Abstract
Increased frontal cortex activation during cognitive task performance is common in aging but remains poorly understood. Here we explored patterns of age-related frontal brain activations under multiple task performance conditions and their relationship to white matter (WM) microstructure. Groups of younger (N = 28) and older (N = 33) participants completed a task-switching paradigm while functional magnetic resonance imaging (fMRI) was performed, and rested while diffusion tensor imaging was performed. Results from fMRI analyses indicated age-related increases in frontal brain activations under conditions of poorer performance in the older group (the nonswitch and switch conditions) and for a contrast in which behavioral performance was equated (older group nonswitch condition vs. younger group switch condition). Within the older adult group, higher frontal activation was associated with poorer behavioral performance under all task conditions. In 2 regions in right frontal cortex, blood oxygen level–dependent (BOLD) magnitudes were negatively correlated with WM integrity in tracts connecting these structures with other task-relevant frontoparietal and striatal regions. Our results link age-related declines in the efficiency of frontal cortex functioning with lower WM integrity in aging.
Keywords: aging, DTI, over-recruitment, task switching, white matter
Introduction
Human aging is associated with altered brain activation patterns on a number of cognitive tasks. Alterations in brain activation associated with aging tend to be especially pronounced on difficult tasks that emphasize cognitive control processes (Drag and Bieliauskas 2010). Cognitive control refers to a set of processes that enable humans to flexibly shape thoughts and behavior in order to accomplish internal goals (Miller and Cohen 2001). Young adults typically recruit a distributed network of frontoparietal regions during the performance of cognitive control tasks (Badre and Wagner 2006; Cole and Schneider 2007; Neumann et al. 2008; Kim et al. 2012). While a variety of age-related alterations in brain activation during cognitive control tasks have been reported, among the most common appears to be increased activation in frontal brain regions (Drag and Bieliauskas 2010; Reuter-Lorenz and Park 2010).
A key goal for the field of aging neuroscience is to understand if age-related increases in frontal activation are compensatory (Grady et al. 1994; Cabeza et al. 1997; Reuter-Lorenz et al. 1999; Cabeza 2002; Park and Reuter-Lorenz 2009) or reflect decreased neural efficiency (Rypma and D'Esposito 2000; Park et al. 2001; Colcombe et al. 2005; Zarahn et al. 2007; Stern 2009). Shedding light on this issue may have implications for testing the efficacy of cognitive intervention programs on frontal brain activations in aging. One challenging issue in this area is that age-related increases in brain activation are typically conflated with performance differences. A possible solution to this issue would be to compare the brain activation patterns of subgroups with matched performance data (i.e., low performing younger adults and high performing older adults). While this approach solves the problem of group performance differences, its results would not be broadly representative of either younger or older adults.
Task-switching paradigms afford a more attractive possibility of comparing brain activation patterns of older and younger adult groups under multiple conditions that vary in their degree of between-group performance differences. In task-switching paradigms, group brain activation patterns can be compared for the nonswitch condition (in which participants make single-task decisions), the switch condition (in which participants alternate between 2 possible task decisions) and for the relative effect of switching (switch–nonswitch contrast). In addition, older adult activation in the nonswitch condition can be compared with younger adult activation in the switch condition. This latter comparison may be informative in that it tends to result in similar accuracy and reaction times (RTs) between groups, likely by reducing explicit switching requirements for the older group (DiGirolamo et al. 2001; Meiran et al. 2001; Hahn et al. 2004; Gold et al. 2010).
The main aim of the present study was to further characterize relationships between age-related increases in frontal cortex activation, behavioral performance, and brain structure. To assess the relationship between age-related increases in activation and performance, we compared group brain activation under multiple behavioral conditions, including a contrast involving matched behavioral performance conditions. Next, we explored the relationship between BOLD magnitudes in frontal regions and behavioral performance in each condition. A finding of negative correlations between BOLD signal change and errors/RT would be consistent with compensation accounts (Cabeza 2002; Park and Reuter-Lorenz 2009). In contrast, positive correlations between BOLD signal change and errors/RT would be more consistent with theories suggesting that age-related activation increases represent declines in neural efficiency (Zarahn et al. 2007; Stern 2009).
In either case, the relationship between age-related increases in frontal cortex activation and age-related structural declines remains unclear. It is possible that age-related alterations in brain activation are in part related to the microstructural integrity of white matter (WM) tracts because many WM tracts undergo age-related decline (Sullivan and Pfefferbaum 2006; Madden et al. 2012), which likely reduces the fidelity of signal transmission between cortical regions (Bartzokis et al. 2010). In the present study, this issue was explored by assessing the relationships between age-related BOLD increases and fractional anisotropy (FA), an in vivo measure of WM integrity derived from diffusion tensor imaging (DTI) data (Basser and Pierpaoli 1996; Le Bihan 2003).
Materials and Methods
Participants
A total of 61 right-handed, monolingual English speakers (28 young adults, 33 older adults) participated. Written informed consent was obtained from each participant under an approved University of Kentucky Institutional Review Board protocol. Participants were community-dwelling individuals with normal or corrected-to-normal visual acuity. Exclusionary criteria for the study included the following: color blindness, a major head injury, stroke, a neurological or psychiatric disorder, high blood pressure, diabetes, heart disease, the use of psychotropic drugs, or the presence of metal fragments and/or metallic implants contraindicated for MRI.
Task-switching performance is known to be correlated with intelligence and digit span (Kray and Lindenberger 2000). The task-switching paradigm employed in the present study involved nonverbal, perceptual switching. Thus, the Cattell Culture Fair Intelligence Test (Cattell RB and Cattell AKS 1960) was used as a measure of intelligence because it assesses nonverbal intelligence associated with perceiving inductive relationships in shapes and figures. Digit Span forward (DF) and backward (DB) were assessed with The Digit Span Subtests of the Wechsler Memory Scale (WMS III) (Wechsler 1997). Totals for the DF and DB sets were based on the number of trials that were accurately reported in the correct order. There was no significant difference between younger and older groups in male/female ratio (X2 = 0.63, P = 0.43), years of education (t(59) = 0.22, P = 0.83), IQ (t(57) = 1.16, P = 0.25), DF (t(57) = 0.21, P = 0.84), or DB (t(57) = 0.53, P = 0.60) (Table 1).
Table 1.
Group means and standard deviations (in brackets) for demographic and neuropsychological scores
| Age group | Age interval (years) | Mean age (years) | N (female) | Years of education | IQ | DF | DB |
|---|---|---|---|---|---|---|---|
| Younger | 25–40 | 32.0 (3.8) | 28 (15) | 16.4 (2.8) | 124.5 (20.8)26 | 10.5 (2.3)26 | 9.7 (2.5)26 |
| Older | 63–83 | 68.4 (5.4) | 33 (21) | 16.5 (2.8) | 130.2 (17.0) | 10.4 (2.0) | 10.0 (2.5) |
Note: IQ, Cattell Culture Fair Intelligence Test; DF, Digit Span forward; DB, Digit Span backward. Values for IQ and DB are age-scaled scores. If score values were missing, the number of participants used in the computation is shown as a subscript.
Task and Procedure
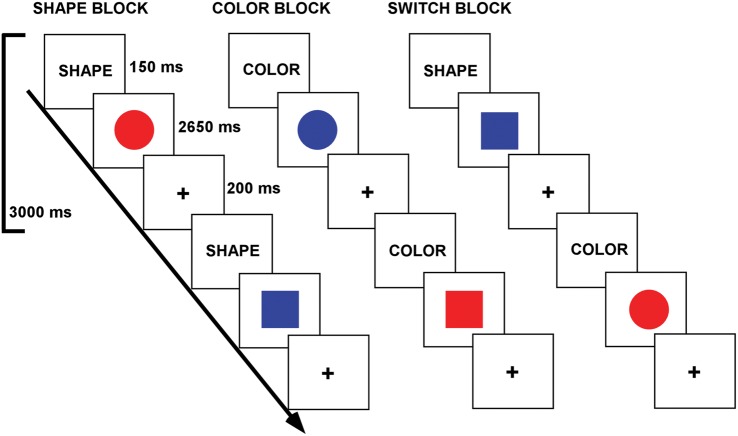
Participants completed a color-shape task-switching paradigm (Fig. 1). The stimuli consisted of 2 possible shapes (circle or square), in 1 of 2 possible colors (red or blue), presented in the center of the screen. The cue was presented for 150 ms and was followed immediately by the stimulus for 2650 ms. A fixation cross then appeared for 200 ms prior to the next cue word. A block design was employed which included 4 types of blocks: shape block, color block, switch (shape/color) block, and baseline fixation block [in which participants focused their vision on a central cross hair (+)]. In the shape block, participants were required to decide if a stimulus was a circle or square. In the color block, participants were required to decide if a stimulus was red or blue. In the switch block, participants alternated between shape and color decisions. Participants were asked to press a response (left or right) button to indicate whether the stimulus was red or blue, or if it was a circle or square, depending upon the cue word. Participants were asked to respond as quickly and accurately as possible.
Figure 1.
Task-switching paradigm. Conditions were indicated via cue words. In the shape condition, participants decided if a stimulus was a circle or square. In the color condition, participants decided if a stimulus was red or blue. In the switch condition, participants alternated between shape and color decisions.
Task blocks were 60 s in duration, and fixation blocks were 30 s in duration. There were 3 runs. Each run contained 4 task blocks and 5 fixation blocks. One run consisted of 2 blocks of each of the color task and shape task. The other 2 runs contained one block each of the color task and shape task and 2 switching blocks (in which the color and shape tasks switched pseudorandomly; every second or third trial on average). The order of runs, task blocks within runs, and stimulus-response mappings were counterbalanced across participants. The experiment was programmed via E-Prime v1.2 (Psychology Software Tool, Pittsburgh, PA, USA). RT and accuracy for subject responses on each trial were recorded by the stimulus presentation program.
Imaging data Acquisition
Imaging data were collected on a 3-Tesla Siemens TIM scanner at the Magnetic Resonance Imaging and Spectroscopy Center of University of Kentucky. Four types of images were collected for each participant: 1) a high-resolution, T1-weighted sequence for the subsequent localization of fMRI activity in standard stereotactic space; 2) T2*-weighted images sensitive to the BOLD signal for estimation of fMRI activity; 3) diffusion tensor images for estimation of fractional anisotropy; 4) a B0 field map sequence for subsequent geometric unwarping of fMRI and DTI images.
High-resolution, 3D anatomic images were acquired using the magnetization-prepared rapid gradient-echo (MP-RAGE) sequence [repetition time (TR) = 1690 ms, echo time (TE) = 2.56 ms, flip angle = 12°, 1-mm isotropic voxels]. T2*-weighted functional images were collected using a gradient-echo echo-planar imaging (EPI) sequence [33 interleaved slices, TR = 3000 ms, TE = 30 ms, flip angle = 83°, field of view (FOV) = 224 mm2, matrix = 64 × 64, 3.5 mm isotropic voxels]. DTI used a double spin-echo EPI sequence (TR = 6900 ms, TE = 105 ms, flip angle = 90°, FOV = 224 mm2, in-plane resolution =1.75 × 1.75 mm voxels, 40 contiguous 3-mm-thick axial slices). The DTI images were acquired with 36 noncollinear encoding directions (b = 1000 s/mm2) and 5 images without diffusion weighting (b = 0 s/mm2, b0). The field map images were collected using a double-echo EPI sequence (TE1 = 5.19 ms, TE2 = 7.65 ms).
fMRI Preprocessing and Voxelwise Analyses
Statistical Parametric Mapping (SPM 8; Wellcome Department of Cognitive Neurology, UCL, London, UK) was used in the preprocessing and statistical analyses of the fMRI data. After discarding the first 3 functional volumes of each run, differences in timing between slices was adjusted using sinc interpolation. The timing-corrected images were then realigned to the first volume in order to correct for head motion. The resulting images were unwarped via B0 field maps to reduce magnetic field distortions. The T1-weighted (MP-RAGE) image was then co-registered to the first functional volume using a mutual information algorithm. This co-registered high-resolution image was then used to determine the nonlinear basis function parameters for transformation into Montreal Neurological Institute (MNI) 2 × 2 × 2 mm standard space. This transformation was then applied to the functional data, which was resliced to 2-mm isotropic voxels and spatially smoothed with an 8-mm full-width at half-maximum Gaussian kernel. Finally, high-pass filtering (a 128-s cutoff) was applied to the images to remove low-frequency drifts.
Statistical analyses at the subject-level were conducted such that predictor variables in the design matrix were composed of epochs representing each task block, with the fixation blocks being modeled implicitly. Each epoch was convolved with a canonical hemodynamic response function producing contrasts for nonswitch condition, switch condition, and switch cost (switch vs. nonswitch).
Group analyses compared activation differences during the nonswitch condition, the switch condition, and the switching cost. In addition, a contrast of older adult nonswitch condition versus younger adult switch condition was conducted to identify age group activation differences under matched behavioral performance conditions. The results of the matched behavioral performance comparison were masked by a switching activation map (switch–nonswitch) conducted across all subjects to identify common “frontal task-switching regions” showing age-related over-recruitment in the absence of age-related performance declines.
Monte Carlo simulations using the AlphaSim program were used to determine the appropriate combination of the significance level and cluster threshold required to reach a corrected significance level of P < 0.05, taking into account both native space voxel dimensions and the effective smoothness estimated directly from our preprocessed data (http://afni.nimh.nih.gov/pub-/dist/doc/manual/AlphaSim.pdf). The Monte Carlo simulations used 1000 iterations and indicated a significance level of P < 0.001 and cluster threshold of 22 voxels in order to reach a corrected significance level of P < 0.05. This threshold was applied to each of the contrasts described above.
BOLD-Behavioral Correlations
These analyses focused on frontal regions showing increased activation in the older group in each of the contrasts described above. BOLD magnitudes in frontal regions of interest (ROIs) were extracted from each older adult subject using Marsbar (http://marsbar.sourceforge.net). The ROIs were defined as 8 mm spheres surrounding peak activation coordinates from each contrast (Tables 3 and 4). Correlations were run between BOLD signal change in these ROIs and older subjects' RT and accuracy data. Participant values larger or smaller than 3 standard deviations from their group mean were excluded from these correlation analyses (the data points removed ranged from 0.9% to 1.8%).
Table 3.
Brain regions showing age-related activation increases in each condition
| Region | Hem | BA | Z-score | X | Y | Z |
|---|---|---|---|---|---|---|
| Nonswitch condition | ||||||
| DLPFC | Left | 9 | 3.37 | −44 | 14 | 34 |
| Precentral gyrus | Left | 6 | 3.76 | −30 | 4 | 32 |
| Precentral gyrus | Left | 4 | 3.85 | −36 | −10 | 54 |
| Precuneus | Left | 7 | 4.48 | −28 | −52 | 58 |
| Middle occipital Gyrus | Left | 37 | 5 | −50 | −66 | −6 |
| Middle frontal Gyrus | Right | 6 | 4.05 | 30 | 0 | 62 |
| DLPFC | Right | 9 | 3.81 | 38 | 10 | 34 |
| Medial frontal Gyrus | Right | 6 | 3.37 | 14 | 2 | 60 |
| Superior parietal lobule | Right | 7 | 4.44 | 28 | −66 | 48 |
| Middle occipital gyrus | Right | 19 | 4.22 | 30 | −88 | 16 |
| Fusiform gyrus | Right | 19 | 4.56 | 42 | 70 | −10 |
| Switch condition | ||||||
| Insula | Left | 13 | 4.42 | −32 | 22 | 6 |
| DLPFC | Left | 9 | 4.32 | −42 | 14 | 30 |
| Precentral gyrus | Left | 6 | 3.79 | −22 | 4 | 58 |
| Precentral gyrus | Left | 4 | 3.61 | −44 | −8 | 50 |
| Superior parietal lobule | Left | 7 | 3.84 | −34 | −46 | 54 |
| Superior parietal lobule | Left | 7 | 3.29 | −24 | −58 | 54 |
| Precuneus | Left | 7 | 3.37 | −22 | −68 | 50 |
| Fusiform gyrus | Left | 37 | 5.18 | −50 | −56 | −14 |
| Thalamus | Left | – | 3.82 | −26 | −28 | 2 |
| Thalamus | Left | – | 3.39 | −10 | −4 | 10 |
| Caudate | Left | – | 3.72 | −16 | −10 | 20 |
| Cerebellum | Left | – | 3.42 | −4 | −64 | −12 |
| Insula | Right | 13 | 4.43 | 36 | 24 | 4 |
| DLPFC | Right | 9 | 3.93 | 44 | 12 | 30 |
| Precentral gyrus | Right | 6 | 5.04 | 24 | 0 | 58 |
| Superior parietal lobule | Right | 7 | 4.48 | 28 | −64 | 50 |
| Cerebellum/lingual gyrus | Right | – | 4.83 | 34 | −68 | −12 |
| Switch cost (switch–nonswitch) | ||||||
| FPC | Left | 10 | 3.69 | −42 | 50 | −4 |
Note: Hem, hemisphere. BA, Brodmann's area. X, Y, and Z presented the stereotaxic coordinates according to Montreal Neurological Institute (MNI) template.
Table 4.
Common “task-switching regions” showing higher activation in the older nonswitch condition than the younger switch condition
| Region | Hem | BA | Z-score | X | Y | Z |
|---|---|---|---|---|---|---|
| DLPFC | Left | 9 | 5.74 | −42 | 12 | 30 |
| Insula | Left | – | 4.89 | −30 | 20 | 6 |
| Precuneus | Left | 7 | 5.51 | −26 | −68 | 48 |
| Caudate | Left | – | 4.74 | −14 | −8 | 18 |
| ACC | Left | 24 | 4.86 | −6 | 8 | 48 |
| DLPFC | Right | 9 | 6.15 | 40 | 12 | 32 |
| Insula | Right | 13 | 5.38 | 36 | 22 | 2 |
| Caudate | Right | – | 4.21 | 16 | −4 | 18 |
Note: Hem, hemisphere. BA, Brodmann's area. X, Y, and Z presented the stereotaxic coordinates according to Montreal Neurological Institute (MNI) template.
DTI Preprocessing
Participants' DTI datasets were normalized to MNI152 (1 × 1 × 1 mm) space using FSL v4.1.5 (Functional MRI of the Brain software library, FMRIB) to enable selection of spatially corresponding WM ROIs across subjects (described below). Registration of FA images into MNI space followed a series of procedures known as Tract-Based Spatial Statistics [TBSS v1.2; (http://www.fmrib.ox.ac.uk/fsl/tbss/), as described in detail in our previous work (Johnson et al. 2012). Briefly, prior to normalization, raw images were corrected for motion and residual eddy current distortion, and corrected for magnetic field distortions using B0 field maps. The FMRIB Diffusion Toolbox (FDT v2.0) was then used to fit the diffusion tensor and calculate FA eigenvalues.
Participants' FA images were then aligned to a common target (the one to which the least amount of warping was required) using a nonlinear registration approach based on free-form deformations and B-Splines. FA datasets were then affine registered and resampled to 1-mm isotropic MNI152 space. All MNI-transformed FA images were then averaged to generate a mean FA image that was used to create a common WM tract skeleton. This skeleton was then thresholded at an FA value of 0.2 in order to minimize partial volume effects after warping across subjects. Each participant's aligned FA image was subsequently projected onto the FA skeleton in order to account for residual misalignments between participants after the initial nonlinear registration.
BOLD-FA Correlations
These analyses focused on frontal ROIs showing age-related increases in BOLD activation under matched behavioral performance conditions (the contrast of older adult nonswitch–younger adult switch). To limit the number of comparisons performed, fMRI ROIs for this analysis were those showing strong evidence of age-related declines in neural efficiency (i.e., those ROIs in which BOLD signal changes were positively correlated with both errors and RT). Two fMRI ROIs met these criteria: Right dorsolateral prefrontal cortex (DLPFC) and right insula. For WM ROIs, we selected tracts with well-established connections between these 2 fMRI ROIs (right DLPFC, right insula) and other regions that also showed age-related over-recruitment. The WM ROIs were the body of the corpus callosum (CC-Body), the genu of the corpus callosum (CC-Genu), and the right corona radiata (R-Radiata).
These WM ROIs were defined using the Johns Hopkins University WM tractography atlas and the International Consortium of Brain Mapping-DTI WM labels atlas. Masks were restricted to the mean skeleton mask of the current participant sample. The CC-Body and CC-Genu were selected because they contain tracts that connect portions of right DLPFC and insula regions with their respective homologous left DLPFC and insula regions, which also showed age-related fMRI over-recruitment. The R-Radiata was selected because it contains tracts connecting the right DLPFC and insula regions with the right caudate nucleus and parietal cortex, structures which also showed age-related over-recruitment. FA values in each of the 3 WM ROIs were extracted from each participant and correlated with BOLD signal change in the older group. Participant values larger or smaller than 3 standard deviations from their group mean were excluded from these analyses (1% of data points were removed).
Results
Behavioral Results
Mean error rates, RTs, and behavioral switch costs are presented in Table 2. Both groups were able to perform the nonswitch and switch condition with high accuracy (both groups ≥95% correct in each condition). For error rates, the main effect of condition was significant (F1,59 = 24.63, P < 0.001), with lower error rates during the nonswitch condition (M = 2.0%, SE = 0.3) than the switch condition (M = 4.1%, SE = 0.5). Both groups showed lower error rates in the nonswitch condition than the switch condition (all P's ≤ 0.01). Conversely, there was no effect of age group (F1,59 = 2.59, P = 0.11), or age × condition interaction (F < 1).
Table 2.
Behavioral performance in the younger and older groups
| Younger | Older | |
|---|---|---|
| Nonswitch condition error rate (%) | 1.7 (0.4) | 2.4 (0.4) |
| Nonswitch condition RT (ms) | 606 (22) | 753 (19) |
| Switch condition error rate (%) | 3.4 (0.7) | 4.9 (0.8) |
| Switch condition RT (ms) | 809 (37) | 1011 (36) |
| Switch cost error rate (%) | 1.7 (0.5) | 2.4 (.7) |
| Switch cost RT (ms) | 203 (19) | 258 (26) |
Note: Standard errors of the mean are presented in brackets.
For RTs, the main effect of condition was significant (F1,59 = 190.05, P < 0.001), due to longer RTs for the switch condition (M = 910 ms, SE = 25) than the nonswitch condition (M = 679 ms, SE = 15). Both groups had longer RTs in the switch condition than the nonswitch condition (all P's < 0.001). The main effect of age group was also significant (F1,59 = 21.53, P < 0.001), due to the older adults having longer RTs (M = 882 ms, SE = 25) than the younger adults (M = 707 ms, SE = 28). The older adults had numerically larger RT switch costs than the younger adults, although this age × condition interaction did not reach significance (F1,59 = 2.73, P = 0.10).
In contrast, direct comparisons between the nonswitch condition in the older group and the switch condition in the younger group indicated numerically but not significantly lower error rates for the older group (t(59) = 1.57, P = 0.12), and numerically but not significantly shorter RTs for the older group (t(59) = 1.46, P = 0.17).
BOLD Activation Increases in the Older Group
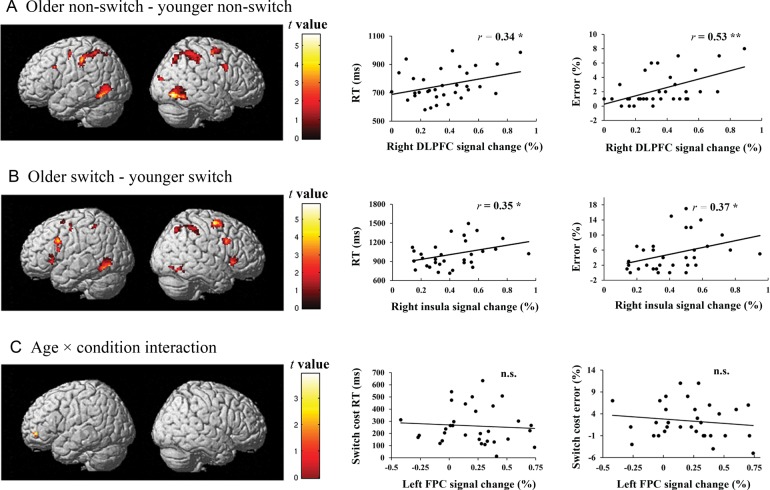
Results showed increased activation in the older group than the younger group in each of the contrasts performed (Table 3). In contrast, the younger group did not show increased activation compared with the older group in any of the contrasts. For the nonswitch condition, age-related over-recruitment was found in regions of each of the 4 lobes, including bilateral DLPFC, bilateral precentral gyri, bilateral parietal lobe, and bilateral occipital lobe (Fig. 2A). For the switch condition, age-related over-recruitment was also found in regions of each of the 4 lobes, including a broad number of specific regions (Fig. 2B). For the switch cost (age × condition interaction), age-related over-recruitment was restricted to a small portion of the left frontopolar cortex (FPC) (Fig. 2C).
Figure 2.
Age-related activation increases under conditions of poorer behavioral performance in the older group. (A) Regions of greater activation in nonswitch condition in the older group than the younger group. (B) Regions of greater activation in switch condition in the older group than the younger group. (C) Regions showing greater neural switch cost activation (switch–nonswitch) in the older group than the younger group. Note: *P < 0.05, **P < 0.01.
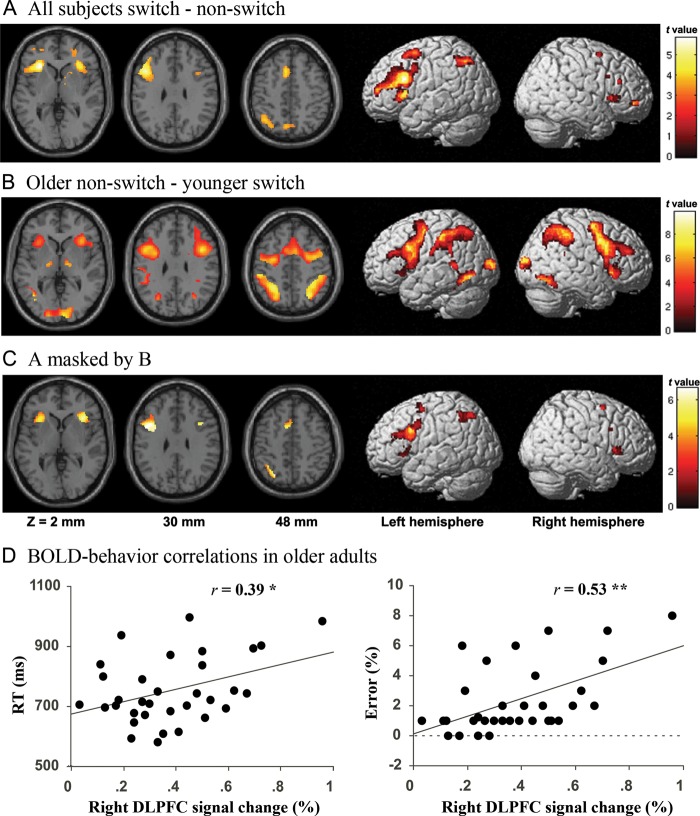
Results from the analysis of the switch condition versus the nonswitch condition across all subjects revealed prominent activation of a bilateral frontoparietal network of regions (Fig. 3A). The matched behavioral contrast (older adult nonswitch condition vs. younger adult switch condition) revealed age-related activation increases in portions of occipitotemporal, parietal, and frontal cortices (Fig. 3B). The masking of regions identified in Figure 3A by those identified in Figure 3B revealed a more circumscribed network of frontoparietal activations reflecting regions used during task switching by both groups and over-recruited by the older group despite equivalent between-group behavioral performance. These activations were observed in bilateral DLPFC, bilateral insula, ACC, parietal cortex, and bilateral caudate nuclei (Fig. 3C and Table 4).
Figure 3.
Age-related activation increases under matched behavioral performance conditions. (A) A primary common task-switching network was defined by the effect of switching (switch condition–nonswitch condition) across participants in both groups. (B) Regions of increased activation in the older group during the nonswitch condition compared with the younger group during the switch condition. (C) Results from A masked by results from B. (D) Correlation between behavioral performance and right dorsolateral prefrontal cortex (DLPFC) BOLD response in the older group. Note: *P < 0.05, ***P < 0.001.
BOLD–Behavior Correlations
Of those frontal ROIs showing age-related activation increases during the nonswitch condition, older adults showed positive correlations between BOLD signal change in the right DLPFC and both RTs (r = 0.37, P = 0.05) and error rates (r = 0.53, P = 0.002) (see Fig. 2A), and marginally between BOLD signal change in the left DLPFC and error rates (r = 0.33, P = 0.07) but not RTs (r = 0.20, P = 0.26). Of those frontal ROIs showing age-related activation increases during the switch condition, older adults showed positive correlations between BOLD signal change in right insula and both RTs (r = 0.35, P = 0.05) and error rates (r = 0.37, P = 0.035) (see Fig. 2B). None of the other correlations between frontal ROIs and behavioral performance in either condition was significant (P's > 0.35). Similarly, in the FPC region showing an age-related activation increase in neural switch cost (switch–nonswitch contrast), there was no correlation between BOLD signal change and behavioral switch costs (Fig. 2C).
Of those frontal ROIs showing age-related activation increases under matched behavioral performance conditions, the strongest relationship between BOLD and behavioral performance in older adults was seen in right DLPFC (Fig. 2D), in which BOLD signal changes were positively correlated with both error rates (r = 0.53, P = 0.001) and RTs (r = 0.39, P = 0.03). Error rates were also positively correlated with BOLD signal changes in the left insula (r = 0.38, P = 0.03), and right insula (r = 0.54, P = 0.001). Error rates were marginally correlated with BOLD signal in the left DLPFC (r = 0.30, P = 0.09), but not in the ACC (r = 0.24, P = 0.18). RTs were also positively correlated with BOLD signal changes in the right insula (r = 0.35, P = 0.049), but not in the left insula (r = 0.23, P = 0.20), left DLPFC (r = 0.23, P = 0.21) nor ACC (r = 0.06, P = 0.75).
FA-Behavior Correlations
In older subjects, error rates in the nonswitch condition were negatively correlated with FA in the CC-Body (r = −0.51, P = 0.003) and marginally negatively correlated with FA in the CC-Genu (r = −0.31, P = 0.08), but not correlated with FA in the R-Radiata (r = −0.26, P = 0.15). No significant RT-FA correlations were observed (P's > 0.15).
BOLD-FA Correlations
As expected, age-related FA declines were observed in each of the WM ROIs (P's < 0.001, Fig. 4A,B). More importantly, age-related FA declines in WM tracts tended to be associated with age-related BOLD increases in connected cortical structures. Specifically, significant negative correlations were observed between BOLD signal change in the right DLPFC and FA in each of the 3 WM ROIs: the CC-Body (r = −0.34, P = 0.05), CC-Genu (r = −0.53, P = 0.002), and R-Radiata (r = −0.37, P = 0.035) (Fig. 3C). In addition, significant negative correlations were observed between BOLD signal change in the right insula and FA in 2 of the 3 WM ROIs: CC-Body (r = −0.38, P = 0.027) and CC-Genu (r = −0.34, P = 0.05), but not the R-Radiata (r = −0.29, P = 0.11).
Figure 4.
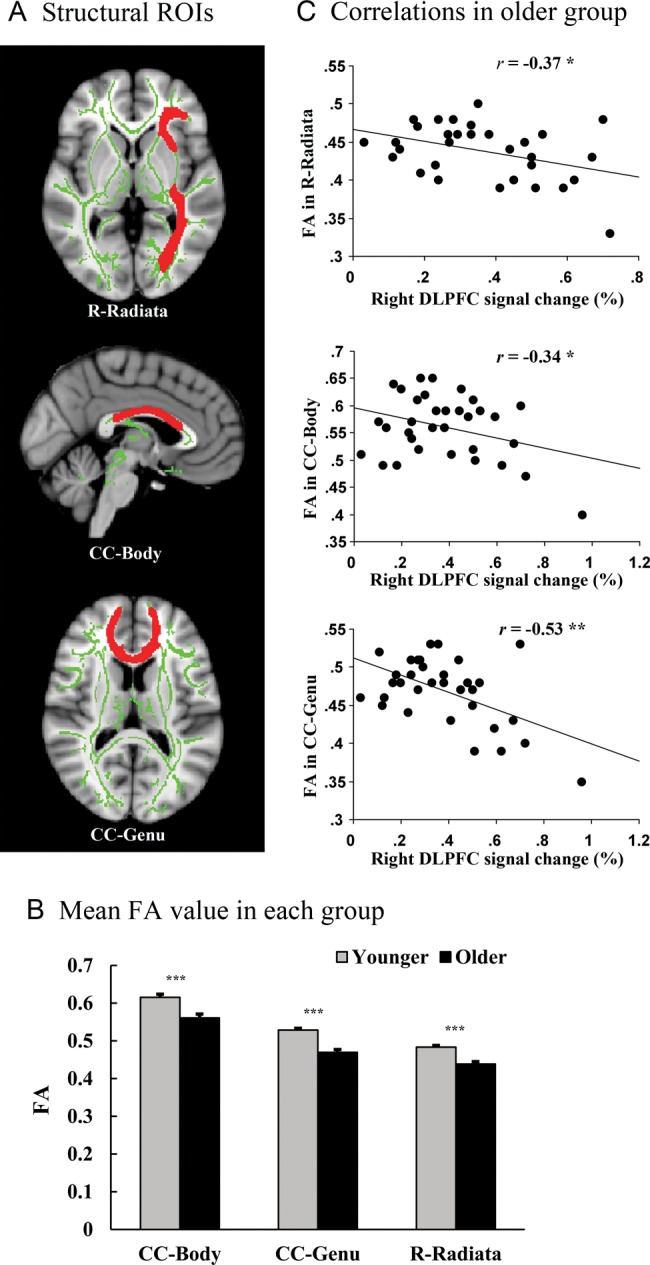
BOLD-FA correlations in the older group. (A) Representations of the white matter ROIs: the right coronal radiata (R-Radiata), the genu of the corpus callosum (CC-Genu) and the body of the corpus callosum (CC-Body). (B) Mean FA values in the white matter ROIs for each group. (C) Regression plots show the significant negative correlations between right DLPFC BOLD response in the nonswitch condition and FA in the R-Radiata, CC-Genu, and CC-Body in the older group. Note: *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
There were 3 main findings of the present study. First, we observed a relatively consistent pattern of age-related brain activation increases across multiple conditions of a task-switching paradigm, including a contrast involving matched behavioral performance between groups. Second, in the frontal regions showing age-related over-recruitment, BOLD magnitudes tended to be positively correlated with error rates and RTs. Finally, our results showed that age-related over-recruitment in right frontal structures tended to be negatively correlated with WM integrity in tracts connecting these structures with their homologous left hemisphere structures and ipsilateral striatal and parietal regions.
Age-related BOLD activation increases in frontal cortex have been reported in a number of cognitive control studies (Drag and Bieliauskas 2010; Reuter-Lorenz and Park 2010). In previous neuroimaging studies using the task-switching paradigm, age-related brain activation increases associated with neural switch costs (switch–nonswitch), have been reported in some studies (Gazes et al. 2012; Gold et al. 2013), but not others (DiGirolamo et al. 2001; Madden et al. 2010). In the present study, age-related neural switch increases were restricted to a very small portion of left FPC. This small effect was likely due in part to the age-related over-recruitment we observed in frontal regions during the “baseline” nonswitch condition in addition to the “experimental” switch condition (see also DiGirolamo et al. 2001). Another variable that may have contributed to the small neural switch costs differences we observed could be the use of a verbal cue, which may aid task switching more than arbitrary symbolic cues.
Age-related over-recruitment during the nonswitch and switch conditions was observed in the context of poorer behavioral performance in the older group, raising the possibility that BOLD increases could merely represent the neural correlates of declining performance. However, age-related activation increases in frontal cortex were even observed for a comparison involving behavioral conditions in which the older adults performed slightly better than younger adults (older adult nonswitch–younger adult switch) (see also Morcom et al. 2007). In addition, in 4 of the 5 frontal ROIs showing age-related over-recruitment under matched performance conditions, there was also a positive correlation in the older group between BOLD magnitude and RT and/or error rate. These results are consistent with several other findings suggesting that age-related over-recruitment in frontal regions may reflect reduced neural efficiency (Zarahn et al. 2007; Stern 2009).
In contrast, age-related activation increases have also been associated with better task performance, a relationship which has most frequently been observed for semantic encoding/memory tasks (cf. Grady and Craik 2000; Cabeza et al. 2002; Davis et al. 2012). It seems likely that the relationship between age-related over-recruitment and behavioral performance depends upon multiple factors. These factors are likely to include but not be limited to the task domain, the specific regions explored, and whether or not those regions are part of a primary task-related network. Future research should explore this issue directly by comparing the relationship between age-related over-recruitment and behavioral performance across multiple task domains in the same younger and older adult groups.
In the present study, age-related declines in neural efficiency were most pronounced in 2 right frontal regions: BOLD signal change in the right DLPFC and insula were positively correlated with both error rate and RT in the older adults. Age-related over-recruitment of right frontal cortex is one of the most common observations in neuroimaging studies of aging, having been reported across a broad range of cognitive domains including perception, attention, memory, and cognitive control (reviewed in Dennis and Cabeza 2008; Reuter-Lorenz and Park 2010). Despite the relative ubiquity of age-related right frontal over-recruitment, little remains known about the neural mechanisms underlying this phenomenon. Our results have implications for mechanisms underlying the link between age-related frontal over-recruitment and decreased neural efficiency.
Specifically, our results suggest a potential link between age-related declines in neural efficiency and WM integrity. The observed negative correlations between BOLD signal changes and FA in older adults are consistent with results from several previous studies (Persson et al. 2006; Madden et al. 2007). Our results extend these previous findings by drawing a critical link to within-scanner behavioral performance: BOLD magnitudes in 2 right frontal regions were positively correlated with errors/RT (suggesting decreased neural efficiency) and negatively correlated with WM integrity (but see Davis et al. 2012). In the right DLPFC and right insula, BOLD signal was negatively correlated with FA in the body and genu of the corpus callosum, tracts that connect portions of these neocortical regions with their left hemisphere homologues. In addition, in the right DLPFC, BOLD signal change was also negatively correlated with FA in the right corona radiata, the dorsal portion of the internal capsule, which contains ipsilateral connections to both striatal and parietal structures (Wakana et al. 2004).
Several of the observed age-related declines in WM integrity were linked with poorer behavioral performance in the older group, a finding that is consistent with previous results, and would be expected to reduce the coordinated activity between cortico-cortical and cortico-striatal regions that contribute to cognitive control (Gold et al. 2010; Madden et al. 2010). Age-related activation increases may thus in part reflect an attempt at compensation for reduction in the communication between neural hubs of the primary task network. Previous work has also suggested that age-related declines in neural efficiency may reflect dedifferentiation, or declining specialization of cortical regions (Baltes and Lindenberger 1997), and this represents a plausible interpretation of many of the present findings.
The present link between age-related declines in neural efficiency in right prefrontal cortex (PFC) and WM integrity reductions in right frontostriatal tracts deserves consideration. There is evidence suggesting that right PFC and right basal ganglia structures are involved in reactive inhibition to an exogenous cue (Robbins 2007), and that right PFC lesions create difficulty in suppressing previous task sets (Aron et al. 2003). The color/shape task-switching paradigm used here emphasizes inhibitory control processes because stimuli contain both color and shape information (e.g., a blue square). Consequently, the distracting, nonrelevant stimulus dimension (e.g., the color blue) must be inhibited based on a cue (e.g., shape task). Given that older adults are vulnerable to interference from distracting information (Hasher and Zacks 1988), the age-related activation increases in right PFC we observed in nonswitch condition may reflect an attempt to suppress proactive interference from the previous set and to inhibit information from the nonrelevant stimulus dimension.
We note several caveats associated with our study. First, it should be emphasized that our results do not necessarily conflict with all versions of the compensation theory. For example, the observed increased frontal activation may have been required by some poor performing older adults simply to reach their current performance levels. Longitudinal studies are required to address this issue. In addition, aging is associated with multiple neurodegenerative processes likely to contribute to our results. Although we focused on WM microstructural integrity, there are likely to be numerous mechanisms contributing to age-related neural efficiency declines including but not limited to alterations in neurochemical transmission (Loerch et al. 2008), reduction in mitochondrial bioenergetics (Hagen et al. 1997), metabolite ratios reflecting various molecular and cellular processes (Kantarci et al. 2011), and vascular changes (Hedden et al. 2012). Future work will be required to comprehensively delineate the biological mechanisms underlying age-related neural efficiency declines.
In summary, we found that age-related activation increases in frontal cortex tended to be correlated with poorer behavioral performance across multiple conditions of a task-switching paradigm. In addition, in several frontal regions, age-related activation increases were correlated with lower WM integrity in older adults. At the broadest level, our results suggest that age-related activation increases in frontal cortex may represent an attempt to compensate for reduced communication between brain regions that typically function together in younger adults.
Funding
This study was supported by the National Institute on Aging of the National Institutes of Health under award number R01AG033036 and the National Science Foundation under award number BCS 0814302. The content is solely the responsibility of the authors and does not necessarily represent the official views of these granting agencies.
Notes
We thank Sara Cilles for her assistance in recruiting, and testing participants. In addition, we thank our study volunteers for their participation in this research. Conflict of Interest: None declared.
References
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci USA. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson Ser B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB, Cattell AKS. Handbook for the individual or group culture fair intelligence test. Champaign, Illinois, USA: IPAT; 1960. [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Davis SW, Kragel JE, Madden DJ, Cabeza R. The architecture of cross-hemispheric communication in the aging brain: linking behavior to functional and structural connectivity. Cereb Cortex. 2012;22:232–242. doi: 10.1093/cercor/bhr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Mahwah, NJ: Lawrence Erlbaum; 2008. pp. 1–54. [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, et al. General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. J Geriatr Psychiatry Neurol. 2010;23:75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Gazes Y, Rakitin BC, Habeck C, Steffener J, Stern Y. Age differences of multivariate network expressions during task-switching and their associations with behavior. Neuropsychologia. 2012;50:3509–3518. doi: 10.1016/j.neuropsychologia.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Kim C, Johnson NF, Kryscio RJ, Smith CD. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J Neurosci. 2013;33:387–396. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Curr Opin Neurobiol. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, Ames BN. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci U S A. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Andersen GJ, Kramer AF. Age influences on multi-dimensional set switching. Aging Neuropsychol C. 2004;11:25–36. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension and aging: a review and a new view. In: Bower GH, editor. The psychology of learning and motivation. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 2012;32:16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59:1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Lowe V, Przybelski SA, Senjem ML, Weigand SD, Ivnik RJ, Roberts R, Geda YE, Boeve BF, Knopman DS, Jr., et al. Magnetic resonance spectroscopy, beta-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77:951–958. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT. Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum Brain Mapp. 2012;33:130–142. doi: 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychol Aging. 2000;15:126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Wang J, Pan Y, Gabuzda DH, Li C, et al. Evolution of the aging brain transcriptome and synaptic regulation. PLoS One. 2008;3:e3329. doi: 10.1371/journal.pone.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R. Adult age differences in functional connectivity during executive control. Neuroimage. 2010;52:643–657. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran N, Gotler A, Perlman A. Old age is associated with a pattern of relatively intact and relatively impaired task-set switching abilities. J Gerontol B Psychol Sci Soc Sci. 2001;56:P88–102. doi: 10.1093/geronb/56.2.p88. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Neumann J, von Cramon DY, Lohmann G. Model-based clustering of meta-analytic functional imaging data. Hum Brain Map. 2008;29:177–192. doi: 10.1002/hbm.20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, Marshuetz C. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues Clin Neurosci. 2001;3:151–165. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Ann Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci. 2010;65:405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller AC. Neural recruitment and cognitive aging: two hemispheres are better than one especially as you age. Psychol Sci. 1999;10:494–500. [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale®, 3rd ed (WMS III) San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging. 2007;28:784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]