Abstract
Background
The development of colon cancer represents a major complication in patients with inflammatory bowel disease (IBD). The importance of microRNAs (miRs) in carcinogenesis is becoming clearer, as miRs have been implicated in the regulation of cancer-related cellular processes to include apoptosis, differentiation, cell cycle progression, and immune function. In the current study, we sought to identify miR dysregulation specific to progression along the normal-inflammation-cancer axis in colonic specimens from IBD patients.
Methods
MiR microarrays and quantitative RT-PCR were used to detect and confirm dysregulated miRs. Receiver-operating characteristic curve analysis was applied to evaluate the potential utility of miR-224 as a neoplastic disease marker in IBD. For miR-224 target mRNA identification, mRNA microarrays were employed in combination with bioinformatic analyses, Western blotting, and luciferase activity measurements.
Results
We identified 30 miRs that were differentially expressed between chronically inflamed mucosae and cancers arising in IBD. MiR-224 levels increased successively at each stage of IBD progression and accurately discriminated cancers from normal or chronically inflamed IBD tissues. Moreover, mRNA arrays combined with bioinformatic analyses suggested participation of miR-224 in cell cycle regulation. Subsequently, cell cycle experiments indicated that miR-224 regulates the G1/S checkpoint. Finally, in silico prediction analyses, confirmed by Western blotting and luciferase assays, identified p21 as a specific direct mRNA target of miR-224.
Conclusions
These findings reveal miR dysregulation specific to IBD-associated colorectal carcinoma. MiR-224 is overexpressed in IBD-cancers and targets p21, a key cell cycle regulator. Moreover, these results establish the participation of miR-224 in IBD-carcinogenesis.
Keywords: microRNA, inflammatory bowel disease, colon cancer
Introduction
The first link between unresolved inflammation and cancer was suggested by Rudolf Virchow in 1863, with the discovery of leukocytes within neoplastic tissues. Subsequently, multiple studies have demonstrated that excessive release of inflammatory mediators creates a tumor-promoting microenviroment that facilitates the survival and proliferation of transforming epithelial cells. One of the prototypes of inflammation-driven carcinogenesis is the progression of colonic chronic inflammation to colorectal cancer that occurs in patients with inflammatory bowel disease (IBD). In this group of patients, colorectal carcinoma has a higher incidence than in the general population1 and accounts for approximately 15% of all IBD-associated deaths 2. Epidemiologic data suggests that in ulcerative colitis (UC), the risk of colon cancer increases 6-fold 3, while the increased risk of colorectal cancer in patients with longstanding Crohn's colitis is similar to that in UC 4.
Over the past decade, the development of new technologies has led to fundamental changes in our understanding of colon cancer biology. Recently, the importance of post-transcriptional regulation has become increasingly recognized as a key determinant in the modulation of gene expression. A relatively new class of RNAs, known as microRNAs (miRs), is now established as a major modulator of gene expression which functions by repressing translation or inducing mRNA degradation 5,6. Numerous studies have identified unique patterns of miR dysregulation inherent to many diseases, suggesting that miRs play important roles in aberrant cellular homeostasis.
In IBD, the participation of miRs has just recently begun to be explored. Initial studies identified miR profiles associated with non-neoplastic UC 7-9. Further reports established that miR dysregulation occurs even in the non-inflamed colonic mucosae of IBD patients 10. One recent paper described elevated expression levels of miRs -21 and -155 in patients with active UC 8. Interestingly, these two miRs belong to a solid tumor miR signature derived from a large-scale miRnome analysis of 540 cancer samples, including tumors of the lung, breast, stomach, prostate, colon, and pancreas 11. Subsequent lines of evidence revealed that in colonocytes, expression of miRs -21 and -155 is induced by activation of the pro-inflammatory genes Akt and NF-κB, and that overexpression of these two miRs promotes tumor growth in mice 12.
Taken together, these publications suggest that miRs may contribute to IBD susceptibility or pathogenesis. These findings also indicate that chronic inflammation results in altered miR expression and permit speculation that a subgroup of miRs that are dysregulated in IBD mucosa may itself have intrinsic carcinogenetic properties, thereby contributing to the increased colon cancer risk in this patient population. This notion is further supported by a study that compared chronically inflammatory vs. genetic (APC(Min/+)) models of colon cancer 13. Upregulation of miRs −215, −708, −31, and −135b was common to both inflammatory model-derived and genetic model-derived tumors, and target messenger RNA prediction coupled with pathway analysis suggested that these 4 miRs probably regulate critical signaling pathways that are central to the early transformation of colonic epithelial cells.
We previously described a signature of miR dysregulation that was associated with intestinal dysplasia in IBD patients 14. However, to date, no studies have yet comprehensively described altered expression of miRs in IBD-associated frank colorectal carcinoma in humans. Thus, the first objective of the current study was to pinpoint alterations in the pattern of miR expression in colon cancers occurring in IBD patients vs. cancer-free IBD subjects. We hypothesized that altered miR expression contributes to carcinogenesis in the setting of chronic inflammatory damage. Our second objective was to ascertain whether dysregulated expression of miR-224 participates in this process by targeting specific tumor suppressor genes, thereby contributing to cancer susceptibility.
Materials and Methods
Human specimens
We evaluated an existing cohort of 162 specimens consisting of 55 normal colonic epithelial specimens from 14 patients without any history of IBD or cancer, 35 chronically inflamed and 23 non-inflamed “matched” specimens from IBD patients, 11 IBD-dysplasias, and 38 frank IBD-cancers. Relevant clinical and pathological information for these specimens is available in Supplementary Table 1 and Supplementary Table 2. All specimens were obtained under approved IRB protocols at the Johns Hopkins University School of Medicine, the University Of Maryland School Of Medicine, or the Mount Sinai School of Medicine.
RNA extraction
TRIzol reagent (Invitrogen, Carlsbad, CA) was used to extract total RNA. One hundred nanograms (ng) of total RNA were used for each microarray, while 10 ng were consumed for each individual miR-RT-PCR assay.
MiR microarrays
Microarray assays were performed on 8 non-neoplastic and 8 IBD-associated cancer specimens using MiR Labeling Reagent and Hybridization Kits (Agilent Technologies, Palo Alto, CA, USA) and Human miR Microarray Kits (Agilent). 100 ng of total RNA from each sample were phosphatase-treated and then labeled with Cyanine 3-pCp. The labeled RNA was purified using Micro Bio-spin columns (Bio-Rad) and subsequently hybridized to a human miR microarray slide at 55°C for 20 hours. After hybridization, the slides were washed with Gene Expression Wash Buffer (Agilent) and scanned on an Agilent Microarray Scanner using Agilent's Scan Control version A.7.0.1 software. Raw hybridization intensities were obtained using Agilent's feature extraction software.
Quantitative RT-PCR (qRT-PCR)
MiR array results were validated via qRT-PCR using TaqMan MicroRNA Assays (Applied Biosystems, Foster City, Ca). RNU6B small nuclear RNA was used as an internal control for normalization, as previously described (34). RNA was diluted to 2 ng/μl, and 10 ng was used as template in each reverse-transcription reaction. Quantitative PCR was carried out in duplicate for each sample for both the RNU6B control and each miR.
mRNA Microarrays
The Illumina mRNA microarray platform was used for mRNA microarray assays. HCT116 DicerKO cells, a generous gift from Dr. Bert Vogelstein, were treated with miR-224 or NSM. 24 hours later, these cells were treated with Doxorubicin (1 ug/ml), and 48 hours later the cells were harvested and RNA was extracted. Array assays were performed at the Johns Hopkins Bayview Genomics Core Facility, as per the manufacturer's protocol.
Transfection of miR mimics and inhibitors
Synthesized RNA duplexes of miR mimics were purchased from Dharmacon (Lafayette, CO). 30~50% confluent cells were transfected with 60nM of each miR mimic using Lipofectamine RNAi MAX (Invitrogen). RNA and protein were harvested after 72 hours of transfection. Nonspecific controls for mimics, miR-31 and miR-21 species were used as negative controls.
Western Blotting
Cells were lysed in Laemmli sample buffer (Bio-Rad, Hercules, CA) with a protease inhibitor, Complete, EDTA-free (Roche). Protein concentration was estimated using a BCA Protein Assay kit (Pierce, Rockford, MA). Cell lysates (20ng) were electrophoresed on 10% polyacrylamide gels (Bio-Rad) and transferred to Immobilon-PSQ polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were blocked with TBS containing 5% skim milk and 0.1% Tween-20, then, incubated with p21 primary antibody (Invitrogen, NY, Catalog No 337000) As an internal control, mouse anti-beta-actin monoclonal antibody from Sigma-Aldrich Inc. (St. Louis, MO, catalog No.: A3854) was used. After washing, membranes were incubated with the secondary antibody, HRP-conjugated goat anti-mouse IgG (Zymed, San Francisco, CA, Catalog No 626620) and analyzed using enhanced chemiluminescence-plus reagent (GE Healthcare, Buckinghamshire, UK).
Cell cycle analysis by flow cytometry
Flow cytometric analysis of DNA content was performed to assess cell cycle phase distribution. After transfection of miRs at day 0, cells were harvested at day 2 and incubated with PI staining buffer (PBS 0.1mg/mL PI, 0.6% NP40, 2mg/mL RNase A for 30 min on ice (Roche Diagnostics, IN). DNA content was analyzed using a FACSCalibur (BD Biosciences, San Jose, CA) and Cell Quest software (BD Biosciences, MD) for histogram analysis.
Luciferase Reporter Assays
The full-length p21 3’-untranslated region (3’-UTR), containing one miR-224 predicted binding site, was amplified from genomic DNA using linker primers containing XbaI restriction sites. Amplicons were cut by XbaI and nondirectionally cloned into vector pGL4 at an XbaI site just downstream of the firefly luciferase structural gene (Promega, Madison, WI). A mutant p21 3’UTR was constructed by mutating three nucleotides within the “seed” sequence of the miR-224 binding site. Plasmid clones that contained the mutated binding site were used as universal negative controls for these assays. Cells were seeded onto 96-well plates on the day prior to transfection, then transfected with miR mimics. The constructed pGL4 vector and an internal control pRL-CMV (Renilla luciferase) vector (Promega) were cotransfected 24 h after miR mimic transfection using Lipofectamine 2000 (Invitrogen). 48 hours after plasmid vector transfection, a luciferase reporter assay was performed using a Dual-Glo luciferase assay kit (Promega). Luminescence intensity was measured by VICTOR2 fluorometry (Perkin Elmer, Waltham, MA), and the luminescence intensity of Firefly luciferase was normalized to that of Renilla luciferase.
Statistical Analyses
For miR microarray data, transformation was applied to set all negative raw values at 0.1, followed by quantile normalization. A filter on low gene expression was used so that only probes expressed (flagged as present) in at least one sample were retained. Then, samples were grouped in accordance with their status and compared using GeneSpring software. A threshold of minimum 2-fold difference was used as an inclusion criterion. For mRNA expression data, candidate genes were filtered as follows: genes with expression levels below 100 units (the array background level) were eliminated from analysis. Next, we eliminated genes that demonstrated less than a 3-fold change upon stimulation with miR-224. At the end of the filtering procedure, from the original 24,527 mRNAs, the list of genes was reduced to 807. These genes were input into Ingenuity Pathway Annalysis (IPA) to identify pathways in which they were involved. qRT-PCR data was analyzed by average fold-change analysis in combination with Student's t-test. Associations between miR expression levels and other tissue or patient demographic parameters (IBD type, grade or stage, anatomic location, age, and gender) were evaluated using Student's t-test.
We used the area under the empirical receiver-operating characteristic (ROC) curve to summarize the ability of the hsa-miR-224 qRT-PCR test result to discriminate an IBD-associated colorectal cancer from normal colonic tissue of a noncancer, non-IBD patient or from uninflamed or chronically inflamed non-neoplastic IBD colonic tissue. We applied bootstrapping with 500 iterations to estimate the 95% confidence interval for the area under the ROC curve (AUROC).
Ethical Considerations
Informed consent was obtained from the patients and all specimens were collected under approved IRB protocols at the Johns Hopkins University, University of Maryland and Mount Sinai School of Medicine.
Results
MiR expression profiles are altered in IBD-associated dysplasias and cancers
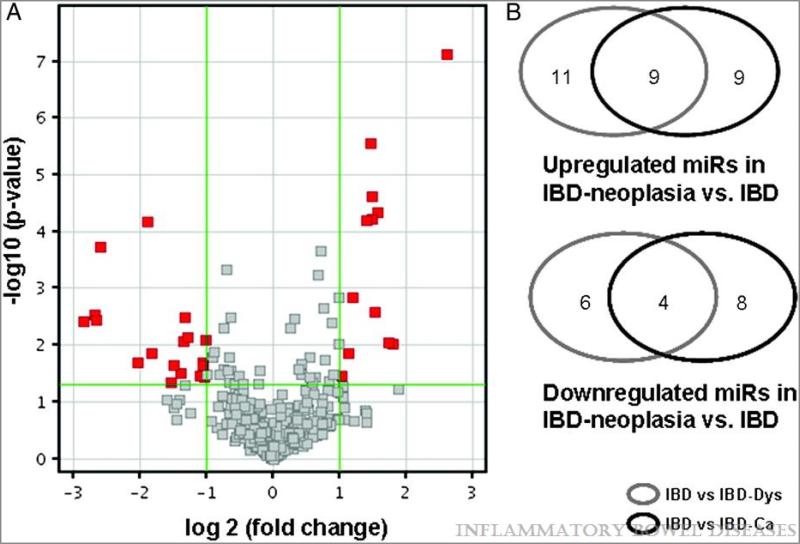
To test our hypothesis that miR expression is altered during IBD-associated neoplastic transformation, as well as to identify specific biomarkers of IBD-associated neoplasia, we performed miR microarray analyses on 8 noncancerous IBD tissues and 8 UC-associated cancers. After filtering out miRs expressed below background intensity in all 16 specimens studied, candidate miRs were further prioritized with GeneSpring software, using a p-value <0.05 and a ≥2-fold change as inclusion criteria. Among the 30 dysregulated miRs selected by these criteria, 18 were upregulated and 12 were downregulated in UC-cancers vs. non-neoplastic UC specimens (Figure 1 A and Table 1).
Figure 1.
A.Volcano plot ilustrating differentially expressed miRs in IBD vs. IBD-Ca as determined by miR microarray annalysis. Samples were grouped in accordance with their status and compared using GeneSpring software. A threshold of minimum 2 fold difference was used as exclusion criteria. B.Venn diagram showing overlapping of miR dysregulation in IBD Dysplasia and IBD Cancer.
Table 1.
| Systematic Name | P | Fold Difference | Regulation |
|---|---|---|---|
| hsa-miR-224 | 0.0001 | 3.68 | Up in IBD-Ca |
| hsa-miR-135b | 0.0002 | 6.05 | Up in IBD-Ca |
| hsa-miR-31* | 0.0028 | 6.40 | Up in IBD-Ca |
| hsa-miR-452 | 0.0032 | 2.51 | Up in IBD-Ca |
| hsa-miR-552 | 0.0036 | 6.26 | Up in IBD-Ca |
| hsa-miR-31 | 0.0037 | 7.22 | Up in IBD-Ca |
| hsa-miR-95 | 0.0073 | 2.44 | Up in IBD-Ca |
| hsa-miR-424* | 0.0080 | 2.02 | Up in IBD-Ca |
| hsa-miR-550* | 0.0085 | 2.55 | Up in IBD-Ca |
| hsa-miR-96 | 0.0135 | 3.52 | Up in IBD-Ca |
| hsa-miR-200a | 0.0196 | 4.06 | Up in IBD-Ca |
| hsa-miR-424 | 0.0201 | 2.07 | Up in IBD-Ca |
| hsa-miR-542-3p | 0.0225 | 2.09 | Up in IBD-Ca |
| hsa-miR-7 | 0.0228 | 2.80 | Up in IBD-Ca |
| hsa-miR-214 | 0.0312 | 2.62 | Up in IBD-Ca |
| hsa-miR-335 | 0.0344 | 2.14 | Up in IBD-Ca |
| hsa-miR-1246 | 0.0356 | 2.06 | Up in IBD-Ca |
| hsa-miR-200b* | 0.0453 | 2.90 | Up in IBD-Ca |
| hsa-miR-1288 | <0.0001 | 2.81 | Down in IBD-Ca |
| hsa-miR-1295 | <0.0001 | 2.69 | Down in IBD-Ca |
| hsa-miR-138 | <0.0001 | 2.98 | Down in IBD-Ca |
| hsa-miR-892b | <0.0001 | 6.09 | Down in IBD-Ca |
| hsa-miR-501-5p | 0.0001 | 2.81 | Down in IBD-Ca |
| hsa-miR-760 | 0.0001 | 2.63 | Down in IBD-Ca |
| hsa-miR-1305 | 0.0014 | 2.30 | Down in IBD-Ca |
| hsa-miR-124 | 0.0025 | 2.90 | Down in IBD-Ca |
| hsa-miR-150 | 0.0089 | 3.32 | Down in IBD-Ca |
| hsa-miR-l39-5p | 0.0096 | 3.50 | Down in IBD-Ca |
| hsa-miR-146b-5p | 0.0139 | 2.18 | Down in IBD-Ca |
| hsa-miR-122 | 0.0342 | 2.05 | Down in IBD-Ca |
IBD-Ca, cancer specimens from patients with IBD.
In a previous study, we had compared miR expression profiles in IBD-dysplastic vs. nondysplastic chronically inflamed IBD mucosae15. As expected, there was a high degree of overlap between the results of the current and previous comparisons, particularly among miRs with high fold changes (Figure 1 B and Supplementary Table 3). For example, six of the seven miRs with the highest fold changes in our current study, miRs-31, −31*, −552, −135b, −200a, and −96, , were also upregulated in IBD-dysplasias vs. non-neoplastic IBD samples in our previous study. MiRs -892b and -139-5p, the two most underexpressed miRs in IBD-dysplasias in our previous study, were also downregulated in IBD-carcinomas vs. non-neoplastic IBD specimens in our new data.
Validation of miRs exhibiting dysregulation in IBD
We next validated the microarray results by employing quantitative RT-PCR (qRT-PCR) on the same RNA specimens assayed with miR microarrays. For this validation step, we selected the 5 most upregulated miRs in IBD-cancer vs. IBD, viz., miRs −31, −552, −135b, −200a and −224. qRT-PCR results accurately matched microarray results, confirming upregulation in IBDN for all 5 of these miRs (Table 2).
Table 2.
| IBD-Ca Versus IBD Fold Difference |
P
|
|||
|---|---|---|---|---|
| Systematic Name | qRT-PCR | Array | qRT-PCR | Array |
| miR-224 | 4.76 | 3.68 | 0.0047 | 0.0001 |
| miR-135b | 10.14 | 6.05 | 0.0090 | 0.0002 |
| miR-200a | 2.43 | 4.06 | 0.0260 | 0.0196 |
| miR-31 | 12.24 | 7.22 | 0.1017 | 0.0037 |
| miR-552 | 12.38 | 6.26 | 0.1226 | 0.0036 |
RNA from the same samples used for miR array analyses was used as a template for qRT-PCR. Signal obtained from qRT-PCR or miR array assays was averaged in the UC and UC-associated cancer groups. Fold change between the 2 groups is displayed.
IBD-Ca, cancer specimens from patients with IBD.
MiR-224 expression is independent of anatomic location
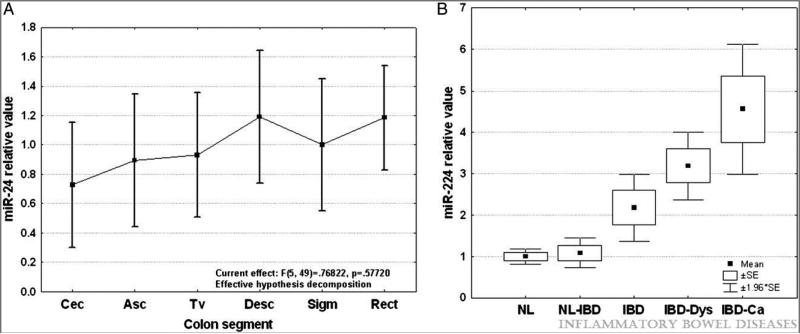
In our miR-array screening experiments, miR-224 exhibited the highest statistical significance in discriminating IBD-cancers from non-cancerous IBD. To verify that miR-224 dysregulation does not reflect sampling error, in the next step, we tested its expression in a larger cohort of colon specimens. This larger cohort included healthy controls as well as IBD, IBD-dysplasia, and IBD-cancer specimens. As a first step in this analysis, to ensure that anatomical location was not introducing any bias, we evaluated miR-224 expression levels throughout the normal colon. RNA was extracted from 55 specimens obtained from 14 patients who lacked any history of IBD or GI cancer. MiR-224 expression was homogeneous in specimens from healthy donors and was independent of anatomic location in the colon. One-way analysis of variance revealed no statistical differences among the 6 anatomic location groups (Figure 2 A and Supplementary Figure 1). These results are in accordance with a study by Wu et al. that evaluated miR levels in the left and right colons of IBD patients 16.
Figure 2.
A. MiR-224 relative expression levels along the normal colon. RNA was extracted from 55 normal specimens collected from patients without any history of IBD or colon cancer. qRT-PCR was performed and signal average for normal specimens was calculated. Values displayed are relative to normal average value. The numbers of specimens for each colon segment are shown in brackets. B. Dynamic changes of miR-224 expression levels during IBD-related neoplastic transformation. qRT-PCR was performed using as template total RNA extracted from 162 patient specimens. Samples were grouped according to their pathologic status, and the average value for each group was calculated. NL = normal from patients without IBD or colorectal cancer history; NL-IBD = normal “unaffected” specimens from IBD patients; IBD = “affected” chronically inflamed specimens from IBD patients; IBD-Dys = dysplastic specimens from IBD patients; IBD-Ca = cancer specimens from IBD-patients.Fold differences relative to the average for normal specimens group are displayed. Error bars represent standard error of the mean.
Mir-224 expression follows a stepwise escalation during neoplastic progression
We further determined miR-224 levels in IBD patients lacking any evidence of neoplasia (Figure 2 B). MiR-224 showed no difference between unaffected colon specimens from IBD patients and normal colonic mucosa. In chronically inflamed specimens from IBD patients, miR-224 levels were significantly elevated compared to normal mucosa (2.17 fold change; p=0.008, Student's t-test) or to unaffected mucosa from IBD patients (2.01 fold change; p=0.019, Student's t-test) Within the IBD group, no differences were observed with regard to age, sex, disease activity, or IBD type (Supplementary Figures 2A and 2B and data not shown).
Next, we assessed miR-224 expression by qRT-PCR in a cohort of specimens consisting of 11 IBD-dysplasias and 38 frank IBD-carcinomas (Figure 2 B). In IBD-dysplasias, expression of miR-224 was elevated compared to normal-appearing mucosa from healthy or IBD patients (3.31- and 3.06-fold increase, respectively; p<0.001, Student's t-test). However, no statistically significant change was observed in IBD-dysplasias vs. chronically inflamed non-neoplastic IBD mucosa. MiR-224 was 4.47-fold higher in IBD-cancers (p<0.001, Student's t-test) than in normal colon from healthy patients. Within the IBD-cancer group, no differences were observed with regard to age, sex, disease duration, or underlying IBD type (Supplementary Figures 2C and 2D and data not shown). Importantly, miR-224 was significantly elevated in IBD-cancers vs. unaffected mucosae (4.13-fold difference, p<0.001, Student's t-test) and chronically inflamed specimens (2.05-fold difference, p=0.012, Student's t-test). Taken together, these results suggest that miR-224 expression exhibits a stepwise escalation during progression from normal to chronic inflammation to neoplasia, suggesting involvement of miR-224 in both chronic inflammation and colonic carcinogenesis.
ROC curve analyses
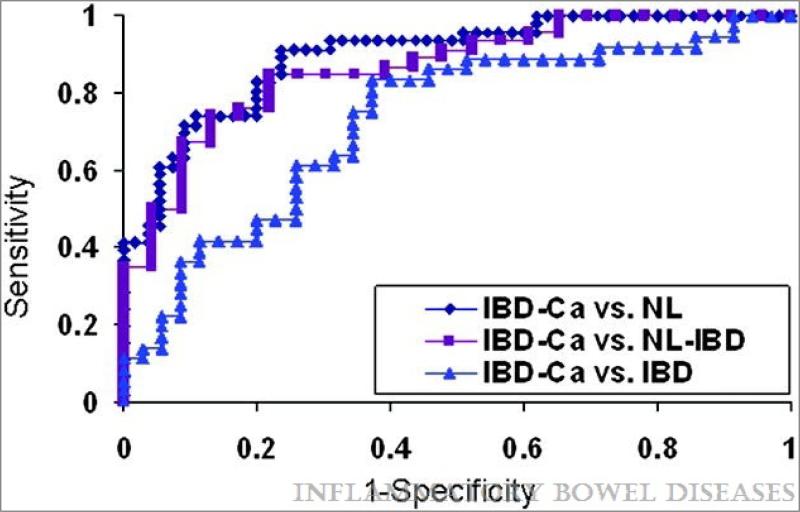
We also evaluated the potential clinical utility of miR-224 expression as a biomarker by using ROC curves (Figure 3). ROC curves evaluated the diagnostic accuracy of miR-224 for IBD-cancers (n = 38) vs. colonoscopy-assessed normals (n=55), IBD (n=35), or N-IBD specimens (n=22). For the discrimination between IBD-cancers and normal specimens, the area under the curve (AUROC) for the miR-224 qRT-PCR assay was 0.896 (sensitivity 82.6%, specificity 80%). For the ability to distinguish IBD-cancers from N-IBDs, the AUROC was 0.865 (sensitivity 84.7%, specificity 78.3%); finally, for the capacity to differentiate between IBD-cancers and IBDs, the AUROC was 0.73 (sensitivity 83.3%, specificity 62.9%).
Figure 3.
ROC curve analysis of miR-224 expression levels determined by qRT-PCR. Individual miR-224 qRT-PCR levels were grouped according to their pathology. NL = normal from patients without IBD or colorectal cancer history; NL-IBD = normal “unaffected” specimens from IBD patients; IBD = “affected” chronically inflamed specimens from IBD patients; IBD-Ca = cancer specimens from IBD patients. AUROC for the IBD-Ca vs. NL, NL-IBDs or IBD was 0.896 (0.83-0.95 95% confidence interval) ,0.865 (0.77-0.95 95% confidence interval) and 0.73 respectively (0.61-0.84 95% confidence interval) x-axis = 1-sensitivity. y-axis = specificity
Pathways triggered by dysregulated miR-224 expression
MiR-224 has been reported to be overexpressed in wide variety of solid tumors, including those of the liver, pancreas, thyroid, and kidney, as well as in WNT signaling-associated medulloblastomas.17-22 These studies support a role for miR-224 in tumor initiation or progression; however, to date, only three genes have been confirmed as being directly controlled by miR-224 17,23,24. MiRs may exert a broad impact on cellular gene expression programs, either directly, or through signaling cascades downstream of their direct target mRNAs. Thus, to identify direct targets of a miR, one strategy is to examine global expression profile changes associated with altered miR expression and to subsequently combine this result with bioinformatic analysis. Although some bona fide miR targets directly regulated by impaired translation may not be identified by this approach, and although some downregulated genes may not be directly regulated by the miR in question, this strategy has successfully identified targets of several mammalian miRs 25-27. Therefore, we performed mRNA expression array analyses of HCT Dicer-KO cells transfected with either a miR-224 mimic or a nonspecific mimic. Dicer-KO cells were used in order to prevent confounding of interpretation due to secondary changes in endogenous miR levels, which require Dicer for their production.
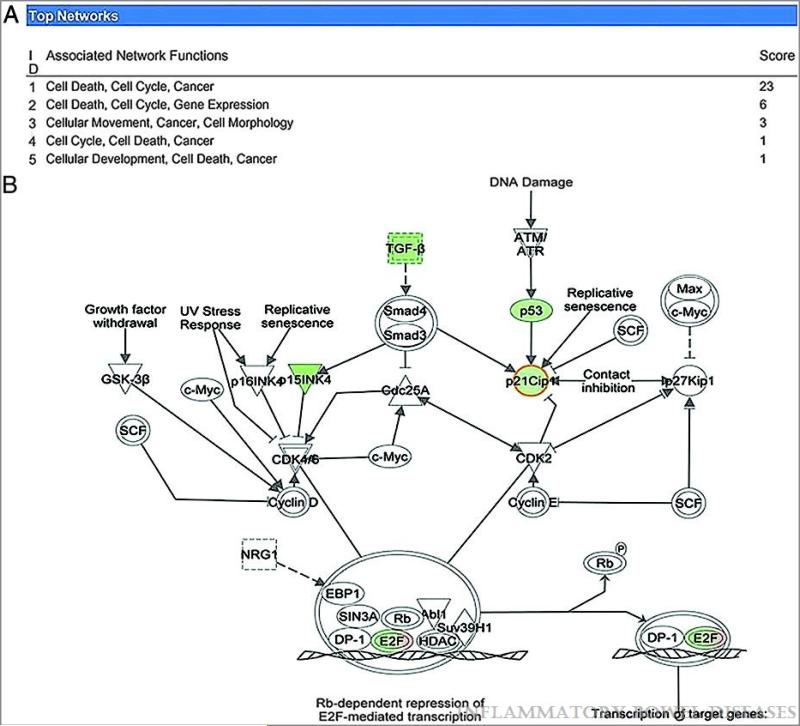
In preliminary experiments, miR induction in cancer cells in the absence of stress factors resulted in a modest shift of gene expression, which was difficult to substantiate (15 and data not shown).Previous reports have linked miR-224 to cell survival and drug resistance 17,28,29; Therefore, to induce a pro-apoptotic response and to enhance the magnitude of changes in mRNA levels, cells were then pretreated with Doxorubicin prior to miR-224 transfection. Data were analyzed with Ingenuity Pathway Analysis (IPA) software, choosing a 3-fold change in mRNA expression as a threshold. From these results, we deduced that miR-224 appears to regulate a network of genes controlling the cell cycle, cancer, and cellular development, the last of which constituted the most-associated network function affected by miR-224 induction. A summary of these analyses is displayed in Figure 4 A.
Figure 4.
A.Genes with altered expression upon miR-224 stimulation are involved in the cell cycle control. The list of genes identified to be downregulated upon miR-224 stimulation was filtered and input into Ingenuity Pathway Analysis with the purpose of identifying general mechanisms of miR function. Top associated network functions are displayed. B. In-silico analysis identifies p21 as a putative direct target of miR-224. The list of dysregulated genes identified by cDNA arrays (downregulated and upregulated genes are highlighted in green and red, respectively) was overlayed on the list of Targetscan predicted miR-224 targets.
MiR-224 induces G1-S release in colon cancer cells
mRNA arrays coupled with IPA analysis suggested that miR-224 exerts its effects via dysregulation of the cell cycle.To verify this hypothesis, we performed cell cycle analyses. In the presence of Doxorubicin, G1/S release occurred in HCT116 Dicer-KO cells (Table 3).
Table 3.
| Cell line | G0-G1 | S | G2-M |
|---|---|---|---|
| CACO-2 | |||
| NSM | 52.70 ± 2.52 | 23.36 ± 6.82 | 22.65 ± 5.62 |
| miR-224 mimic | 46.16 ± 3.16 | 28.26 ± 5.85 | 24.76 ± 3.36 |
| P | 0.049 | 0.432 | 0.653 |
| HCT-116 Dicer-KO doxorubicin | |||
| NSM | 14.21 ± 1.58 | 62.47 ± 0.83 | 23.31 ± 3.55 |
| miR-224 mimic | 10.02 ± 0.62 | 66.38 ± 2.59 | 23.58 ± 2.82 |
| P | 0.013 | 0.068 | 0.923 |
Interestingly, this cell cycle effect was abolished in the absence of Doxorubicin, in agreement with results observed by Wang et al. 17. We also performed cell cycle analyses in a second colon cancer cell line, CaCO-2. We chose this cell line because it exhibited the lowest miR-224 expression levels among 7 colon cancer cell lines tested. Forced overexpression of miR-224 in CaCo2 cells, even in the absence of genotoxic stress (Doxorubicin), led to a marked decrease in the G0/G1 cell population (Table 3).
p21 is a direct target of miR-224
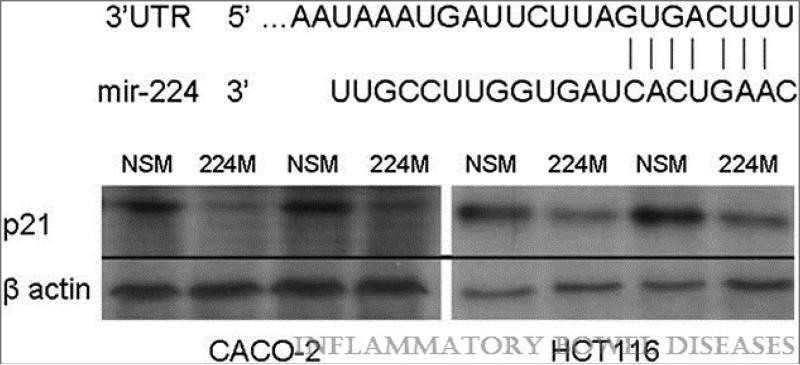
To investigate possible mechanisms conferring oncogenic properties on miR-224, we performed in silico analyses using search engines to predict biological targets of this miR. Using IPA software, we overlayed the target genes predicted by TargetScan onto miR-224-dysregulated genes involved in the G1/S checkpoint (from IPA analysis of our mRNA array data). This approach identified p21 as the sole G1/S checkpoint candidate for direct miR-224 regulation (Figure 4 B). Transfection of CaCO-2 and HCT-116 colon cancer cells with miR-224 mimics produced dramatic p21 downregulation vs. cells transfected with a synthetic nonspecific mimic (Figure 5).
Figure 5.
Protein expression of p21 decreases upon miR-2244 stimulation. Representative western blots of p21protein in CACO-2 and HCT116 cell lines are shown. Equal protein loading was performed, as shown by b-actin. Predicted miR-224 binding site within p21 3’UTR is shown.
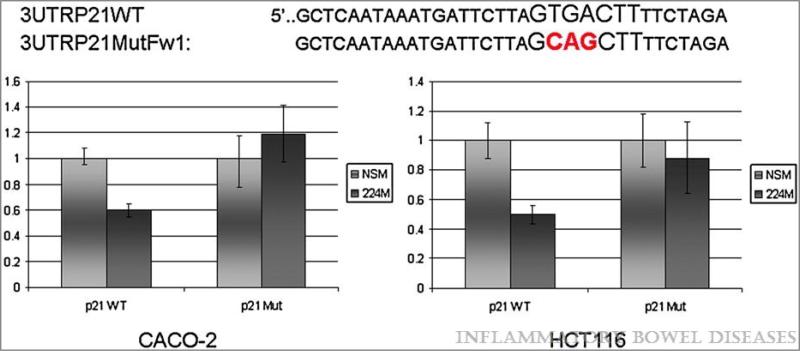
To investigate whether miR-224 directly interacts with p21, we measured the luciferase activity of plasmids containing the p21-3’UTR in cells transfected with either a miR-224 mimic or a nonspecific mimic (NSM; Figure 6). Cells transfected with the miR-224 mimic demonstrated 50% and 40% decreased luciferase activity in HCT116 and CaCO-2 cells, respectively (p=0.001, Student's t-test) vs. NSM -transfected cells. Site-directed mutageneis of three nucleotides within the predicted miR-224 binding site within the p21-3’UTR eliminated this effect of miR-224 overexpression on luciferase activity. These results indicate that p21 is directly targeted and inhibited by miR-224.
Figure 6.
miR-224 directly interacts with binding site in the 3’UTR of p21. Y-axis – relative luminescence normalized to the luminescence level in NSM treatment. X-axis – treatment conditions. NSM – non-specific mimic, 224M – miR-224 mimic; p21WT – p21 wild type 3’UTR containing miR-224 binding site; p21 Mut – p21 3’UTR containing a mutated miR-224 binding site. Shown is the Standard Error of the Mean. miR-224 induces a statistically significant decrease in luminescence (p-value < 0.05, Student's t- test) of the forward p21 3’UTR fragment vs. NSM.
Discussion
Post-transcriptional control of gene expression by miRs has emerged as an important mechanism in the maintenance of cellular homeostasis. A growing body of evidence supports an important role for miRs in tumor initiation, progression and metastasis. In gastrointestinal cancers, numerous reports have highlighted frequent dysregulation of miRs that are tumor-suppressive (e.g., miR-34 or miR-143/145) as well as oncogenic (e.g., miR-21 or the miR-17-92 cluster) 30-34. However, despite numerous studies describing miR expression profiles in sporadic colon cancer, to date no published studies have emerged to describe global miR expression alterations in IBD-associated frank colon cancers, and only one study delineated altered miR profiles in tumors from a chronically inflamed murine colon cancer model 13. Herein, we now report global miR dysregulation in human IBD-associated colon cancers.
Several authors have reported that IBD-related CRC is frequently diagnosed at an advanced stage 35-37. MiRs have been studied for their potential as biomarkers in the detection of a range of diseases, including inflammatory diseases as well as cancers 38. One feature that makes miRs useful as biomarkers is their apparent ability to discriminate diseases arising in different organs or anatomic locations. For example, in IBD, miR expression patterns differ between ileal CD, proximal and distal colonic CD, and UC 16. In addition to their presence in tissues, miRs may also occur in peripheral blood or fecal samples, increasing their ultimate potential utility as non-invasive biomarkers 39,40. In peripheral blood, microRNAs can distinguish between healthy controls and patients with ulcerative colitis or Crohn's disease 41. Perhaps more clinically relevant, miRs can also discriminate colorectal cancer patients from patients with inflammatory bowel disease 39. Thus, our novel panel of altered miRs in IBD-associated colon cancer establishes a potential foundation for the future development of non-invasive cancer screening strategies in IBD. Further studies are needed to confirm the feasibility of such an approach in the management of IBD patients.
In a previous study, we reported altered miR profiles associated with colonic IBD-dysplasias 14. It should be emphasized that dysplasia represents an earlier step in the IBD progression continuum. While dysplasia found during colonoscopy is a strong predictor of concomitant or future carcinoma, this stage may not remain stable and may even regress, in contrast to frank carcinoma 42-44. Many biologic features of dysplasia are not shared by frank carcinoma, and vice versa. In the current study, we now report miR species manifesting altered expression in IBD cancers vs. non-neoplastic IBD mucosae. While the miR profile of IBD-associated cancers shares many similarities with that of IBD-associated dysplasia, dysregulation of new miR species was also observed. One possible explanation for our new findings is that dysregulation of one subset of miRs may occur early in the colitis-associated dysplasia-carcinoma timeline, while alteration of a different subset represents a later event. Alternatively, some miRs may display modest but steady increases at each subsequent stage of neoplastic progression. Indeed, miR-224 levels gradually increased during progression from non-inflamed, to chronically inflamed non-neoplastic, to dysplastic, and finally to frankly cancerous mucosae. However, this increase in miR-224 levels was relatively minimal at the earlier dysplastic stage, reaching statistical significance only at the transition to frank cancer.
The dominant paradigm of cancer development is embodied in the multi-hit model of tumorigenesis, which invokes successive events (“hits”) leading to the inactivation of tumor-suppressive genes and the activation of oncogenes 45-47. In addition to genetic changes, protein levels of tumor suppressor genes can be influenced by other mechanisms, including miR-mediated dysregulation. These additional molecular events may prove critical in triggering malignant transformation or conferring a survival advantage on cancerous or pre-cancerous cells. This concept underscores the importance of understanding the mechanisms regulating gene expression by miRs. MiRs interact with their mRNA targets through base-pairing with, and subsequently inhibiting expression of, their target genes. Since prediction engines can yield hundreds of potential candidates, identifying these mRNA targets still remains a challenge. Using mRNA microarrays, coupled with pathway analysess, we determined that miR-224 appears to coordinate a network of genes involved in cell cycle control, cancer, and cellular development. Subsequently, our cell cycle analyses confirmed that miR-224 overexpression indeed induces release from the G1/S check-point, thereby circumventing a key cellular defense mechanism against uncontrolled proliferation. The ability of miR-224 to increase proliferation of HCT116 colon cancer cells was previously reported by Wang et al., although a specific mechanism or mRNA target for this miR was not identified in their study 17. Our own in silico analyses identified p21 as the sole mRNA candidate that simultaneously is downregulated by miR-224, controls the cell cycle, and possesses a miR-224 binding site within its 3’UTR. p21 is a tumor suppressor gene that acts as a major regulator of cell cycle progression from G1 to S by inhibiting cyclin-dependent kinases 2 and 4. This is an important defence mechanism by which cells exposed to damaging agents are prevented from further dividing. Indeed, our luciferase assays confirmed p21 mRNA as a bona fide direct miR-224 target, suggesting one possible mechanism by which miR-224 could contribute to tumorigenesis.
This study represents, to our knowledge, the first systematic analysis of miR expression alterations associated with the progression of chronic inflammation to frank colorectal carcinoma in IBD patients. Moreover, it provides direct evidence that miR-224, the most significantly overexpressed miR in IBD-associated cancers, dysregulates cell cycle control by targeting p21. Thus, these results uncover a new miR signaling pathway and substantiate the etiologic role of miRs in the development of IBD-related carcinoma.
Supplementary Material
Acknowledgements
We would like to thank Dr. Bert Vogelstein for generously providing the HCT-116 Dicer-KO cells.
Grant Support: The Broad Medical Research Program of the Broad Foundation IBD-0271R PI Olaru AV; NIH RO1CA133012 PI Meltzer SJ. Grant
Abbreviations
- IBD
inflammatory bowel disease
- IBDN
IBD-related neoplasia
- miR
microRNA
- FIH1
factor inhibiting hypoxia inducible factor 1
- ROC
receiver operating characteristic
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
Footnotes
Competing interests: None of the authors has any disclosures that are relevant to the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 3.Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 4.Averboukh F, Ziv Y, Kariv Y, et al. Colorectal carcinoma in inflammatory bowel disease: a comparison between Crohn's and ulcerative colitis. Colorectal Dis. 2011;13:1230–1235. doi: 10.1111/j.1463-1318.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 5.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 6.Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Pekow JR, Dougherty U, Mustafi R, et al. miR-143 and miR-145 are downregulated in ulcerative colitis: Putative regulators of inflammation and protooncogenes. Inflamm Bowel Dis. doi: 10.1002/ibd.21742. Published Online First 6 May 2011; doi: 10.1002/ibd.21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takagi T, Naito Y, Mizushima K, et al. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol. 2010;25(Suppl 1):S129–133. doi: 10.1111/j.1440-1746.2009.06216.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu F, Zikusoka M, Trindade A, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635. e1624. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 10.Fasseu M, Treton X, Guichard C, et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One. 5:e13160. doi: 10.1371/journal.pone.0013160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakirtzi K, Hatziapostolou M, Karagiannides I, et al. Neurotensin Signaling Activates MicroRNAs-21 and -155 and Akt, Promotes Tumor Growth in Mice, and Is Increased in Human Colon Tumors. Gastroenterology. 2011;141:1749–1761. e1741. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Necela BM, Carr JM, Asmann YW, et al. Differential expression of microRNAs in tumors from chronically inflamed or genetic (APC(Min/+)) models of colon cancer. PLoS One. 2011;6:e18501. doi: 10.1371/journal.pone.0018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olaru AV, Selaru FM, Mori Y, et al. Dynamic changes in the expression of MicroRNA-31 during inflammatory bowel disease-associated neoplastic transformation. Inflamm Bowel Dis. 2011;17:221–231. doi: 10.1002/ibd.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olaru AV, Ghiaur G, Yamanaka S, et al. A microRNA downregulated in human cholangiocarcinoma controls cell cycle through multiple targets involved in the G1/S checkpoint. Hepatology. doi: 10.1002/hep.24591. Published Online First 1 Aug 2011. doi: 10.1002/hep.24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu F, Zhang S, Dassopoulos T, et al. Identification of microRNAs associated with ileal and colonic Crohn's disease. Inflamm Bowel Dis. 2010;16:1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Lee AT, Ma JZ, et al. Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J Biol Chem. 2008;283:13205–13215. doi: 10.1074/jbc.M707629200. [DOI] [PubMed] [Google Scholar]
- 18.Ladeiro Y, Couchy G, Balabaud C, et al. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 19.Mees ST, Mardin WA, Sielker S, et al. Involvement of CD40 targeting miR-224 and miR-486 on the progression of pancreatic ductal adenocarcinomas. Ann Surg Oncol. 2009;16:2339–2350. doi: 10.1245/s10434-009-0531-4. [DOI] [PubMed] [Google Scholar]
- 20.Nikiforova MN, Tseng GC, Steward D, et al. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93:1600–1608. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gokhale A, Kunder R, Goel A, et al. Distinctive microRNA signature of medulloblastomas associated with the WNT signaling pathway. J Cancer Res Ther. 2010;6:521–529. doi: 10.4103/0973-1482.77072. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Brannon AR, Reddy AR, et al. Identifying mRNA targets of microRNA dysregulated in cancer: with application to clear cell Renal Cell Carcinoma. BMC Syst Biol. 2010;4:51. doi: 10.1186/1752-0509-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White NM, Chow TF, Mejia-Guerrero S, et al. Three dysregulated miRNAs control kallikrein 10 expression and cell proliferation in ovarian cancer. Br J Cancer. 102:1244–1253. doi: 10.1038/sj.bjc.6605634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao G, Yin M, Lian J, et al. MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol. 2010;24:540–551. doi: 10.1210/me.2009-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 26.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson CD, Esquela-Kerscher A, Stefani G, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 28.Arthington BA, Bennett LG, Skatrud PL, et al. Cloning, disruption and sequence of the gene encoding yeast C-5 sterol desaturase. Gene. 1991;102:39–44. doi: 10.1016/0378-1119(91)90535-j. [DOI] [PubMed] [Google Scholar]
- 29.Gmeiner WH, Reinhold WC, Pommier Y. Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol Cancer Ther. 2010;9:3105–3114. doi: 10.1158/1535-7163.MCT-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulda V, Pesta M, Topolcan O, et al. Relevance of miR-21 and miR-143 expression in tissue samples of colorectal carcinoma and its liver metastases. Cancer Genet Cytogenet. 2010:154–160. doi: 10.1016/j.cancergencyto.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Suzuki H, Hibi T. The role of microRNAs in gastrointestinal cancers. J Gastroenterol. 2009;44(Suppl 19):18–22. doi: 10.1007/s00535-008-2285-3. [DOI] [PubMed] [Google Scholar]
- 32.Slaby O, Svoboda M, Fabian P, et al. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 33.Tazawa H, Kagawa S, Fujiwara T. MicroRNAs as potential target gene in cancer gene therapy of gastrointestinal tumors. Expert Opin Biol Ther. 2011;11:145–155. doi: 10.1517/14712598.2011.542749. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchida A, Ohno S, Wu W, et al. miR-92 is a key oncogenic component of the miR-17-92 cluster in colon cancer. Cancer Sci. Dec. 2011;102:2264–2271. doi: 10.1111/j.1349-7006.2011.02081.x. [DOI] [PubMed] [Google Scholar]
- 35.Brackmann S, Andersen SN, Aamodt G, et al. Relationship between clinical parameters and the colitis-colorectal cancer interval in a cohort of patients with colorectal cancer in inflammatory bowel disease. Scand J Gastroenterol. 2009;44:46–55. doi: 10.1080/00365520801977568. [DOI] [PubMed] [Google Scholar]
- 36.Greenstein AJ. Cancer in inflammatory bowel disease. Mt Sinai J Med. 2000;67:227–240. [PubMed] [Google Scholar]
- 37.Kersting S, Bruewer M, Laukoetter MG, et al. Intestinal cancer in patients with Crohn's disease. Int J Colorectal Dis. 2007;22:411–417. doi: 10.1007/s00384-006-0164-z. [DOI] [PubMed] [Google Scholar]
- 38.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 39.Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 40.Wu CW, Ng SS, Dong YJ, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. doi: 10.1136/gut.2011.239236. Published Online First: 27 Sep 2011 doi:10.1136. [DOI] [PubMed] [Google Scholar]
- 41.Wu F, Guo NJ, Tian H, et al. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2011;17:241–250. doi: 10.1002/ibd.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albert MB, Nochomovitz LE. Dysplasia and cancer surveillance in inflammatory bowel disease. Gastroenterol Clin North Am. 1989;18:83–97. [PubMed] [Google Scholar]
- 43.Axon AT. Cancer surveillance in ulcerative colitis--a time for reappraisal. Gut. 1994;35:587–589. doi: 10.1136/gut.35.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311–1319. doi: 10.1016/j.gastro.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Arends JW. Molecular interactions in the Vogelstein model of colorectal carcinoma. J Pathol. 2000;190:412–416. doi: 10.1002/(SICI)1096-9896(200003)190:4<412::AID-PATH533>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 46.Bozic I, Antal T, Ohtsuki H, et al. Accumulation of driver and passenger mutations during tumor progression. Proc Natl Acad Sci U S A. 2010;107:18545–18550. doi: 10.1073/pnas.1010978107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerstung M, Eriksson N, Lin J, et al. The temporal order of genetic and pathway alterations in tumorigenesis. PLoS One. 2011;6:e27136. doi: 10.1371/journal.pone.0027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.