Abstract
Lateralization of the processing of positive and negative emotions and pain suggests an asymmetric distribution of the neurotransmitter systems regulating these functions between the left and right brain hemispheres. By virtue of their ability to selectively mediate euphoria, dysphoria, and pain, the μ-, δ-, and κ-opioid receptors and their endogenous ligands may subserve these lateralized functions. We addressed this hypothesis by comparing the levels of the opioid receptors and peptides in the left and right anterior cingulate cortex (ACC), a key area for emotion and pain processing. Opioid mRNAs and peptides and 5 “classical” neurotransmitters were analyzed in postmortem tissues from 20 human subjects. Leu-enkephalin-Arg (LER) and Met-enkephalin-Arg-Phe, preferential δ-/μ- and κ-/μ-opioid agonists, demonstrated marked lateralization to the left and right ACC, respectively. Dynorphin B (Dyn B) strongly correlated with LER in the left, but not in the right ACC suggesting different mechanisms of the conversion of this κ-opioid agonist to δ-/μ-opioid ligand in the 2 hemispheres; in the right ACC, Dyn B may be cleaved by PACE4, a proprotein convertase regulating left–right asymmetry formation. These findings suggest that region-specific lateralization of neuronal networks expressing opioid peptides underlies in part lateralization of higher functions, including positive and negative emotions and pain in the human brain.
Keywords: anterior cingulate cortex, emotions, endogenous opioid system, lateralization, pain
Introduction
A fundamental property of the human brain is lateralization of higher functions. The left hemisphere (LH) is dominant for language, mathematical, and logical reasoning; while the right hemisphere (RH) is specialized in shape recognition, spatial attention, and musical and artistic functions (Joseph 1988; Dehaene et al. 1999; Levy et al. 1999; Mesulam 1999; Toga and Thompson 2003; Craig 2005; Gazzaniga 2005; Bever and Chiarello 2009; Gier et al. 2010). According to “the right hemisphere hypothesis,” emotions are predominantly processed in the RH. The left side of the face is emotionally more expressive (Sackeim et al. 1978). Similarly, the emotional intonation (prosody) of words is more easily recognized if stimuli are presented to the left ear (Erhan et al. 1998), and stimuli presented to the left visual field are perceived with greater emotion (Levine and Levy 1986) and elicit greater autonomic response (Spence et al. 1996). Consistently, patients with frontal damage to the RH demonstrate deficits both in prosody (Ross and Mesulam 1979) and in the recognition of emotional facial expressions (Weddell 1994; Mandal et al. 1999). The alternative, “valence hypothesis” postulates that emotions are lateralized depending on their valence. The RH is dominant for negative emotions and pain, whereas the LH predominantly processes positive emotions (Sackeim et al. 1982; Davidson 1992). Patients who suffered trauma to the left frontal lobe are more frequently depressed (Sackeim et al. 1982; Morris et al. 1996), while those with right frontal damage show inappropriate signs of cheerfulness and mania (Starkstein et al. 1989). The valence hypothesis is supported by behavioral, neuroimaging, and electrophysiological evidence (Lugo et al. 2002; Symonds et al. 2006; Carrasquillo and Gereau 2008; Ji and Neugebauer 2009). Yet, a number of studies did not support both the right hemisphere and valence hypotheses and, instead, proposed region-specific contralateral processing of positive and negative emotions (Demaree et al. 2005; Barber et al. 2012). Importantly, dichotomous lateralization as a dimension of frontal brain organization is a function not only of emotional valence, but also approach/avoidance motivation (Davidson 2003; Harmon-Jones et al. 2010; Miller et al. 2013). Specialization and activation of subregions of the frontal cortex with their ipsi- and contralateral connectivity to each other, with respect to emotional valence and approach/avoidance motivation, may be considerably differentiated (Miller et al. 2013). Thus, positive valence and approach motivation are apparently associated with different areas in the LH. Interestingly, the valence of emotions, or a learning event, may modulate the activation of the contralateral subregions (Young and Williams 2010; Enticott et al. 2012).
A distributed network of cortical regions including the prefrontal (PFC), insular, and anterior cingulate (ACC) cortices is involved in the presentation and processing of emotional stimuli (Lane et al. 1998; Craig 2005; Corradi-Dell'Acqua et al. 2011; Fan et al. 2011; Ansell et al. 2012). The ACC may function as a generator of physiological or behavioral responses and may be involved in the top-down modulation of limbic and endocrine systems to regulate positive and negative emotions (Phan et al. 2002; Murphy et al. 2003; Vytal and Hamann 2010). The ACC is a part of neural cortical network processing emotional–affective aspects of pain (Casey et al. 1997; Kulkarni et al. 2005; Wiech et al. 2006). The left ACC may predominantly process positive emotions or happiness (Dolan et al. 1996; Phillips et al. 1998), whereas the right ACC negative emotions and pain (Hsieh et al. 1995, 1996; Brooks et al. 2002; Symonds et al. 2006; Brügger et al. 2011; Kim et al. 2012) that is consistent with the emotional lateralization model (Craig 2005).
The endogenous opioid system (EOS) is a critical neurotransmitter system regulating the presentation and processing of emotions and pain. This system includes 3 opioid receptors, μ- (MOR), δ- (DOR), and κ- (KOR) types, that are preferentially activated by β-endorphin, enkephalins, and dynorphins, respectively. The endogenous opioid peptides derive from proopiomelanocortin (POMC), proenkephalin (PENK), and prodynorphin (PDYN). Endorphins and enkephalins are positive reinforcers and their interactions with MOR and DOR modulate positive emotional states (Bals-Kubik et al. 1993; Filliol et al. 2000; Leknes and Tracey 2008). Activation of MOR and DOR can be counterbalanced by the stimulation of KOR by dynorphins, which can induce a dysphoric state. KOR agonists produce aversive effects in animals and dysphoria in humans, and dynorphin upregulation may lead to anhedonia and depression (Narita et al. 2006; Land et al. 2008; Schwarzer 2009; Shippenberg 2009; Knoll and Carlezon 2010). KOR blockade attenuates the development of depressive-like behaviors; and the activation of the PDYN/KOR system is critical for the escalated intake of addictive substances including alcohol in dependent animals (Ebner et al. 2010; Chartoff et al. 2012; Sirohi et al. 2012; Kissler et al. 2013). Furthermore, the OPRK1 (KOR) and PDYN genes are associated with both mood disorders (Deo et al. 2013) and increased propensity to drink alcohol in negative emotional states (Karpyak et al. 2012). Opioid receptors are densely distributed in brain regions, including the ACC and PFC, that have a central role in the integration of emotionally salient stimuli and in processing the affective components of pain (Zubieta et al. 2001, 2003). Alterations in MOR in the ACC and other cortical regions may contribute to affective disorders, including major depression and borderline personality disorders (Kennedy et al. 2006; Walter et al. 2009; Prossin et al. 2010; Pizzagalli 2011).
Lateralization of positive and negative emotions and pain suggests that the neurotransmitter systems regulating these functions may be asymmetrically distributed between the LH and RH. By virtue of their ability to selectively mediate euphoria and dysphoria, as well as pleasure and pain, the EOS is an ideal candidate for neurochemical system to subserve these lateralized brain functions. The present study addressed this hypothesis by comparing the levels of 6 EOS genes and 4 opioid peptides in postmortem human specimens from symmetric areas of the LH and RH. Leu-enkephalin-Arg (LER) and Met-enkephalin-Arg-Phe (MEAP), the neuropeptide markers of PDYN and PENK, respectively, as well as dynorphin A (Dyn A) and dynorphin B (Dyn B) derived from PDYN, were analyzed. Dyn A and Dyn B function as KOR ligands; LER is potent DOR and MOR agonist, whereas MEAP may activate KOR and MOR (Iyengar et al. 1987; Kamei et al. 1994; Mansour et al. 1995; Benyhe et al. 1997). The neurotransmitters dopamine (DA), serotonin (5-HT), noradrenaline (NA), glutamate (Glu), and aspartate (Asp), previously proposed to be lateralized in the human brain (Tucker and Williamson 1984; Fitzgerald 2012), were analyzed for comparison. We focused on the ACC, and specifically the pregenual area (pgACC) within the ACC, for multiple reasons. (1) The pgACC is involved in the processing and expression of positive (Vogt 2005; Lindgren et al. 2012) and negative (Etkin et al. 2011; Amemori and Graybiel 2012) emotions. (2) The pgACC is the site where negative affect is associated with opioid receptor occupancy (Zubieta et al. 2003). (3) The pgACC mediates the analgesic and placebo effects of opioids (Petrovic et al. 2002; Eippert et al. 2009). (4) Opioid receptors are expressed at high levels in the pgACC (Vogt 2005). (5) The processing of emotions and pain signals may be lateralized in the pgACC (Hsieh et al. 1996; Brügger et al. 2011). For example, the right pgACC was found to have a role in self-conscious emotional reactivity. Neurodegeneration in this region may underlie the emotional decline seen in patients with frontotemporal dementia (Sturm et al. 2012), and abnormalities in the pgACC are present in patients with borderline personality disorder (Tebartz van Elst et al. 2003; Goodman et al. 2011). In addition, endogenous opioid functions were selectively dysregulated in the right pgACC in individuals with borderline personality disorders (Prossin et al. 2010). Furthermore, neurometabolic abnormalities associated with autism spectrum disorders were lateralized to the right pgACC (Oner et al. 2007; Bejjani et al. 2012). Prompted by our preliminary evidence that opioid peptides displayed lateralization in the pgACC, we additionally assessed their levels in other brain regions involved in the processing of emotions and pain, including the left and right dorsolateral PFC (dl-PFC; Padberg et al. 2001; Killgore et al. 2007), and the caudate and putamen (Bartels and Zeki 2000; Starkman et al. 2007) for comparison.
Materials and Methods
Subjects
Brain tissues were collected from 32 deceased subjects with no evidence of neuropathology and no known history of neuropsychiatric disease (Supplementary Table 1A,B) by KI Donatum at Forensic Medicine, Department of Oncology and Pathology, Karolinska Institutet, Stockholm, Sweden, by qualified pathologists under the full ethical clearance from the Stockholm Ethical Review Board. Donation was performed after an informed consent by the next of kin or by a registration as a donor by the deceased. Samples were taken from symmetric areas of the left and right pgACC that comprises of Brodmann areas 24a–c and 32 (Bush et al. 2000; Vogt 2009; Shackman et al. 2011), dl-PFC, caudate, and putamen. The discovery sample (Sample 1) was comprised of 20 subjects in which the warm and cold periods of the postmortem interval (PMI) did not exceed 12 and 48 h, respectively, and in which the tissue characteristics were in range acceptable for molecular analyses [Supplementary Table 1A; brain pH values >5.0; RNA quality indicator (RQI) values of >5.0] (Kingsbury et al. 1995; Fleige et al. 2006). The replication sample (Sample 2) consisted of 12 subjects in which the longest warm, cold, and total PMI exceeded those for Sample 1 by 1.8-, 1.3-, and 1.4-fold, respectively, and had an RQI of <5.0 in 5 subjects (Supplementary Table 1B).
Radioimmunoassay
The procedure for peptide extraction and antibodies has been described elsewhere (Christensson-Nylander et al. 1985; Merg et al. 2006). Briefly, 1 M hot acetic acid was added to finely powdered, frozen brain tissues, and samples were boiled, sonicated, and centrifuged. Tissue extracts were run through a SP-Sephadex ion exchange C-25 column, and peptides were eluted and analyzed by radioimmunoassay (RIA). Anti-Dyn A antiserum demonstrated 100% molar cross-reactivity with Dyn A (9–17) and <0.1% molar cross-reactivity with Dyn B, Dyn A (1–8), α-neoendorphin, Leu-enkephalin, and big dynorphin. Anti-Dyn B antiserum showed 100% molar cross-reactivity with big dynorphin, 0.8% molar cross-reactivity with Leu-morphine (29 amino acid C-terminally extended Dyn B), and <0.1% molar cross-reactivity with Dyn A (1–17), Dyn A (1–8), α-neoendorphin, and Leu-enkephalin. Cross-reactivity of LER antiserum with Dyn A, Dyn B, and Leu- and Met-enkephalin was <0.1% molar, α-neoendorphin 0.5% molar, Dyn A (1–8) 0.7% molar, MEAP 1% molar, and Met-enkephalin-Arg 10% molar. Cross-reactivity of MEAP antiserum with Met-enkephalin, Met-enkephalin-Arg, Met-enkephalin-Arg-Gly-Leu, Leu-enkephalin, and LER was < 0.1% molar (Nylander et al. 1997).
Dyn A, Dyn B, and LER RIA readily detected these peptides in the striatum, hippocampus, and frontal cerebral cortex of wild-type mice (Nguyen et al. 2005) and rats (Christensson-Nylander et al. 1985), but not in Pdyn knockout mice (for details, see, Merg et al. 2006). This indicated that the assay was highly specific and not sensitive to the presence of contaminants in brain tissues. Protein concentrations were estimated by DC protein assay (Bio-Rad Laboratories, Hercules, CA, USA). The peptide content was calculated with the GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA) and presented in fmol/mg protein.
HPLC Determination of Monoamines and Acidic Metabolites
Chemicals
The chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA), with the exception of methanol and tetrahydrofuran which were obtained from Merck (Darmstadt, Germany).
Tissue Preparation
The brain tissue samples (20–30 mg) were mixed at a ratio of 1:10 (w/v) with 0.2 M perchloric acid including 100 µM EDTA-2Na and homogenized at 0 °C in a glass-pestle microhomogenizer. Following standing for 30 min on ice, the homogenates were centrifuged at 12 000 × g and 4 °C for 15 min. The supernatants were carefully aspirated and mixed with 1 M Na-acetate buffer, pH 3.0 at a ratio 5:1 (v/v), and filtered through a 0.22-µm centrifugal filter (Merck Millipore, Darmstadt, Germany) at 12 000 × g and 4 °C for 4 min. The filtrates were stored at −80 °C before high performance liquid chromatography (HPLC) analysis.
HPLC Analysis
The concentrations of NA, DA, and 5-HT were determined by HPLC with electrochemical detection (Kehr and Yoshitake 2006). The HPLC system included a HTEC500 chromatograph (Eicom, Kyoto, Japan) and a CMA/200 Refrigerated Microsampler (CMA Microdialysis, Stockholm, Sweden) equipped with a 20-µL loop and operating at +4 °C. The potential of the glassy carbon working electrode was +450 mV versus the Ag/AgCl reference electrode. The separation was achieved on a 200 × 2.0-mm (i.d.) Eicompak CAX column (Eicom). The mobile phase consisted of a mixture of methanol and 0.1 M phosphate buffer (pH 6.0, 30:70, v/v) containing 40 mM potassium chloride and 0.13 mM EDTA-2Na. The chromatograms were recorded and integrated using Clarity (DataApex, Prague, The Czech Republic), a computerized data acquisition system. Asp and Glu concentrations were determined by gradient elution reversed-phase column liquid chromatography with fluorescence detection, following precolumn derivatization with ortho-phthaldialdehyde/mercaptoethanol (OPA/MCE) reagent, as described elsewhere (Kehr 1998). Briefly, the HPLC system included a gradient pump Spectra Physics SP8800 (Spectra Physics, USA), a CMA/260 degasser (CMA Microdialysis), a CMA/280 Fluorescence detector (CMA Microdialysis) operating at excitation and emission wavelengths of 350 and 495 nm, respectively. Typically, 10-µL samples were mixed with 10 µL of the OPA/MCE reagent by use of a CMA/200 Refrigerated Microsampler (CMA Microdialysis) equipped with a 20 μL loop and operating at +6 °C. After 60 s, 10 µL of the reaction product was injected onto a HPLC column (60 × 4 mm i.d., Nucleosil 100 C18, 5 μm; Knauer GmbH, Berlin, Germany). The mobile phase A consisted of a 0.03 M sodium acetate buffer (pH 6.95) containing 2.5% (v/v) of methanol and 2% (v/v) of tetrahydrofuran pumped at a flow rate of 1 mL/min. The amino acids were eluted by the use of a linear gradient of methanol used as a mobile phase B and from 0% to 60% at 4 to 28 min. The chromatograms were recorded and integrated by using EZ Chrom (Scientific Software, Inc., CA, USA).
Gene Expression Analysis by qRT-PCR
Total RNA was purified with a RNeasy Lipid Tissue Mini kit (QIAGEN, Maryland, USA) using TRIzol Reagent (QIAGEN) and treated with RNase-free DNase I on-column. An RQI was measured using a Bio-Rad Experion system (Bio-Rad Laboratories) and a Eukaryote Total RNA StdSens assay according to the manufacturer's protocol. RNA samples with the RQI values of >5.0 are generally considered suitable for quantitative real-time polymerase chain reaction (qRT-PCR) (Fleige et al. 2006). The average value of RQI in Sample 1 was 6.70 ± 0.55 (mean ± SD), demonstrating the high quality of the isolated RNA. Reverse transcription of total RNA was performed with a cDNA iScript kit (Bio-Rad Laboratories) using a CFX96™ Real-Time Detection System (Bio-Rad Laboratories) according to the manufacturer's protocol.
qRT-PCR was performed on a CFX96™ Real-Time Detection System (Bio-Rad Laboratories). For SYBR Green-based assays, the reaction mixture consisted of cDNA, 5 × HOT FIREPol® EvaGreen® qPCR Mix Plus (Solis BioDyne, Tartu, Estonia) and forward and reverse primers (Supplementary Table 2). Samples were analyzed in triplicate. The following conditions were applied for the 3-step qRT-PCR reactions; 95 °C for 15 min followed by 40 cycles of amplification at 95 °C for 15 s, annealing temperature for 61.6 °C for 20 s, and elongation at 70 °C for 20 s. Melting curves were analyzed to ensure primer specificity and the lack of primer dimers. To ensure correct amplification, PCR products were separated on agarose gel and sequenced in both directions. Proprotein convertase subtilisin/kexin type 6 (PCSK6) mRNA was quantified using the TaqMan assay. The reaction mixture contained cDNA, 2 × iTaq Universal Probe Supermix (Bio-Rad Laboratories) and TaqMan® probe (Applied Biosystems Europe BV, The Netherland, Assay ID Hs00159844_m1). The following conditions were applied for the 2-step qRT-PCR reactions: 95 °C for 30 s followed by 40 cycles of amplification at 95 °C for 5 s and 60 °C for 30 s. The carboxyfluorescein fluorescence of individual samples was measured at the end of every cycle. Samples were analyzed in triplicate. Expression levels were normalized to TATA-box binding protein (TBP), beta-actin (ACTB), and glyceraldehyde-3-phophate dehydrogenase (GAPD) genes in both assays. Data analysis was performed using Bio-Rad CFX Manager (Bio-Rad Laboratories). Fluorescent product was not observed in samples lacking template cDNA (no template control) or in negative controls prepared by the omission of reverse transcriptase.
Statistical Analysis
The assumption of conducting analyses of variance (ANOVAs) was met for all data that have (1) normal distributions according to the Kolmogorov–Smirnov test, and (2) equal error variance of the dependent variables across sexes (homogeneity of error variances tested by the Levene's test). Lateralization was examined by (1) comparing the levels of analyzed substances for the LH and RH, respectively; and (2) analyzing the asymmetry index (AI) for each substance. An AI was defined as the ratio of differences in the levels between the right (R) and left (L) hemispheres divided by the sum of the levels in 2 hemispheres, AI = [(R − L)/(R + L)], where −1.0 and +1.0 values correspond to the completely left or right lateralization, respectively, and 0.0 corresponds to a symmetric distribution. The AI analyses assumed the data were distributed normally and the variances were homogeneous. In addition to analyzing the AI, the log-odds ratio (log of the odds ratio, L/R) was also analyzed. The L/R is a common transformation when assumptions of normality and homogeneity are not fulfilled. The ratio converts proportions to odds followed by taking the log of the odds. The AI and the log-odds analyses resulted in the same or very similar significance levels. For simplicity, the paper presents significant differences in the AI that also demonstrated significant differences in their odds ratios. Analyses were conducted in 4 general steps. First, data were subjected to ANOVAs with sex as the between factor (2 levels: females and males). This included analyzing (1) the LH and RH (2-way ANOVA) and (2) the AI indices (multivariate analysis of variance, MANOVA) as dependent measures. Effects deemed significant were subsequently examined by analyses of covariance (ANCOVA) to control for potential confounds. Demographic parameters and tissue characteristics (age, brain pH, PMI, and RQI) were correlated with the dependent variables to identify potential covariates (COVs). Significant effects identified by ANOVA or MANOVA followed by ANCOVA are reported when appropriate. Secondly, all dependent variables were subjected to Spearman correlation analyses. (1) Each parameter was correlated between the 2 hemispheres. Thus, 15 correlations were examined in the pgACC, while 4 were assessed each for the dl-PFC, caudate, and putamen. (2) In the pgACC, all 105 pair-wise correlations between 15 parameters were compared between the LH and RH. Similarly, all 6 correlations between the 4 parameters were compared between the left and right parts for each other 3 areas. (3) Associations between Dyn B and LER identified as significant in the pgACC at the second step were compared across 4 brain regions for the LH and RH separately. (4) To determine whether opioid peptide levels were affected by tissue quality, correlations between these levels with tissue characteristics and PMI, and with respective mRNAs, were examined. PMIs (the warm and cold periods combined) were included as a COV in statistical analyses. Analyzing the warm and cold periods separately did not significantly affect the outcome. Differences between correlations were evaluated using the Fisher's z-transformation test. In ANOVAs and correlations, α levels of P < 0.05 and <0.01 were considered as significant, respectively. The P-value was adjusted to compensate for multiple comparisons using Bonferroni correction. Data are presented as the mean ± standard deviation (SD). Analyses conducted with the same set of subjects, but after removing outliers that were at the 2 SD or greater distance from the mean values, produced the same levels of significance.
Results
Postmortem tissue samples were collected from subjects with no evidence of neuropathology and no known history of neuropsychiatric disease (Sample 1; Supplementary Table 1A). Analyses of lateralization were performed using the combined sample of females and males, as well as of each sex subgroup separately. Effect sizes for significant effects in males were calculated based on partial eta-squared, and the power to detect these effects was estimated for the male (0.838) and female (0.406) sample size separately. Evidently, the female sample afforded moderate power to detect effects characteristic of the male sample, whereas a failure to find an effect in the female sample may be due to power reduced by a factor 2, and must be viewed with caution. At the same time, only the large effect size may underlie several significant findings detected for the female sample. Analyses of the demographic characteristics for the combined sample of males (N = 14) and females (N = 6) (Supplementary Table 1A) showed no significant differences in age [t(18) = −0.27, P = 0.78], PMI (total time) [t(18) = −0.50, P = 0.62)], brain pH values [t(18) = 0.77, P = 0.44], and RQI [t(18) = 0.06, P = 0.96] between females and males for the pgACC.
pgACC
Analyses of Variances
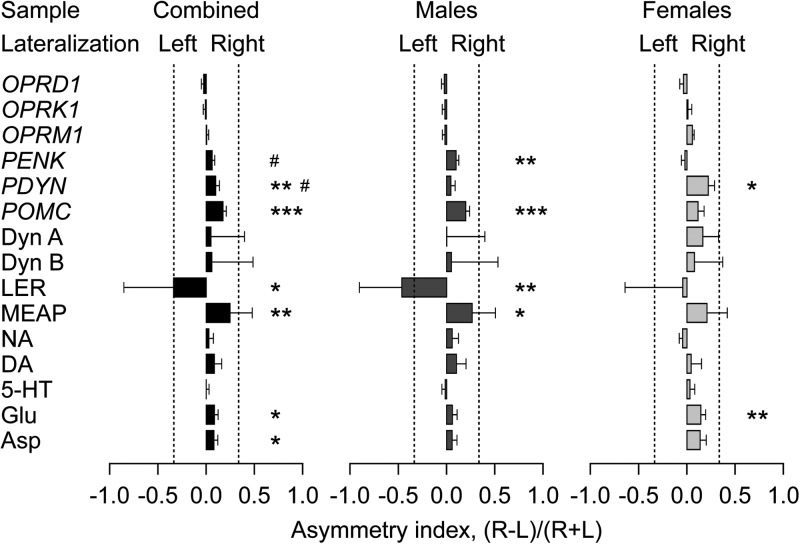
The levels of µ- (OPRM1), δ- (OPRD1), and κ- (OPRK1) opioid receptors, and POMC, PENK, and PDYN opioid peptide precursor mRNAs; the PDYN-derived opioid peptides, Dyn A, Dyn B, LER, and PENK-derived MEAP; and the neurotransmitters DA, 5-HT, NA, Glu, and Asp, were analyzed in the left and right pgACC. Data are displayed as normalized levels in the left (LH) and right (RH) hemispheres in Figure 1, and as the AI in Figure 2. Analyses of expression levels across hemispheres using 2-way ANOVAs followed by ANCOVAs revealed significant main effects of hemisphere for MEAP and PDYN, significant sex differences in Glu, and a significant sex × hemisphere interaction for LER (Fig. 1). The MEAP levels were significantly higher in the RH (the combined sample: ANCOVA: F1,16 = 9.6, P < 0.01; when COVs included brain pH in the LH, ρ = −0.56, P < 0.01; and RQI, ρ = −0.58, P < 0.01). The significant hemisphere effect was evident in males (ANCOVA: F1,11 = 7.4, P < 0.05), but not in females. Greater PDYN levels were identified in the RH in the combined sample (F1,18 = 6.8, P < 0.05; no significant COVs) and females (F1,5 = 19.2, P < 0.01). A significant effect of sex (the combined sample: F1,18 = 8.7, P < 0.01) was observed in the Glu left–right distribution (no significant COVs). Females, but not males, showed a significant difference among hemispheres (F1,5 = 7.5, P < 0.05), with Glu levels elevated in the RH. LER revealed a significant interaction effect (the combined sample: F1,18 = 5.1, P < 0.05; no significant COVs) and was lateralized to the left in males (F1,13 = 9.3, P < 0.01), but not in females.
Figure 1.
Comparison of the opioid mRNA, opioid peptide, and neurotransmitter levels between the left and the right pgACC. Data are shown as mean ± SD of the normalized levels in the LH [L/(L + R)] and RH [R/(L + R)], where L and R are levels in the LH and RH, respectively. Dashed lines at a 0.66 value mark 2-fold differences between the hemispheres. Significance levels in ANOVAs followed by ANCOVAs when appropriate: Lateralization effects, *P < 0.05, **P < 0.01; sex effects, ##P < 0.01; lateralization × sex interactions, §P < 0.05.
Figure 2.
The AI of the opioid mRNAs, opioid peptides, and neurotransmitters in the pgACC. The AI [(R − L)/(R + L)] is a measure of hemispheric differences, which the −1.0 and +1.0 values indicate a complete left or right lateralization, respectively, and the 0.0 value symmetric distribution. Data bars represent the means ± SD. Dashed lines at the −0.33 and +0.33 AI values mark 2-fold differences between hemispheres. Significance in the MANOVA followed by ANCOVAs when appropriate: Lateralization effect, *P < 0.05, **P < 0.01, ***P < 0.001; sex effects, #P < 0.05.
Differences in PDYN and Glu levels between the LH and RH were minor; they did not exceed 1.2-fold in the combined sample. Differences in MEAP and LER were greater, 1.5- and 1.6-fold for all subjects, respectively, and 1.6- and 2.5-fold in the male group, respectively. The 2 enkephalins demonstrated different directions in the lateralization with LER displaying greater levels in the LH and MEAP in the RH.
Analysis of the AI for LER and MEAP by the MANOVA followed by ANCOVAs when appropriate revealed a significant main effect of the hemisphere. LER was left lateralized in the combined sample (F1,18 = 4.6, P < 0.05; AI = −0.33; no significant COVs) and males (F1,13 = 15.7, P < 0.01, the AI = −0.49). MEAP showed right lateralization [ANCOVA: F1,17 = 9.1, P < 0.01, the AI = +0.24 when COVs included brain pH (ρ = 0.62, P < 0.001)] in the combined sample and males (F1,12 = 7.5, P < 0.05; AI = +0.27). Much smaller (0.06–0.17 AI values) significant lateralization effects for Asp, Glu, POMC, and PDYN were found. Asp and Glu were lateralized to the RH when male and female subjects were considered together (F1,18 = 4.9, P < 0.05, AI = +0.08; and F1,18 = 6.1, P < 0.05, AI = +0.08, respectively; no significant COVs). Glu lateralization was more pronounced in females (F1,5 = 8.9, P < 0.01, AI = +0.14) than in males who did not show significant differences. POMC and PDYN revealed significant differences among hemispheres (F1,18 = 18.9, P < 0.001, right lateralized AI = +0.17; and F1,18 = 11.6, P < 0.01, right lateralized AI = +0.10, respectively; no significant COVs) in the combined sample; within this group, POMC showed asymmetry in males (F1,13 = 26.0, P < 0.001, right lateralized AI = +0.14). Further, PDYN displayed significant differences among males and females (F1,18 = 5.1, P < 0.05, males: AI = +0.04, females: AI = +0.21); only females showed significant differences among LH and RH (F1,5 = 11.7, P < 0.05, right lateralized AI = +0.21). A significant sex effect was also noted for PENK (F1,18 = 5.7, P < 0.05, males: AI = +0.10, females: AI = −0.02; no significant COVs) with only males, displaying a significant PENK lateralization (F1,13 = 12.1, P < 0.01, AI = +0.10).
Correlations of Individual Molecular Parameters Between the Hemispheres
Individual opioid mRNAs, NA, and 5-HT demonstrated strong and significant correlations between the LH and RH in the combined sample and males (Table 1). In females, the correlations were strong but did not reach significance except for those of OPRM1 and 5-HT. No significant correlations between opioid peptides, Glu, Asp, and DA were evident. Comparisons of the PDYN correlation between the LH and RH with such of Dyn A, Dyn B, and LER, respectively, as well as that of the PENK correlation between the LH and RH with such of MEAP (altogether 4 comparisons), revealed significant difference between the left–right PDYN and the left–right LER correlations (P < 0.0014; corrected for 4 comparisons P < 0.0057). Thus, LER biosynthesis and/or degradation had greater variations than the expression of the parent PDYN gene when comparing the left and right pgACC.
Table 1.
Correlations of individual opioid mRNA, opioid peptides, and neurotransmitters between the left and right pgACC
| Spearman's rank coefficient, ρ |
|||
|---|---|---|---|
| Combined sample | Males | Females | |
| OPRD1 | 0.75*** | 0.72** | 0.77 |
| OPRK1 | 0.84*** | 0.77*** | 0.83 |
| OPRM1 | 0.87*** | 0.85*** | 0.94** |
| PENK | 0.77*** | 0.77*** | 0.83 |
| PDYN | 0.70** | 0.54 | 0.60 |
| POMC | 0.79*** | 0.89*** | 0.83 |
| Dyn A | 0.56 | 0.29 | 0.83 |
| Dyn B | 0.07 | −0.27 | 0.37 |
| LER | −0.24 | −0.23 | −0.20 |
| MEAP | 0.47 | 0.38 | 0.71 |
| NA | 0.78*** | 0.74** | 0.71 |
| DA | 0.56 | 0.54 | 0.31 |
| 5-HT | 0.86*** | 0.87*** | 0.94** |
| Glu | 0.18 | −0.03 | 0.14 |
| Asp | 0.47 | 0.59 | 0.54 |
**P < 0.01, ***P < 0.001.
Comparison of Pair-Wise Intrahemispheric Correlations Between the LH and RH
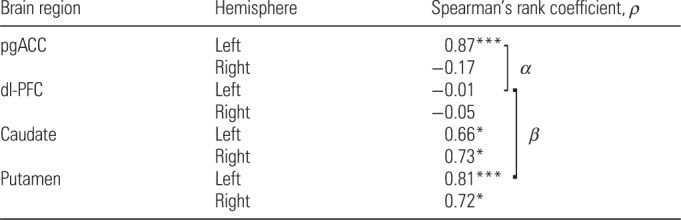
The neurotransmitter systems analyzed may interact with each other within each hemisphere, which may be reflected in the intrahemispheric correlation between the components of these systems. Some interactions may be lateralized, and consequently, the respective correlations would be anticipated to differ between the LH and RH. To test this hypothesis, we compared all intrahemispheric Spearman correlations (altogether, 105 pair-wise comparisons of each of the 15 parameters were performed between the 2 hemispheres). The Dyn B and LER correlations were found to be significantly different between the 2 hemispheres (P < 0.000011; corrected for 105 comparisons P < 0.0012); this correlation was significant in the LH (ρ = 0.87, P < 0.001), but not in the RH (ρ = −0.17), in the combined sample (Table 2). The difference was also significant for males (P < 0.0000068; corrected P < 0.00071), showing a significant positive correlation in the LH (ρ = 0.91, P < 0.001), but not in the RH (ρ = −0.36).
Table 2.
Comparison of the correlation between Dyn B and LER across the brain regions for each hemisphere separately
 |
Note: Data are shown for the combined sample of males and females.
Significance was corrected for 12 comparisons made for 2 hemispheres.
Significance of individual correlations; *P < 0.05, ***P < 0.001.
Significance of differences between 2 correlations; α, P < 0.00012 (corrected P < 0.0014) between the left pgACC and left dl-PFC; β, P < 0.0032 (corrected P < 0.039) between the left dl-PFC and left putamen.
dl-PFC, Caudate, and Putamen
For the comparison with the pgACC, we next analyzed the distribution of 4 opioid peptides between the left and right dl-PFC, caudate, and putamen, respectively. The dl-PFC and the 2 areas of dorsal striatum are characterized by moderate and high PDYN and PENK expression levels (Healy and Meador-Woodruff 1994).
Analyses of Variances
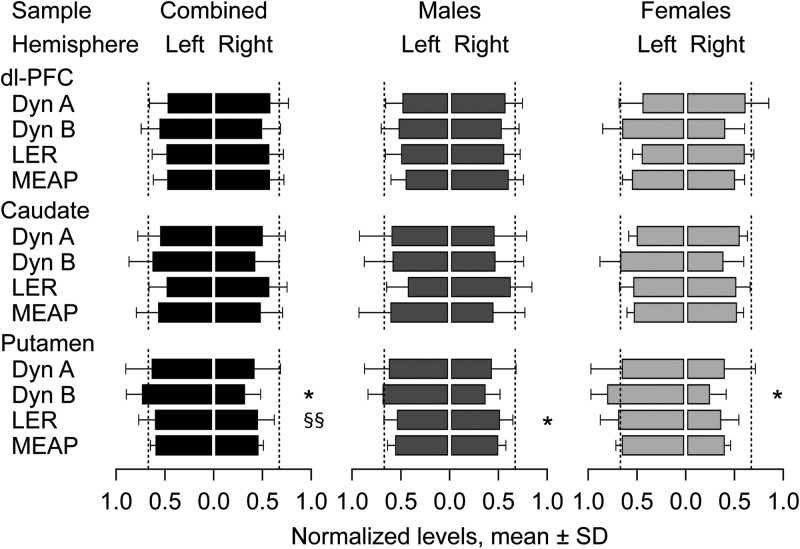
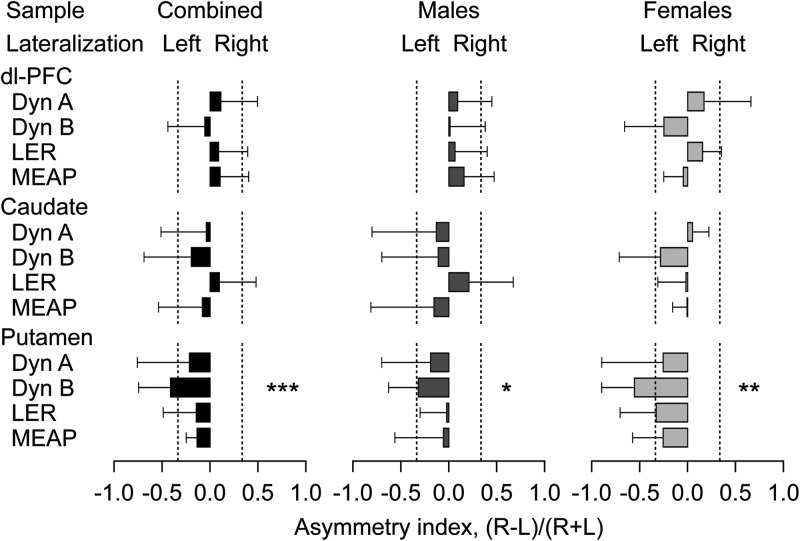
The levels of Dyn A, Dyn B, LER, and MEAP in each brain region studied within the LH and RH are displayed in Figure 3, while the respective AIs are presented in Figure 4. No significant effects were evident for the dl-PFC and the caudate. A significant hemisphere × sex interaction effect was noticed for LER in the putamen [the combined sample; ANCOVA: F1,12 = 11.0, P < 0.01; covaried with brain pH in the LH and RH (ρ = −0.66, P < 0.01 and ρ = −0.71, P < 0.01, respectively)]; significant asymmetry was evident in the males (ANCOVA: F1,7 = 6.7, P < 0.05). Dyn B displayed a strong and significant difference between the right and left putamen in the combined sample (F1,13 = 8.0, P < 0.05; no significant COVs) and females (F1,5 = 7.1, P < 0.05). Analysis of the AI revealed no significant effects in the dl-PFC and caudate, while the putamen showed a significant lateralization of Dyn B in the combined (F1,13 = 26.75, P < 0.001, left lateralized AI = −0.41; no significant COVs), male (F1,8 = 9.66, P < 0.05, left lateralized AI = −0.32), and female (F1,5 = 15.94, P < 0.01, left lateralized AI = −0.55) groups (Fig. 4).
Figure 3.
Comparison of the opioid peptide levels between the left and the right hemispheres of the dl-PFC, caudate, and putamen. Data are shown as means ± SD of the normalized levels in the LH [L/(L + R)] and RH [R/(L + R)]. Dashed lines at a 0.66 value mark 2-fold differences between hemispheres. Significance levels in ANOVAs followed by ANCOVAs when appropriate: Lateralization effects, *P < 0.05; lateralization × sex interactions, §§p < 0.01.
Figure 4.
The AI of the opioid peptides in the dl-PFC, caudate, and putamen. The −1.0 and +1.0 values of the AI [(R − L)/(R + L)] indicate a complete left or right lateralization, respectively, and the 0.0 value symmetric distribution. Data bars represent the means ± SD. Dashed lines at the −0.33 and +0.33 AI values mark 2-fold differences between hemispheres. Significance in MANOVAs followed by ANCOVAs when appropriate: Lateralization effects, *P < 0.05, **P < 0.01, ***P < 0.001.
Analyses of Correlations
No significant correlations in each of 4 neuropeptides between the left and right dl-PFC, caudate, and putamen, respectively (4 correlations for each area) were evident. In contrast to the pgACC, the Dyn B and LER correlations were significant in each of the left and right putamen and caudate, but not significant in the left and right dl-PFC (Table 2). No significant differences in pair-wise intrahemispheric correlations of the 4 neuropeptides (6 correlations for each hemisphere) between the 2 hemispheres were found.
Comparison of the Dyn B and LER Correlation Between Brain Areas
The relationship between Dyn B and LER was compared across the pgACC, dl-PFC, caudate, and putamen for each hemisphere separately. Differences in the correlation were significant between (1) the left pgACC and left dl-PFC (P < 0.00012, corrected for 12 comparisons P < 0.0014); and (2) the left dl-PFC and left putamen (P < 0.0032, corrected P < 0.039; Table 2). Partial correlation analyses, to control for any confounding effects, indicated that most of the comparisons were significantly different from one another (Supplementary Table 3).
Validity of the Opioid Peptide Analysis
No significant correlations between peptide levels and tissue characteristics including brain pH, total PMI, and RQI were generally evident, indicating that these levels did not substantially change during postmortem period (Supplementary Table 4). Furthermore, the high correlation between PDYN mRNA versus Dyn A and PDYN mRNA versus Dyn B levels (Table 3) suggested that interindividual variations between PDYN transcription and PDYN processing to dynorphins were small and, therefore, their analyses were not impaired by differential degradation of RNA and peptide molecules during the PMI.
Table 3.
Correlations between opioid mRNAs and respective opioid peptide products in the pgACC
| Correlation | Hemisphere | Spearman's rank coefficient, ρ |
|---|---|---|
| PDYN and Dyn A | Left | 0.85*** |
| Right | 0.73*** | |
| PDYN and Dyn B | Left | 0.75*** |
| Right | 0.70*** | |
| PDYN and LER | Left | 0.43 |
| Right | −0.13 | |
| PENK and MEAP | Left | 0.13 |
| Right | 0.24 |
The combined sample of males and females was analyzed. ***P < 0.001.
Demographic Correlations
For the pgACC, age-dependent alterations in OPRM1 and PENK mRNA levels were noted; OPRM1 mRNA levels correlated positively with age in both the LH and RH (ρ = 0.77, P < 0.001; and ρ = 0.59, P < 0.01, respectively), whereas PENK mRNA correlated negatively with age (LH: ρ = −0.74, P < 0.001; RH: ρ = −0.58, P < 0.01, respectively).
Proprotein Convertase in the pgACC
The only peptidase that is involved in the left–right asymmetry and cleaves neuropeptides at pairs of basic amino acid residues is the paired basic amino acid cleaving enzyme 4 or proprotein convertase PACE4 encoded by the PCSK6 gene (Beck et al. 2002; Seidah and Prat 2012). To test whether this convertase is involved in Dyn B cleavage, we analyzed PCSK6 mRNA in the pgACC in the male sample that demonstrated greater left–right differences in the Dyn B versus LER correlation. The correlation between Dyn B and PCSK6 was negative, strong and significant in the right pgACC (ρ = −0.68; P = 0.008), but not in the left pgACC (ρ = −0.40), suggesting a role of PACE4 in Dyn B conversion in the RH. No asymmetry in PCSK6 mRNA distribution between the LH and RH and no PCSK6 and LER correlation were evident.
Replication Study
The availability of specimens from the LH and RH of the same subjects is a limitation for analyzing the lateralization of neurochemical systems in the human brain. Brain banks generally collect one hemisphere of each brain for molecular/biochemical studies and the other side for morphological/histochemical analyses. Because we were unable to find necessary specimens at the major human brain banks, we proceeded with the analyses of the pgACC specimens from 12 subjects, including 9 males and 3 females (Supplementary Table 1B), that were excluded from the primary analysis. These samples had warm or cold PMIs exceeded 12 or 48 h, respectively, or their RQI values were <5.0 (5 subjects). There was no significant difference in age [t(30) = 0.04, P = 0.96] and in the proportion of males and females (Fisher's exact test, P = 0.30) between the discovery and replication samples.
The levels of Dyn B, LER, and MEAP were analyzed in the left and right pgACC (Supplementary Fig. 1A,B). The levels of LER were higher in the LH by 2.8-fold (2-way ANOVA: F1,10 = 4.9, P = 0.05; no significant COVs), whereas the MEAP levels were 2.0-fold higher in the RH (2-way ANOVA: F1,10 = 6.7, P < 0.05; no significant COVs). A significant lateralization of MEAP was evident in males (2-way ANOVA: F1,8 = 13.8, P < 0.01; no significant COVs). Analysis of the AI for LER and MEAP by the MANOVA revealed a significant main effect of the hemisphere. LER was lateralized to the LH in the combined male and female sample (F1,10 = 15.2, P < 0.01; AI = −0.56; no significant COVs), and in samples from males (F1,8 = 14.2, P < 0.01, the AI = −0.54). MEAP showed a right lateralization in the combined sample (F1,17 = 11.1, P < 0.01, the AI = +0.24; no significant COVs) and in males (F1,8 = 19.0, P < 0.01; the AI = +0.27). Dyn B and LER levels showed significant correlation in the LH (ρ = 0.78; P < 0.01), but not in the RH (ρ = 0.18), while their difference was at the trend level (P = 0.07).
Discussion
The principal findings of the present study are that (1) the opioid peptides LER and MEAP are markedly lateralized to the left and right pgACC, respectively; and (2) Dyn B and LER strongly correlate in the left, but not in the right pgACC. No substantial lateralization >20% was evident in the levels of 6 EOS mRNAs and 5 neurotransmitters in the pgACC. No considerable asymmetry in the levels of 4 opioid peptides was detected in the dl-PFC and caudate, while Dyn B showed strong lateralization in the putamen.
A few studies compared the distribution of neurotransmitters and their receptors between the LH and RH in the human brain. Postmortem analyses revealed higher NA in the left compared with right thalamus (Oke et al. 1978), higher 5-HT receptor levels in the right frontal cortex (Arato et al. 1991), and nondirectional asymmetry of DA in the basal ganglia (Glick et al. 1982). Positron emission tomography studies demonstrate right–left differences in DA receptor expression levels in the striatum and in the frontal and temporal cortices that varied among individuals in both magnitude and direction (Tomer et al. 2008, 2012). 5-HT1A receptors in the cortical region associated with language exhibit higher levels in the right frontal gyri and in the left auditory cortex (Fink et al. 2009). It has been proposed that the LH is organized around a DA activation system, which made it superior for complex motor programming (leading to a right manual preference) and speech, while the RH is structured around a NA arousal system (Tucker and Williamson 1984). This view was challenged by the hypothesis that the preferential activation of the LH is mediated by NA and the RH is regulated by 5-HT (Fitzgerald 2012). Molecular analysis identified 27 genes that are differentially expressed in the left and right human embryonic hemispheres. This suggests that cortical asymmetry is preceded by early transcriptional asymmetries (Sun et al. 2005; Sun and Walsh 2006). Other genome-wide studies found no interhemispheric asymmetry in gene expression in the developing neocortex during midgestation (Johnson et al. 2009) or in the adult human brain (Hawrylycz et al. 2012). However, a fewer number of brains analyzed in these 3 studies (from 2 to 5) did not allow sound conclusions. In the present study, the number of subjects analyzed was sufficient to support the notion on the lateralization phenomenon at the population level.
The pgACC, cytoarchitecturally and physiologically distinct subregion of the ACC, is involved in the processing of negative and positive emotions and affective component of pain and has anatomical connections to other core-emotional processing regions as amygdala, PAG, and hypothalamus (An et al. 1998; Beckmann et al. 2009). Imaging studies using the selective μ-opioid receptor agonist [11C]carfentanyl, or nonselective opioid antagonist naloxone, identified a critical role of the EOS in control of these processes in the pgACC (Kennedy et al. 2006; Wager et al. 2007; Eippert et al. 2009). Analysis of the mechanisms underlying the neurochemical basis of lateralized function in the pgACC is clinically important, because abnormalities in this area are associated with depression, schizophrenia, borderline personality, and anxiety disorders (Fahim et al. 2005; Walter et al. 2009; Prossin et al. 2010; Pizzagalli 2011), and autism spectrum disorder including Asperger syndrome (Oner et al. 2007). Thus, an aberrant neuronal activation of the pgACC may contribute to anhedonia in major depression (Walter et al. 2009). Many functional and structural changes in psychiatric patients were identified specifically in the right pgACC. They include a correlation between reductions in gray matter volume and self-conscious emotional reactivity in patients with dementia (Sturm et al. 2012); reduced gray matter volume in patients with borderline personality disorders (Soloff et al. 2008); hyperglutamatergic metabolism in subjects with autism spectral disorder (Bejjani et al. 2012); a higher N-acetylaspartate: choline ratio in Asperger syndrome (Oner et al. 2007); and abnormal blood oxygen level-dependent responses in nonparanoid schizophrenia patients expressing fear (Williams et al. 2004). Thus, the right pgACC may contribute to emotional dysregulation in psychiatric disorders with negative emotional states.
LER has high binding affinity for both δ- and µ-opioid receptors (Mansour et al. 1995). Endogenous µ- and δ-agonists are involved in the regulation of positive emotional states and pleasure (Bals-Kubik et al. 1993; Filliol et al. 2000; Leknes and Tracey 2008; Jutkiewicz and Roques 2012). We hypothesize that the preferential activation of δ- and µ-opioid receptors by LER in the left pgACC has a role in the lateralization of positive emotions to the LH (Dolan et al. 1996; Phillips et al. 1998). In contrast to LER, MEAP is lateralized to the right pgACC. This peptide binds to both κ- and µ-opioid receptors, but only weakly to the δ-receptor (Iyengar et al. 1987; Kamei et al. 1994; Mansour et al. 1995; Benyhe et al. 1997). The κ-system is critical for the modulation of negative emotional states and control of pain processing (Narita et al. 2006; Land et al. 2008; Schwarzer 2009; Shippenberg 2009; Knoll and Carlezon 2010) that both may be preferentially presented and processed in the RH and specifically in the right pgACC (Hsieh et al. 1995, 1996; Brooks et al. 2002; Symonds et al. 2006; Brügger et al. 2011; Kim et al. 2012). The lateralization of MEAP, which may activate κ-opioid receptor, along with the differences between the LH and RH in the conversion of the κ-opioid receptor agonist Dyn B into δ-/µ-agonist LER may provide molecular basis for the lateralization of negative emotions and pain to the right pgACC in the human brain.
While cortical lateralization has been a focus of multiple papers, less is known about the lateralization of the striatum. DA levels were found to be higher in the left putamen and asymmetries in the nigrostriatal DArgic system were suggested to have a role in lateralized motor function (de la Fuente-Fernández et al. 2000; Barber et al. 2012). Dynorphins inhibit DA release acting through the presynaptic κ-opioid receptor (Butelman et al. 2012). Therefore, dynorphin elevation may lead to increase in DA levels in the left compared with the right putamen. Dyn B lateralization in this area along with the asymmetry of LER and MEAP in the pgACC supports the notion on brain region-specific EOS lateralization in the human brain.
Difference between the 2 hemispheres in the Dyn B and LER correlation that is significant in the LH suggests the complex regulation of the conversion of Dyn B into shorter enkephalins in the RH compared with the LH. The only known peptidase that is involved in the left–right asymmetry and cleaves neuropeptides at the pairs of basic amino acid residues is the proprotein convertase PACE4. PACE4 is encoded by the PCSK6 gene (Beck et al. 2002; Seidah and Prat 2012). In a proportion of mouse embryos lacking the Pcsk6 gene, the Nodal, Lefty, and Pitx2 genes are expressed bilaterally in contrast to wild-type animals in which they are expressed asymmetrically. Some embryos consequently display laterality defects including situs ambiguous. The GWAS study identified single-nucleotide polymorphisms in PCSK6 that are associated with left handedness and dyslexia (Scerri et al. 2011). This association may originate from the PACE4 activity in the left–right axis formation (Constam 2009) and brain development. Our analysis revealed a strong and significant negative correlation between Dyn B and PCSK6 in the right, but not in the left pgACC. Thus, PACE4 may be involved in conversion of Dyn B to enkephalins in the RH, where the variations in the expression or activity of this enzyme may contribute to high interindividual variability in Dyn B cleavage.
The present study has several limitations. Firstly, the replication sample was made of subjects characterized by a prolonged PMI (10 subjects), and/or a RQI of <5.0 (5 subjects). Yet, the results obtained with the discovery sample were replicated. Secondly, information on the lateralization of language, emotions and pain, and handedness for analyzed individuals was missing and, therefore, no functional associations could be analyzed. Because multiple neurobiological, genetic, and environmental mechanisms differentially control asymmetry for distinct brain systems (Liu et al. 2009; Ocklenburg et al. 2011), the application of combined genetic, neuroimaging, and behavioral approach is required for understanding a physiological role of the lateralized opioid peptides that is a subject for future studies.
The presence of pharmaceutical drugs, alcohol, and opioid analgesic in the blood of 3 subjects, and a high number of suicide death (36% in the male sample), may be the factors influencing the EOS lateralization. The high number of male suicide death is not surprising, because the average age in male samples was 49 years. Suicide rates are much higher among middle-aged individuals (from 45 to 54 years old) compared with other ages, and also among males compared with females (report of the World Health Organization 2010). Exclusion of either 3 subjects with drugs/alcohol in the blood, or 6 subjects that committed suicide, did not affect the significance of the lateralization effects for the combined male and female samples (N = 14), and for the male sample (N = 9) in most of the cases. Thus, the EOS lateralization is robust and is not affected by suicide as a cause of death or the consumption of pharmaceutical drugs and alcohol.
The EOS complexity is high and as such has not been comprehensibly addressed in any single animal or human “case–control” study. A “complete profiling” of opioid receptors and peptides requires the analysis of multiple splicing variants, opioid peptides and receptor proteins and their modifications, and functional receptor characteristics. We limited our study to the EOS characteristics that may be reliably analyzed in postmortem human tissues such as mRNAs and peptide levels including 13 of 17 OPRM1 mRNA variants, 4 major PDYN splicing variants that generate opioid peptides, and PDYN and PENK neuropeptide levels. We did not focus on more functional characteristics such as activation of G-proteins that are more sensitive to the quality of postmortem human tissues and for which rigorous criteria for analysis in the postmortem human brain have not yet been established. We cannot rule out that some of the individual splicing variants that were or were not included in the analyses, as well as functional opioid receptor characteristics, may be lateralized in the ACC.
Postmortem analyses may be complicated due to RNA and peptide degradation during the PMI. The following steps were taken to address this problem. Firstly, only cases in which the PMI was <48 h and the tissue characteristics were in the range acceptable for postmortem analyses (brain pH >5.0 and RQI >5.0; Kingsbury et al. 1995; Fleige et al. 2006) were included in the primary analysis. Secondly, demographic and tissue characteristics were analyzed as COVs that did not affect the significance of the left–right differences. Thirdly, despite the susceptibility to undergo degradation through different mechanisms, dynorphin peptides and PDYN mRNA highly correlated, thus demonstrating that both types of analyses were relevant. Fourthly, the experimental design based on the pair-wise comparison of the LH and RH from the same subjects diminished effects of both interindividual variations and demographic/tissue characteristics on statistical outcome. Fifthly, the analyses of the replication sample consisting of cases with long PMI and low RQI reproduced the findings obtained with the discovery sample. Overall, these data strongly suggest that, despite limitations, the EOS lateralization phenomenon is robust and characteristic for the population analyzed.
Our previous work identified an asymmetric distribution of opioid receptors and their endogenous ligands in the brain and spinal cord of naïve, stressed, or injured animals (Bakalkin et al. 1982, 1986, 1989; Bakalkin and Kobylyansky 1989; Hussain et al. 2012). In the frontal cortex and striatum, injury to the RH affected Dyn A levels to a greater extent than that to the LH. The Dyn A-expressing neuronal networks differentially responding to the left and right brain injury may mediate effects of unilateral trauma on lateralized brain functions (Hussain et al. 2012). In the spinal cord, the lateralized opioid receptors may be a part of the hypothetical neurochemical mechanism that controls the processing of nociceptive stimuli and motor reflexes selectively on the left or right side (Bakalkin et al. 1982, 1986; Bakalkin and Kobylyansky 1989). The present study is the first that demonstrates the lateralization of the EOS in the human brain. This asymmetry may underlie the lateralization of higher brain functions including emotions and affective components of pain. It should be emphasized that the laterality for emotional valence is not absolute and may be region-specific, and that a physiological role of the endogenous opioid peptides LER and MEAP is not well established yet. Further verification and functional analysis of the lateralization of the EOS in the pgACC are necessary for understanding a role of the endogenous opioid peptides in the lateralized processing of positive and negative emotions and pain in the normal brain and in mood disorders. This could be accomplished through the use of selective μ-, δ-, and κ-opioid ligands in neuroimaging experiments, through the analysis of associations of genes regulating opioid peptide conversion with functional brain asymmetry, and via the functional analysis of enkephalins and dynorphins found as lateralized in the present study.
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by grants from the Swedish Council for Working Life and Social Research (FAS), Swedish Science Research Council (VR), Swedish Research Council FORMAS to G.B.; the Swedish Institute (Visby Program) to F.N., O.K., and G.B.; and from NIH “NIH K02 DA027374” to K.F.H.
Supplementary Material
Notes
We acknowledge Dr Olga Yamskova for help with experiments. Conflict of Interest: None declared.
References
- Amemori K, Graybiel AM. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat Neurosci. 2012;15:776–785. doi: 10.1038/nn.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arato M, Frecska E, Maccrimmon DJ, Guscott R, Saxena B, Tekes K, Tothfalusi L. Serotonergic interhemispheric asymmetry: neurochemical and pharmaco-EEG evidence. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:759–764. doi: 10.1016/0278-5846(91)90004-k. [DOI] [PubMed] [Google Scholar]
- Bakalkin GY, Kobylyansky AG. Opioids induce postural asymmetry in spinal rat: the side of the flexed limb depends upon the type of opioid agonist. Brain Res. 1989;480:277–289. doi: 10.1016/0006-8993(89)90193-5. [DOI] [PubMed] [Google Scholar]
- Bakalkin GY, Kobylyansky AG, Nagornaya LV, Yarygin KN, Titov MI. Met-enkephalin-induced release into the blood of a factor causing postural asymmetry. Peptides. 1986;7:551–556. doi: 10.1016/0196-9781(86)90025-2. [DOI] [PubMed] [Google Scholar]
- Bakalkin GY, Krivosheev OG, Stolyarov GK. Postural asymmetry in rats induced by stress and pain stimuli. Life Sci. 1982;30:779–783. doi: 10.1016/0024-3205(82)90613-0. [DOI] [PubMed] [Google Scholar]
- Bakalkin GY, Pivovarov AS, Kobylyansky AG, Nesterenko PN, Yarygin KN. Lateralization of opioid receptors in turtle visual cortex. Brain Res. 1989;480:268–276. doi: 10.1016/0006-8993(89)90192-3. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Mostofsky SH. Motor “dexterity”: evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cereb Cortex. 2012;22:51–59. doi: 10.1093/cercor/bhr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Beck S, Le Good JA, Guzman M, Ben Haim N, Roy K, Beermann F, Constam DB. Extraembryonic proteases regulate nodal signalling during gastrulation. Nat Cell Biol. 2002;4:981–985. doi: 10.1038/ncb890. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjani A, O'Neill J, Kim JA, Frew AJ, Yee VW, Ly R, Kitchen C, Salamon N, McCracken JT, Toga AW, et al. Elevated glutamatergic compounds in pregenual anterior cingulate in pediatric autism spectrum disorder demonstrated by 1H MRS and 1H MRSI. PLoS One. 2012;7:e38786. doi: 10.1371/journal.pone.0038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyhe S, Farkas J, Tóth G, Wollemann M. Met5-enkephalin-Arg6-Phe7, an endogenous neuropeptide, binds to multiple opioid and nonopioid sites in rat brain. J Neurosci Res. 1997;48:249–258. doi: 10.1002/(sici)1097-4547(19970501)48:3<249::aid-jnr7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Bever TG, Chiarello RJ. Cerebral dominance in musicians and nonmusicians. 1974. J Neuropsychiatry Clin Neurosci. 2009;21:94–97. doi: 10.1176/jnp.2009.21.1.94. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Brügger M, Ettlin DA, Meier M, Keller T, Luechinger R, Barlow A, Palla S, Jäncke L, Lutz K. Taking sides with pain—lateralization aspects related to cerebral processing of dental pain. Front Hum Neurosci. 2011;5:12. doi: 10.3389/fnhum.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, Kreek MJ. kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35:587–596. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW., IV. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain. 2008;4:24. doi: 10.1186/1744-8069-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30:61–69. [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensson-Nylander I, Nyberg F, Ragnarsson U, Terenius L. A general procedure for analysis of proenkephalin B derived opioid peptides. Regul Pept. 1985;11:65–76. doi: 10.1016/0167-0115(85)90032-1. [DOI] [PubMed] [Google Scholar]
- Constam DB. Running the gauntlet: an overview of the modalities of travel employed by the putative morphogen nodal. Curr Opin Genet Dev. 2009;19:302–307. doi: 10.1016/j.gde.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Corradi-Dell'Acqua C, Hofstetter C, Vuilleumier P. Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. J Neurosci. 2011;31:17996–18006. doi: 10.1523/JNEUROSCI.2686-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernández R, Kishore A, Calne DB, Ruth TJ, Stoessl AJ. Nigrostriatal dopamine system and motor lateralization. Behav Brain Res. 2000;112:63–68. doi: 10.1016/s0166-4328(00)00165-0. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Everhart DE, Youngstrom EA, Harrison DW. Brain lateralization of emotional processing: historical roots and a future incorporating “dominance”. Behav Cogn Neurosci Rev. 2005;4:3–20. doi: 10.1177/1534582305276837. [DOI] [PubMed] [Google Scholar]
- Deo AJ, Huang YY, Hodgkinson CA, Xin Y, Oquendo MA, Dwork AJ, Arango V, Brent DA, Goldman D, Mann JJ, et al. A large-scale candidate gene analysis of mood disorders: evidence of neurotrophic tyrosine kinase receptor and opioid receptor signaling dysfunction. Psychiatr Genet. 2013;23:47–55. doi: 10.1097/YPG.0b013e32835d7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher P, Morris J, Kapur N, Deakin JF, Frith CD. Neural activation during covert processing of positive emotional facial expressions. Neuroimage. 1996;4:194–200. doi: 10.1006/nimg.1996.0070. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Harrison BA, Arnold SL, Nibaldi K, Segrave RA, Fitzgibbon BM, Kennedy HA, Lau K, Fitzgerald PB. Emotional valence modulates putative mirror neuron activity. Neurosci Lett. 2012;508:56–59. doi: 10.1016/j.neulet.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Erhan H, Borod JC, Tenke CE, Bruder GE. Identification of emotion in a dichotic listening task: event-related brain potential and behavioral findings. Brain Cogn. 1998;37:286–307. doi: 10.1006/brcg.1998.0984. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim C, Stip E, Mancini-Marie A, Mensour B, Boulay LJ, Leroux JM, Beaudoin G, Bourgouin P, Beauregard M. Brain activity during emotionally negative pictures in schizophrenia with and without flat affect: an fMRI study. Psychiatry Res. 2005;140:1–15. doi: 10.1016/j.pscychresns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Fan J, Gu X, Liu X, Guise KG, Park Y, Martin L, de Marchena A, Tang CY, Minzenberg MJ, Hof PR. Involvement of the anterior cingulate and frontoinsular cortices in rapid processing of salient facial emotional information. Neuroimage. 2011;54:2539–2546. doi: 10.1016/j.neuroimage.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Fink M, Wadsak W, Savli M, Stein P, Moser U, Hahn A, Mien LK, Kletter K, Mitterhauser M, Kasper S, et al. Lateralization of the serotonin-1A receptor distribution in language areas revealed by PET. Neuroimage. 2009;45:598–605. doi: 10.1016/j.neuroimage.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ. Whose side are you on: does serotonin preferentially activate the right hemisphere and norepinephrine the left? Med Hypotheses. 2012;79:250–254. doi: 10.1016/j.mehy.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Fleige S, Walf V, Huch S, Prgomet C, Sehm J, Pfaffl MW. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol Lett. 2006;28:1601–1613. doi: 10.1007/s10529-006-9127-2. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Forty-five years of split-brain research and still going strong. Nat Rev Neurosci. 2005;6:653–659. doi: 10.1038/nrn1723. [DOI] [PubMed] [Google Scholar]
- Gier VS, Kreiner DS, Solso RL, Cox SL. The hemispheric lateralization for processing geometric word/shape combinations: the Stroop-shape effect. J Gen Psychol. 2010;137:1–19. doi: 10.1080/00221300903293022. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ross DA, Hough LB. Lateral asymmetry of neurotransmitters in human brain. Brain Res. 1982;234:53–63. doi: 10.1016/0006-8993(82)90472-3. [DOI] [PubMed] [Google Scholar]
- Goodman M, Hazlett EA, Avedon JB, Siever DR, Chu KW, New AS. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid major depression. J Psychiatr Res. 2011;45:803–807. doi: 10.1016/j.jpsychires.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol Psychol. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DJ, Meador-Woodruff JH. Prodynorphin-derived peptide expression in primate cortex and striatum. Neuropeptides. 1994;27:277–284. doi: 10.1016/0143-4179(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63:225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Hannerz J, Ingvar M. Right-lateralised central processing for pain of nitroglycerin-induced cluster headache. Pain. 1996;67:59–68. doi: 10.1016/0304-3959(96)03066-7. [DOI] [PubMed] [Google Scholar]
- Hussain ZM, Fitting S, Watanabe H, Usynin I, Yakovleva T, Knapp PE, Scheff SW, Hauser KF, Bakalkin G. Lateralized response of dynorphin a peptide levels after traumatic brain injury. J Neurotrauma. 2012;29:1785–1793. doi: 10.1089/neu.2011.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Kim HS, Wood PL. Mu-, delta-, kappa- and epsilon-opioid receptor modulation of the hypothalamic-pituitary-adrenocortical (HPA) axis: subchronic tolerance studies of endogenous opioid peptides. Brain Res. 1987;435:220–226. doi: 10.1016/0006-8993(87)91604-0. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol. 2009;102:2253–2264. doi: 10.1152/jn.00166.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanović D, Geschwind DH, Mane SM, State MW, Sestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R. The right cerebral hemisphere: emotion, music, visual-spatial skills, body-image, dreams, and awareness. J Clin Psychol. 1988;44:630–673. doi: 10.1002/1097-4679(198809)44:5<630::aid-jclp2270440502>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Roques BP. Endogenous opioids as physiological antidepressants: complementary role of delta receptors and dopamine. Neuropsychopharmacology. 2012;37:303–304. doi: 10.1038/npp.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Iwamoto Y, Misawa M, Nagase H, Kasuya Y. Antitussive effect of [Met5]enkephalin-Arg6-Phe7 in mice. Eur J Pharmacol. 1994;253:293–296. doi: 10.1016/0014-2999(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Karpyak VM, Winham SJ, Preuss UW, Zill P, Cunningham JM, Walker DL, Lewis KA, Geske JR, Colby CL, Abulseoud OA, et al. Association of the PDYN gene with alcohol dependence and the propensity to drink in negative emotional states. Int J Neuropsychopharmacol. 2012 doi: 10.1017/S1461145712001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J. Determination of glutamate and aspartate in microdialysis samples by reversed-phase column liquid chromatography with fluorescence and electrochemical detection. J Chromatogr B Biomed Sci Appl. 1998;708:27–38. doi: 10.1016/s0378-4347(97)00677-4. [DOI] [PubMed] [Google Scholar]
- Kehr J, Yoshitake T. Monitoring brain chemical signals by microdialysis. In: Grimes CA, Dickey EC, Pishko MV, editors. Encyclopedia of sensors. USA: American Scientific Publishers; 2006. pp. 287–312. [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Gruber SA, Yurgelun-Todd DA. Depressed mood and lateralized prefrontal activity during a Stroop task in adolescent children. Neurosci Lett. 2007;416:43–48. doi: 10.1016/j.neulet.2007.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Mátyás F, Lee S, Acsády L, Shin HS. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proc Natl Acad Sci USA. 2012;109:15497–15501. doi: 10.1073/pnas.1213903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ, Lees AJ, Marsden CD. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res Mol Brain Res. 1995;28:311–318. doi: 10.1016/0169-328x(94)00219-5. [DOI] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM. The One-Two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Youell P, Watson A, Derbyshire SW, Frackowiak RS, Friston KJ, Jones AK. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci. 2005;21:3133–3142. doi: 10.1111/j.1460-9568.2005.04098.x. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Levine SC, Levy J. Perceptual asymmetry for chimeric faces across the life span. Brain Cogn. 1986;5:291–306. doi: 10.1016/0278-2626(86)90033-3. [DOI] [PubMed] [Google Scholar]
- Levy LM, Reis IL, Grafman J. Metabolic abnormalities detected by 1H-MRS in dyscalculia and dysgraphia. Neurology. 1999;53:639–641. doi: 10.1212/wnl.53.3.639. [DOI] [PubMed] [Google Scholar]
- Lindgren L, Westling G, Brulin C, Lehtipalo S, Andersson M, Nyberg L. Pleasant human touch is represented in pregenual anterior cingulate cortex. Neuroimage. 2012;59:3427–3432. doi: 10.1016/j.neuroimage.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci USA. 2009;106:20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo M, Istúriz G, Lara C, García N, Eblen-Zaijur A. Sensory lateralization in pain subjective perception for noxious heat stimulus. Somatosens Mot Res. 2002;19:207–212. doi: 10.1080/0899022021000009125. [DOI] [PubMed] [Google Scholar]
- Mandal MK, Borod JC, Asthana HS, Mohanty A, Mohanty S, Koff E. Effects of lesion variables and emotion type on the perception of facial emotion. J Nerv Ment Dis. 1999;187:603–609. doi: 10.1097/00005053-199910000-00003. [DOI] [PubMed] [Google Scholar]
- Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98. doi: 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- Merg F, Filliol D, Usynin I, Bazov I, Bark N, Hurd YL, Yakovleva T, Kieffer BL, Bakalkin G. Big dynorphin as a putative endogenous ligand for the kappa-opioid receptor. J Neurochem. 2006;97:292–301. doi: 10.1111/j.1471-4159.2006.03732.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Crocker LD, Spielberg JM, Infantolino ZP, Heller W. Issues in localization of brain function: the case of lateralized frontal cortex in cognition, emotion, and psychopathology. Front Integr Neurosci. 2013;7:2. doi: 10.3389/fnint.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PL, Robinson RG, Raphael B, Hopwood MJ. Lesion location and poststroke depression. J Neuropsychiatry Clin Neurosci. 1996;8:399–403. doi: 10.1176/jnp.8.4.399. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Nguyen XV, Masse J, Kumar A, Vijitruth R, Kulik C, Liu M, Choi DY, Foster TC, Usynin I, Bakalkin G, et al. Prodynorphin knockout mice demonstrate diminished age-associated impairment in spatial water maze performance. Behav Brain Res. 2005;161:254–262. doi: 10.1016/j.bbr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Nylander I, Stenfors C, Tan-No K, Mathé AA, Terenius L. A comparison between microwave irradiation and decapitation: basal levels of dynorphin and enkephalin and the effect of chronic morphine treatment on dynorphin peptides. Neuropeptides. 1997;31:357–365. doi: 10.1016/s0143-4179(97)90072-x. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S, Arning L, Hahn C, Gerding WM, Epplen JT, Güntürkün O, Beste C. Variation in the NMDA receptor 2B subunit gene GRIN2B is associated with differential language lateralization. Behav Brain Res. 2011;225:284–289. doi: 10.1016/j.bbr.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Oke A, Keller R, Mefford I, Adams RN. Lateralization of norepinephrine in human thalamus. Science. 1978;200:1411–1413. doi: 10.1126/science.663623. [DOI] [PubMed] [Google Scholar]
- Oner O, Devrimci-Ozguven H, Oktem F, Yagmurlu B, Baskak B, Munir KM. Proton MR spectroscopy: higher right anterior cingulate N-acetylaspartate/choline ratio in Asperger syndrome compared with healthy controls. AJNR Am J Neuroradiol. 2007;28:1494–1498. doi: 10.3174/ajnr.A0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg F, Juckel G, Prässl A, Zwanzger P, Mavrogiorgou P, Hegerl U, Hampel H, Möller HJ. Prefrontal cortex modulation of mood and emotionally induced facial expressions: a transcranial magnetic stimulation study. J Neuropsychiatry Clin Neurosci. 2001;13:206–212. doi: 10.1176/jnp.13.2.206. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, Williams SC, Simmons A, Andrew C, Brammer M, David AS. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Res. 1998;83:127–138. doi: 10.1016/s0925-4927(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. Dysregulation of regional endogenous opioid function in borderline personality disorder. Am J Psychiatry. 2010;167:925–933. doi: 10.1176/appi.ajp.2010.09091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ED, Mesulam MM. Dominant language functions of the right hemisphere? Prosody and emotional gesturing. Arch Neurol. 1979;36:144–148. doi: 10.1001/archneur.1979.00500390062006. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic evidence. Arch Neurol. 1982;39:210–218. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Gur RC, Saucy MC. Emotions are expressed more intensely on the left side of the face. Science. 1978;202:434–436. doi: 10.1126/science.705335. [DOI] [PubMed] [Google Scholar]
- Scerri TS, Brandler WM, Paracchini S, Morris AP, Ring SM, Richardson AJ, Talcott JB, Stein J, Monaco AP. PCSK6 Is associated with handedness in individuals with dyslexia. Hum Mol Genet. 2011;20:608–614. doi: 10.1093/hmg/ddq475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C. 30 Years of dynorphins—new insights on their functions in neuropsychiatric diseases. Pharmacol Ther. 2009;123:353–370. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS. The dynorphin/kappa opioid receptor system: a new target for the treatment of addiction and affective disorders? Neuropsychopharmacology. 2009;34:247. doi: 10.1038/npp.2008.165. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Bakalkin G, Walker BM. Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front Mol Neurosci. 2012;5:95. doi: 10.3389/fnmol.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff P, Nutche J, Goradia D, Diwadkar V. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Res. 2008;164:223–236. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S, Shapiro D, Zaidel E. The role of the right hemisphere in the physiological and cognitive components of emotional processing. Psychophysiology. 1996;33:112–122. doi: 10.1111/j.1469-8986.1996.tb02115.x. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in mood and ideation associated with increase in right caudate volume. J Affect Disord. 2007;101:139–147. doi: 10.1016/j.jad.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Robinson RG, Honig MA, Parikh RM, Joselyn J, Price TR. Mood changes after right-hemisphere lesions. Br J Psychiatry. 1989;155:79–85. doi: 10.1192/bjp.155.1.79. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, Levenson RW. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, Orkin SH, Geschwind DH, Walsh CA. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7:655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- Symonds LL, Gordon NS, Bixby JC, Mande MM. Right-lateralized pain processing in the human cortex: an FMRI study. J Neurophysiol. 2006;95:3823–3830. doi: 10.1152/jn.01162.2005. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, Lieb K, Bohus M, Hennig J, Ebert D. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol Psychiatry. 2003;54:163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Slagter HA, Christian BT, Fox AS, King CR, Murali D, Davidson RJ. Dopamine asymmetries predict orienting bias in healthy individuals. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM, Williamson PA. Asymmetric neural control systems in human self-regulation. Psychol Rev. 1984;91:185–215. [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. In: Cingulate neurobiology and disease. Vogt BA, editor. New York: Oxford University Press; 2009. pp. 3–30. [Google Scholar]
- Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J Cogn Neurosci. 2010;22:2864–2885. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- Weddell RA. Effects of subcortical lesion site on human emotional behavior. Brain Cogn. 1994;25:161–193. doi: 10.1006/brcg.1994.1029. [DOI] [PubMed] [Google Scholar]
- Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci. 2006;26:11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Das P, Harris AW, Liddell BB, Brammer MJ, Olivieri G, Skerrett D, Phillips ML, David AS, Peduto A, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Suicide rates (per 100,000), by gender, Sweden, 1950–2010. 2010. Available from: http://www.who.int/mental_health/media/swed.pdf. Accessed July 31, 2013.
- Young EJ, Williams CL. Valence dependent asymmetric release of norepinephrine in the basolateral amygdala. Behav Neurosci. 2010;124:633–644. doi: 10.1037/a0020885. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.