrated a significantly higher alcohol preference over the entire data set, and significant differences on individual days are marked by *’s (p < .05). Data in these figures and others are expressed as mean + SEM.
Table 1. Experiment 1 Exposure Phase Ethanol Intake by Sex (g/kg/day).
Alcohol drinking in g/kg/day throughout the first 12 days of Experiment 1 Exposure Phase, separated by sex. These data suggest higher intakes by females regardless of age, though a significant effect is only seen in adult animals.
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adol M | 12.9 | 6.7 | 10.9 | 14.1 | 11.6 | 12.7 | 15.9 | 16.6 | 18.4 | 16.0 | 18.2 | 18.9 |
| Adol F | 9.1 | 12.1 | 13.9 | 14.5 | 12.7 | 17.6 | 16.4 | 20.5 | 21.0 | 17.4 | 18.9 | 23.3 |
| Adult M | 11.8 | 12.2 | 13.3 | 13.8 | 16.5 | 19.2 | 16.8 | 17.0 | 22.2 | 20.4 | 22.0 | 21.9 |
| Adult F | 15.8 | 18.7 | 18.4 | 20.7 | 20.1 | 22.8 | 20.3 | 22.8 | 24.8 | 23.7 | 25.6 | 27.5 |
Table 2. Experiment 1 Exposure Phase Water Intake (ml/kg/day).
Water drinking in ml/kg/day throughout the first 12 days of Experiment 1 Exposure Phase. These data are suggestive of an increase in water drinking in adolescents, who consume more water than adults, and steady water drinking in adults that were not offered ethanol, and a decline in water drinking in all animals that were given a choice of ethanol or water.
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adol W | 385.7 | 378.4 | 404.8 | 399.8 | 372.8 | 418.5 | 421.6 | 443.8 | 424.2 | 375.9 | 441.0 | 440.0 |
| Adult W | 361.5 | 335.5 | 337.4 | 348.9 | 318.6 | 342.1 | 350.5 | 341.6 | 351.3 | 330.3 | 361.3 | 362.0 |
| Adol E | 183.1 | 229.3 | 173.6 | 166.6 | 171.9 | 145.4 | 117.8 | 112.9 | 79.5 | 132.2 | 121.6 | 90.6 |
| Adult E | 132.9 | 87.3 | 79.4 | 67.7 | 37.4 | 23.3 | 28.4 | 17.9 | 9.4 | 19.9 | 16.1 | 13.5 |
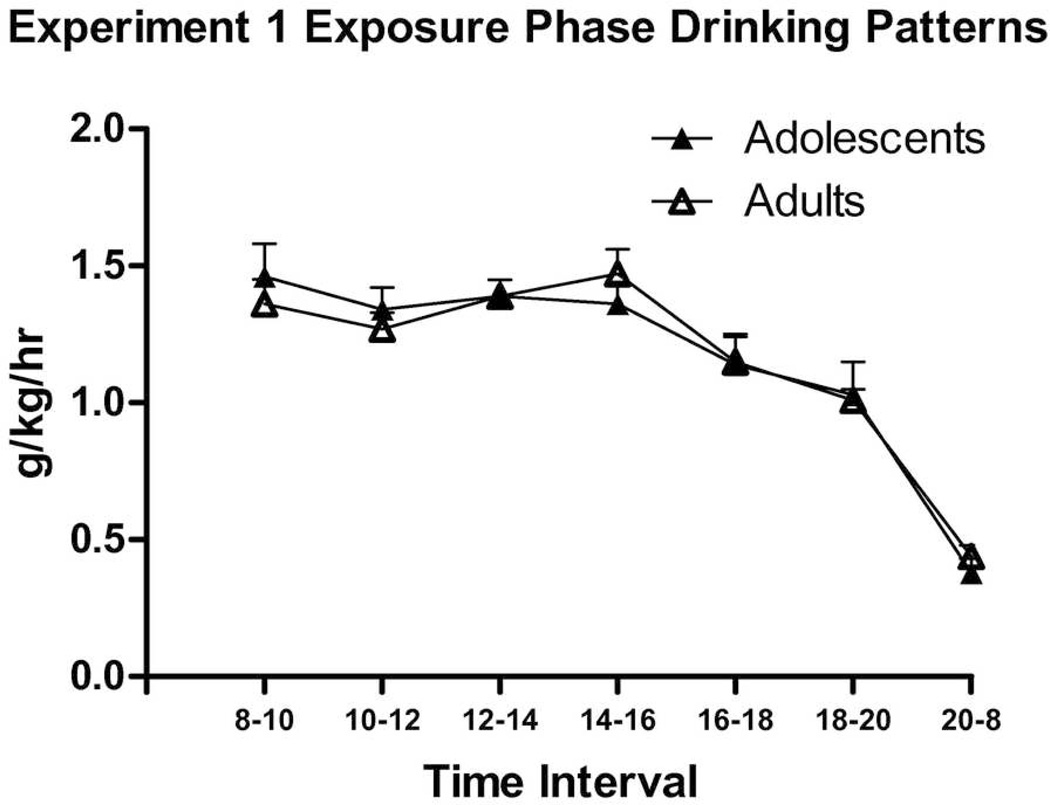
On days 13–14 of acquisition, surprisingly, no difference between ages was found. This lack of effect may be due to the change in methodology (the addition of intake readings every 2 hours), but it is additionally predicted by the non-significant age difference on Day 12. It may be that as adolescent mice aged, their intakes became more similar to adults, although as stated above, the Age × Day interaction missed significance. The similarity between adolescent and adult drinking patterns (Fig. 3; no significantly different time intervals) justified our decision to take bloods from adolescents and adults at the same time of day. Peak consumption was observed from 08:00–16:00 hours. Retro-orbital BEC data taken at 16:00 hours on Day 14 showed remarkably high BECs in adolescents (M = 141.8 ± 25.3 mg/dl) and adults (M = 154.3 ± 10.0 mg/dl); the difference between age groups was not statistically significant.
Figure 3.
Experiment 1 Exposure Phase g/kg/hr drinking patterns over 2 days, from P40–41 or P72–73. No significant differences were observed between adolescent (n = 15) and adult (n = 15) groups during any time interval.
An ANOVA comparing averaged weights on Days 3, 6, 9, and 12 revealed effects of Day (F(3,156) = 88.58, p < .001), Sex (F(1,52) = 21.48, p < .001), Age (F(1,52) = 76.65, p < .001), Day × Sex (F(3,156) = 3.21, p = .024), and Day × Age (F(3,156) = 8.59, p < .001). Importantly, no Group effects were seen. Data suggests that adolescents gained more weight than adults over the course of the Exposure Phase (seen in Table 3).
Table 3. Experiment 1 Exposure Phase Weights (g).
Weights in grams throughout Experiment 1 Exposure Phase, assessed on experimental days 3, 6, 9, and 12, which correspond to, on average, P30, P33, P36, and P39 for adolescents and P62, P65, P68, and P71 for adults. Significant differences were assessed between ages and sexes, and as a result of the progression of time, but no differences were seen between the alcohol and water groups.
| Day 3 | Day 6 | Day 9 | Day 12 | ||
|---|---|---|---|---|---|
| Males | Adult E | 26.1 | 26.4 | 26.2 | 27.0 |
| Adol E | 20.3 | 21.2 | 21.6 | 22.3 | |
| Adult W | 25.4 | 26.0 | 26.0 | 26.2 | |
| Adol W | 21.0 | 21.5 | 22.2 | 22.7 | |
| Females | Adult E | 22.8 | 23.2 | 23.5 | 24.2 |
| Adol E | 18.9 | 19.5 | 20.4 | 20.7 | |
| Adult W | 22.5 | 23.7 | 23.7 | 24.5 | |
| Adol W | 18.6 | 19.4 | 20.2 | 21.1 | |
| Overall | Adult E | 24.6 | 24.9 | 24.9 | 25.7 |
| Adol E | 19.5 | 20.3 | 21.0 | 21.5 | |
| Adult W | 23.9 | 24.8 | 24.7 | 25.3 | |
| Adol W | 19.8 | 20.5 | 21.3 | 21.9 |
Testing Phase – Adult Drinking
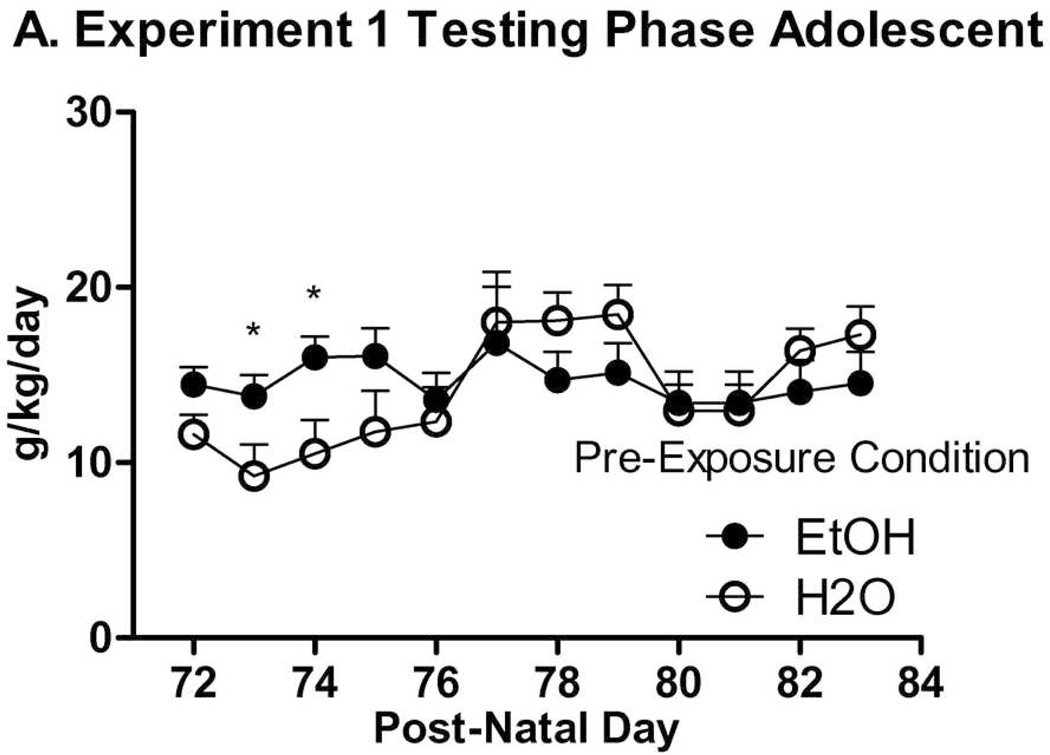
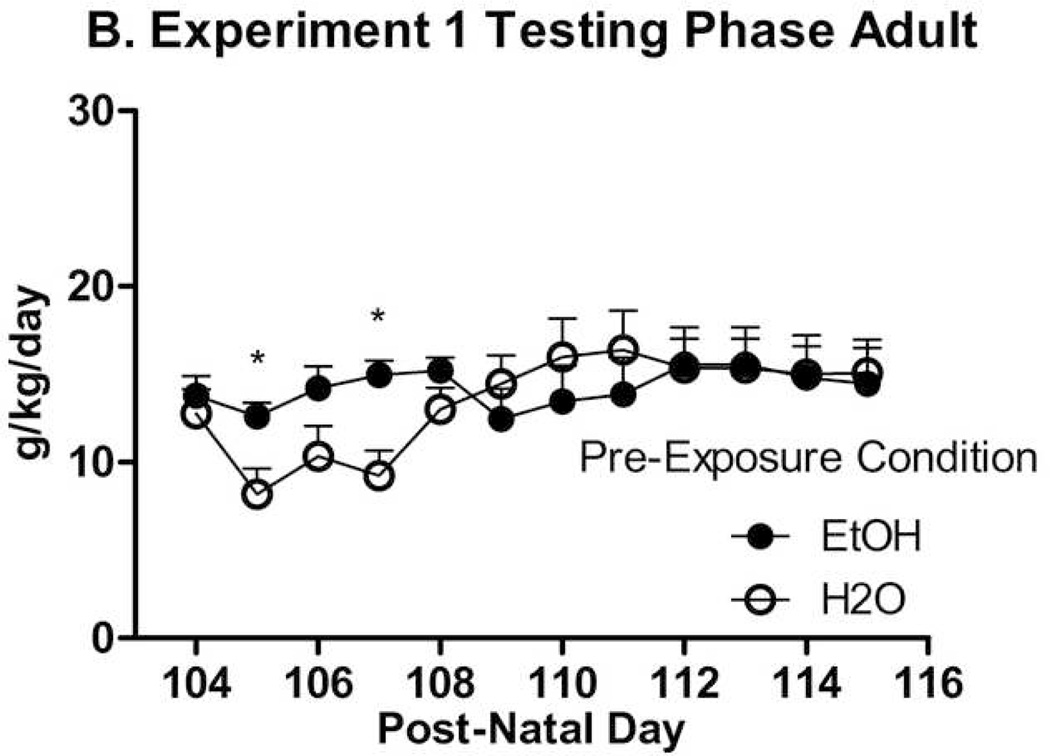
During this phase, data outliers representative of fluid spills were adjusted to the median of the day they occurred twice on Day 6 and once on Day 8. Inspection of data indicated that the groups converged after 8 days of alcohol access, so the first 8 days of alcohol access in the Testing Phase were statistically analyzed in isolation. ANOVA results revealed significant effects of Day (F(7,364) = 6.47, p < .001), Sex, and Group (Fs(1,52) ≥ 6.66, ps ≤ .013), and a Day × Group interaction (F(7,364) = 2.13, p = .040). As shown in Figure 4-A and -B, groups previously exposed to ethanol consumed significantly more ethanol on two of the first four days of post-exposure testing than ethanol-naïve groups as shown by individual ANOVAs. During the Testing Phase in general, animals failed to reach the typical level of intake for HAPIIs, although intake levels were comparable to the high-drinking inbred strain, C57Bl/6J (Yoneyama et al., 2008). Overall means and SEMs for exposed groups (n = 7 or 8): Adult female: 17.84 ± 0.46; adult male: 12.52 ± 0.69; adolescent female: 18.07 ± 0.55; adolescent male: 15.55 ± 0.50. Overall means and SEM’s for non-exposed groups (n = 7 or 8): Adult female: 14.95 ± 0.66; adult male: 11.73 ± 0.78; adolescent female: 16.20 ± 0.76; adolescent male: 11.59 ± 0.82.
Figure 4.
Experiment 1 Testing Phase. Alcohol exposed mice drank more alcohol than water exposed mice in both age groups, though non-exposed groups increased consumption over the first 8 days. Significant differences on individual days are marked by *’s (p < .05). A. Animals exposed to alcohol (n = 15) or not (n = 15) as adolescents. B. Animals exposed to alcohol (n = 15) or not (n = 15) as adults.
Experiment 2
Exposure Phase
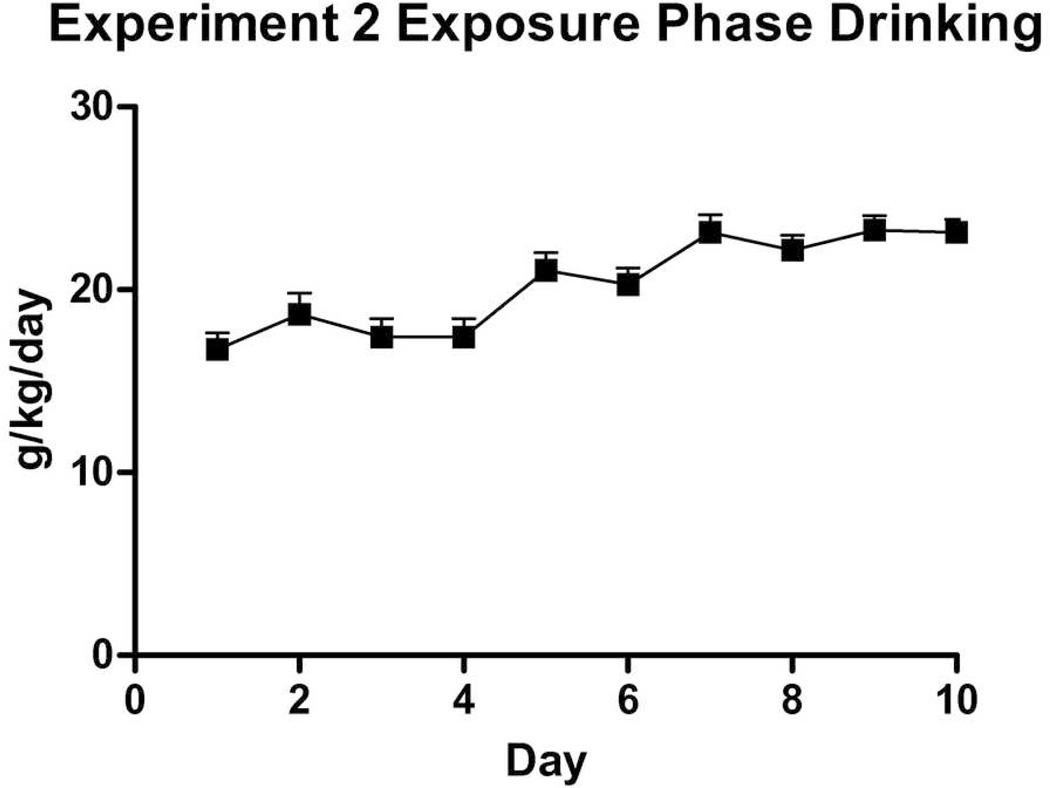
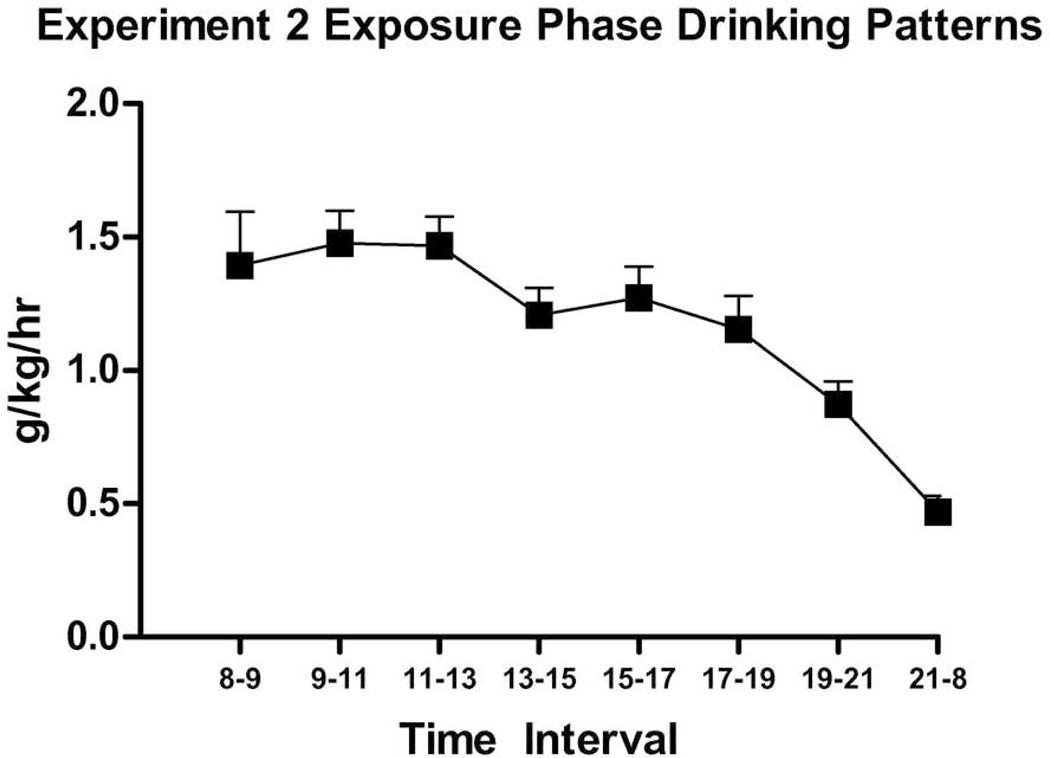
All subjects proceeded through to completion of this phase. A visibly less-pronounced increase in drinking over 10 days was observed in this experiment compared to Experiment 1 (Fig. 5). Nonetheless, a repeated-measures ANOVA revealed an effect of Day on ethanol consumption (F(9,198) = 13.03, p < .001) and as with Experiment 1 adolescents, no effect of Sex nor Sex × Day interaction (ps > 0.05). Bihourly readings showed high consumption from 08:00–13:00 hours and peak intakes at 11:00–13:00 hours (Fig. 6). Retro-orbital bloods were taken earlier in the day than in Experiment 1, at 12:00 hours, because of the observed peak in drinking levels from 11:00–13:00 hours. Bloods again showed high BECs in adolescents (M = 108.8 ± 13.3 mg/dl), though somewhat lower likely due to the four-hour difference in time. There was no sex difference observed in BECs.
Figure 5.
Experiment 2 Exposure Phase adolescent g/kg/day ethanol intake in adolescent mice (n = 24) over 10 days, from P28–P37. No sex effect was observed in these data.
Figure 6.
Experiment 2 Exposure Phase g/kg/hr drinking patterns over 3 days, from P38–P40. No sex effect was observed in these data.
Testing Phase – Adult Impulsivity
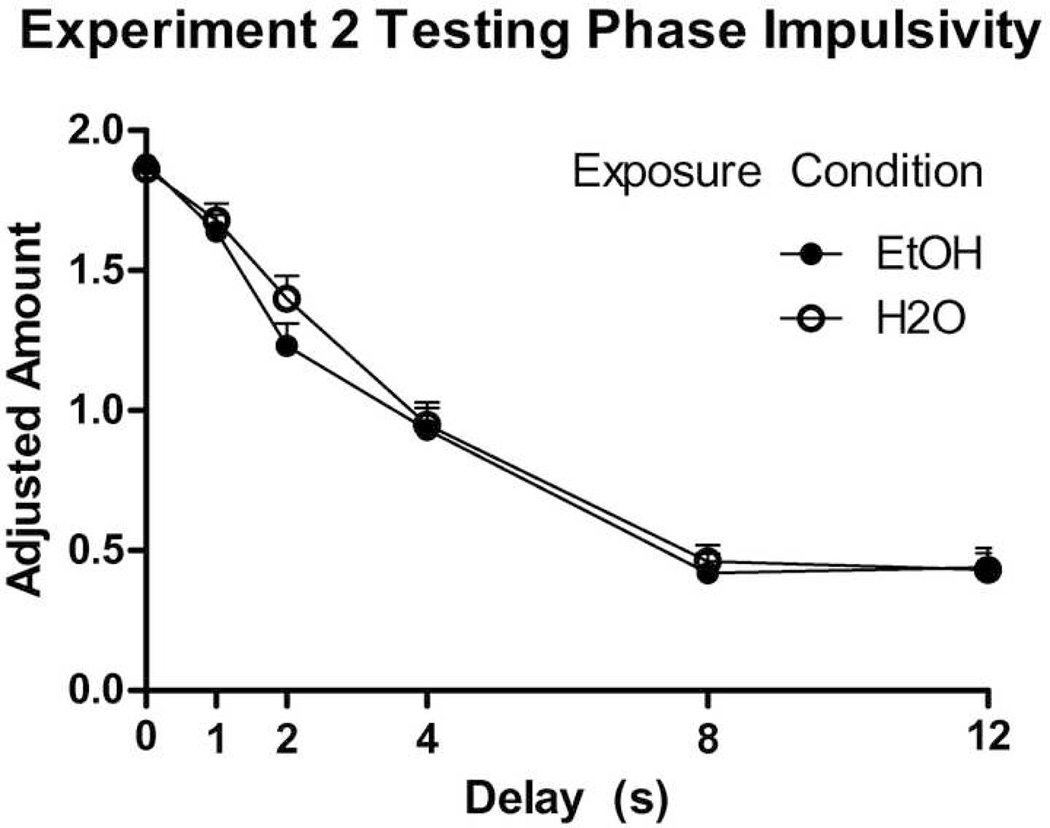
Three mice, all in the alcohol group, were not tested due to shaping difficulties. Nonetheless, there were not any Group or Sex differences throughout shaping. Significant Group (or Sex) differences were not observed at any delay (Fig. 7). A univariate ANOVA also revealed no Group (or Sex) differences in k values. However, each increase in delay decreased subjective valuation for all delays save the increase from 8 to 12 seconds (ps < .001). Pearson correlation analysis showed no relationship between k-values and either ethanol intake during the first day or total ethanol intake over the Exposure Phase (ps ≥ .343).
Figure 7.
Experiment 2 Testing Phase group mean adjusted amounts + SEM in seconds of access shown at each delay tested. While a trend toward increased impulsivity in the EtOH group is seen at the 2-second delay (p = .12), no significant group differences were observed at any delays measured.
Discussion
This research demonstrates that pharmacologically relevant consumption of ethanol during adolescence or adulthood facilitates later drinking, but that exposure to alcohol during adolescence does not increase adult cognitive impulsivity. Experiment 1 shows that free choice alcohol drinking facilitates later ethanol consumption in HAPII mice, regardless of age. Both adolescent and adult mice achieved high BECs throughout the dark cycle, characteristic of sustained intoxication. However, intake was only increased in the exposed groups during the first 4 days of re-exposure access. Results are complicated by the fact that all mice failed to reach intakes characteristic of HAPII mice. Overall, Experiment 1 only partly supports previous studies demonstrating sustained increased intakes in adulthood following ethanol exposure in C57BL/6J mice (Moore et al., 2010; Strong et al., 2010). Instead, these findings are more consistent with studies using lower-drinking populations such as Wistar rats (Siegmund et al., 2005), DBA/2J mice (Moore et al., 2010), WSC-1 mice (Tambour et al., 2008), and a vapor exposure study using Sprague-Dawley rats (Slawecki and Betancourt, 2002), in showing no or modest effects of adolescent exposure on adult intake. Altogether, these findings suggest genetics are a prime contributor to heavy ethanol consumption in these animal models, and genetic differences in baseline intake don’t predict which populations will show long-term increases in alcohol consumption following adolescent exposure.
Because P rats are bred to drink high levels of ethanol, like HAPII mice, results of adolescent drinking studies using these animals should be analogous to this research. Although Bell et al. (2006) showed significantly higher intakes in adolescents as compared to adult P rats, no age effects on BECs were observed. In addition, adolescent BECs were much lower than in the present findings, less than 60 mg/dl in spite of the use of concurrent access to multiple concentrations of alcohol. Another study with P rats showed that adolescents exposed to ethanol acquired EtOH operant responding quicker than non-exposed animals, a result in agreement with our transient increase in drinking (Rodd-Henricks et al., 2002). Our results uniquely suggest that ethanol exposure in adolescence or adulthood in a selectively bred high-drinking population such as HAPII mice causes elevated initial drinking levels relative to non-exposed animals, but that the non-exposed mice quickly show similar drinking levels. This effect may be due to established tolerance in exposed animals, or a behavioral or neurobiological difference that rapidly manifests itself in non-exposed mice upon ethanol exposure.
This experiment was also important because it was the first to assess adolescent and adult 24-hour free choice drinking concomitantly in a high-drinking mouse population. Other similar experiments looked at adolescent and adult ethanol consumption using the drinking-in-the-dark (DID) paradigm (Metten et al., 2011; Moore et al., 2010), and intermittent drinking (Melendez, 2011). Melendez (2011) and Moore et al. (2010) showed that adolescent C57BL/6J mice had higher intakes of ethanol using each paradigm than their adult counterparts, but without differences in BECs. Given these data along with increased intakes in adulthood seen following adolescent exposure in this strain, C57BL/6J mice appear to be uniquely affected by ethanol consumption during adolescence. Metten et al. (2011) demonstrated that adult mice selectively bred for high alcohol consumption using the DID drank more than adolescents, and once adolescent mice entered adulthood their intakes matched the ones that initiated as adults. Thus, their results were in agreement with our current research. Findings suggest that whether the adolescent or adult animal voluntarily consumes more ethanol varies based upon drinking procedure and between populations.
Our research also showed that HAPII adolescent and adult mice reach roughly the same BECs during free-choice access. Adolescent BECs were higher than those previously mentioned in the study by Moore et al. (2010). Additionally, g/kg/day consumptions were the highest observed in any study assessing adolescent intake, including Siegmund et al. (2005), Melendez et al. (2011), and Metten et al. (2011). Furthermore, consumption was higher than those observed in forced administration studies that have demonstrated neurological changes from adolescent exposure (Coleman et al., 2011; Crews et al., 2000), although these studies used rats. Our procedure resulted in high BECs with no food, water, or alcohol deprivation, important considerations in relatively polydipsic and hyperphagic adolescent animals. Therefore, we had a valid prospect of detecting an effect in adulthood based on adolescent free-choice exposure. Our results also support the adolescent HAPII mouse as a candidate for future free-choice drinking developmental research. The observation that body weights were not affected by ethanol consumption is consistent with low stress levels as expected with a voluntary access model. Other rodent models that cause similar sustained BECs, such as liquid diet or vapor chamber, are sources of stress to animals and/or cause marked weight loss (Anji and Kumari, 2008; Kang et al., 2004). We do note that although our animals reached pharmacologically relevant BECs, behavior could also be influenced by changes in taste hedonics that occur during free-choice access (e.g., Kiefer et al., 1995). Thus, increases in subsequent drinking observed could be due to taste familiarity, pharmacological/neuroadaptive changes, or a combination of these.
Interesting post-hoc results of Experiment 1 were that ethanol intakes resumed at lower levels following abstinence, and animals never reached the expected intake levels for HAPII mice during the Testing Phase. Given that the adolescent water group commenced ethanol consumption at about the same age as the adult alcohol group, it was expected that each group would show similar drinking patterns, but this was not supported. The lack of an acquisition effect is particularly interesting, as HAPII mice usually show a gradual increase in drinking (Oberlin et al., 2011). The results of Experiment 1 may suggest an effect of being single-housed for an extended period of time prior to drinking leading to diminished ethanol intake, as HAPII mice usually drink ethanol shortly after single-housing. Previous research using adult rats suggests that social isolation suppresses ethanol consumption (Doremus et al., 2005). These findings suggest a potential shortcoming in our design, which called for a period of abstinence after the end of adolescence following exposure, as is commonly done to assess effects specific to this developmental period (Metten et al., 2011; Moore et al., 2010; Vetter et al., 2007). However, the results of developmental exposure are potentially confounded because all mice underwent an abstinence period following the termination of their initial free-choice drinking phase. There is currently no other information about the effects of forced abstinence in HAPII mice. Overall, our unexpected results warrant consideration of social, environmental, and epigenetic factors in the drinking behaviors of high-alcohol-preferring lines.
Both experiments 1 and 2 showed that adults had larger sex differences in drinking than adolescents, although this didn’t result in differences in BECs between the sexes in either age. Adult sex differences in drinking have been seen repeatedly in HAPII mice (Oberlin et al., 2011; Trujillo et al., 2011). Together with the current findings, these data suggest that adult sex differences in intake may result from late-adolescence hormonal changes. These findings are consistent with a study showing that estrogen increased alcohol intake in ovariectomized adult C57Bl/6J mice (Rajasingh et al., 2007). Speculatively, these findings suggest that post-pubertal estrogen release may elevate alcohol consumption in female HAPII mice, but further studies are needed to validate this observation.
In spite of the high BECs encountered during adolescence, and despite our ability to detect systematic effects on subjective reward valuation of increasing delays to the delayed reinforcer, we were unable to detect effects of adolescent exposure on impulsivity as measured by DD. Previous unpublished results from our lab suggest that adult ethanol exposure using forced injections also doesn’t affect DD in HAP mice. These results imply that past or present ethanol consumption does not increase cognitive impulsivity, as assessed by subjective sensitivities to delayed rewards, in HAP mice. We cannot prove a null effect, but if the effect size were similar to the HAP/LAP line difference we previously observed (about 25% of the less impulsive group mean; Oberlin and Grahame, 2009), this experiment would have been sensitive to it. Additionally, our group sizes were consistent with several prior studies that detected significant effects (Helms et al., 2006; Pinkston and Lamb, 2011; Wilhelm and Mitchell, 2009). Finally, we detected effects of delay duration on the subjective value of the reinforcer, demonstrating that this study possessed sufficient power to observe changes in behavior in response to a manipulation.
However, our results could be specific to impulsivity measured with by DD, as a study of adult performance in the 5-choice serial reaction time test showed a significant effect of adolescent alcohol intake (Semenova, 2012). Using DD, several experiments suggest that populations of animals that display high alcohol intake are more impulsive than low-drinking and non-selected populations, including the aforementioned study using HAP/LAP mice (Oberlin and Grahame, 2009) and another using inbred strains derived from selectively bred High- and Low- Alcohol Drinking rats (Wilhelm and Mitchell, 2008). Additionally, research using outbred mice has shown that animals displaying greater levels of impulsivity later show greater locomotor sensitization to ethanol than non-impulsive animals (Mitchell et al., 2006). Taken together with the present findings, these results suggest that innately high cognitive impulsivity is a predispositional factor for high drinking behavior, rather than a trait resulting from prior alcohol use.
This interpretation of the animal findings is consistent with longitudinal human research that has failed to observe significant impulsivity differences between alcohol drinkers and non-drinkers in adolescence. However, other cognitive deviations that appear to result from adolescent binge drinking are observed (Goudria et al., 2007; Medina et al., 2008; Squeglia et al., 2009). Teenage drinkers show decrements in verbal encoding tasks, such as failure to encode the quantity of words of non-drinkers (Schweinsburg et al., 2010). Deficits in spatial working memory function and abnormalities in brain response to the task are also seen in adolescents with AUDs (Tapert et al., 2004). Future animal research should seek differences in parallel tasks resulting from adolescent alcohol exposure to further validate the human findings. Our results show that ethanol exposure during adolescence fails to increase impulsivity in HAPII mice. Thus, the observed transient increase in drinking in adolescents and adults is seemingly mediated by an effect other than higher levels of impulsivity. The causes of changes in drinking should be addressed by future research.
In sum, this study suggests that alcohol exposure promotes later alcohol use regardless of the age at which it occurs, although this effect doesn’t appear to be mediated by changes in cognitive impulsivity. Evidence suggests that different animal populations are differentially susceptible to these effects of alcohol exposure on subsequent drinking, a result that may translate to humans. Conclusions have the greatest generality when studies examining adolescent and adult ethanol consumption utilize a variety of genotypes and a range of drinking onset ages in each genotype, and the current findings contribute a new population, with very high innate levels of drinking, to the literature.
Acknowledgments
Support: AA07911 to David Crabb
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Anji A, Kumari M. Supplementing the liquid alcohol diet with chow enhances alcohol intake in C57BL/6 mice. Drug Alcohol Depen. 2008;97:86–93. doi: 10.1016/j.drugalcdep.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce E, Santisteban C. Impulsivity: a review. Psicothema. 2006;18:213–220. [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJK, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Be. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belkanp JK. The Complexity of Alcohol Drinking: Studies in Rodent Genetic Models. Behav Genet. 2010;40:737–750. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Be. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol Biochem Be. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Ernst M, Luckenbaugh DA, Moolchan ET, Leff MK, Allen R, Eshel N, London ED, Kimes A. Behavioral predictors of substance-use initiation in adolescents with and without attention-deficit/hyperactivity disorder. Pediatrics. 2006;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Be. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotypes concept in psychiatry: etymology and strategic intentions. Am J Psychiat. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Goudria AE, Grekin ER, Sher KJ. Decision making and binge drinking: A longitudinal study. Alcohol Clin Exp Res. 2007;31:928–938. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Huang B. Co-occurrence of 12-month mood and anxiety disorders and personality disorders in the US: results from the national epidemiologic survey on alcohol and related conditions. J Psychiat Res. 2005;39:1–9. doi: 10.1016/j.jpsychires.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Selective breeding for high and low alcohol preference in mice. Behav Genet. 1999;29:47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Grunbaum J, Kaun L, Kinchen S, et al. Youth Risk Behavior Surveillance-United States, 2003. MMWR Surv Sum. 2004;53:1–96. [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, et al. Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology. 2006;188(2):144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Kang SS, Cole M, Lee S, Rivier C. Development of individual alcohol inhalation chambers for mice: Validation in a model of prenatal alcohol. Alcohol Clin Exp Res. 2004;28:1549–1556. doi: 10.1097/01.alc.0000141639.79278.5e. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, N. Badiaelder N, Bice PJ. Taste reactivity in high alcohol-drinking and low alcohol-drinking rats. Alcohol Clin Exp Res. 1995;19:279–284. doi: 10.1111/j.1530-0277.1995.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Bucholz KK, Madden PAF, Waldron M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: A female twin study. Pharmacol Biochem Be. 2009;93:313–321. doi: 10.1016/j.pbb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson L, Grahame N. Pharmacologically relevant intake during chronic, free- choice drinking rhythms in selectively bred high alcohol preferring mice. Addict Biol. 2011 Nov 29; doi: 10.1111/j.1369-1600.2011.00412.x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur J. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. The Effect of Delay and of Intervening Events on Reinforcement Value. Vol. 5. Hillsdale, N.J.: Lawrence Erlbaum; 1987. pp. 55–73. Quantitative analysis of behavior series. [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez R. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Brown LL, Crabbe JC. Limited access ethanol drinking in the dark in adolescent and adult mice. Pharmacol Biochem Be. 2011;98:279–285. doi: 10.1016/j.pbb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–437. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC, Boehm SL. Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Best C, Matson LM, Henderson A, Grahame NJ. Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav Genet. 2011;41:288–302. doi: 10.1007/s10519-010-9394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1–10. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Pinkston JW, Lamb RJ. Delay Discounting in C57BL/6J and DBA/2J Mice: Adolescent-Limited and Life-Persistent Patterns of Impulsivity. Behav Neurosci. 2011;125(2):194–201. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasingh J, Bord E, Qin GJ, Ii M, Silver M, Hamada H, Ahluwalia D, Goukassian D, Zhu Y, Losordo DW, Kishore R. Enhanced voluntary alcohol consumption after estrogen supplementation negates estrogen-mediated vascular repair in ovariectomized mice. Endocrinology. 2007;148:3618–3624. doi: 10.1210/en.2006-1357. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Salimov RM, McBride WJ, McKinzie DL, Lumeng L, Li TK. Effects of ethanol consumption by adolescent alcohol-preferring P rats on subsequent behavioral performance in the cross-maze and slip funnel tests. Alcohol. 1996;13:297–300. doi: 10.1016/0741-8329(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Schulenberg J, O'Malley PM, Bachman JG, Wadsworth KN, Johnston LD. Getting drunk and growing Up: trajectories of frequent binge drinking during the transition to young adulthood. J Stud Alcohol. 1996;57:189–304. doi: 10.15288/jsa.1996.57.289. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S. Attention, impulsivity, and cognitive flexibility in adult male rats exposed to ethanol binge during adolescence as measured in the five-choice serial reaction time task: the effects of task and ethanol challenges. Psychopharmacology. 2012;219:433–444. doi: 10.1007/s00213-011-2458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav R. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The Influence of Substance Use on Adolescent Brain Development. Clin EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Trujillo JL, Do DT, Grahame NJ, Roberts AJ, Gorman MR. Ethanol consumption in mice: relationships with circadian period and entrainment. Alcohol. 2011;45:147–159. doi: 10.1016/j.alcohol.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]