Abstract
Obstructive sleep apnea (OSA) is a disorder of repetitive sleep disruption caused by reduced or blocked respiratory airflow. Although an anatomically compromised airway accounts for the major predisposition to OSA, a patient's arousal threshold and factors related to the central control of breathing (ventilatory control stability) are also important. Arousal from sleep (defined by EEG desynchronization) may be the only mechanism that allows airway re-opening following an obstructive event. However, in many cases arousal is unnecessary and even worsens the severity of OSA. Mechanisms for arousal are poorly understood. However, accumulating data are elucidating the relevant neural pathways and neurotransmitters. For example, serotonin is critically required, but its site of action is unknown. Important neural substrates for arousal have been recently identified in the parabrachial complex (PB), a visceral sensory nucleus in the rostral pons. Moreover, glutamatergic signaling from the PB contributes to arousal caused by hypercapnia, one of the arousal-promoting stimuli in OSA. A major current focus of OSA research is to find means to maintain airway patency during sleep, without sleep interruption.
Introduction: Importance of arousal in obstructive sleep apnea (OSA)
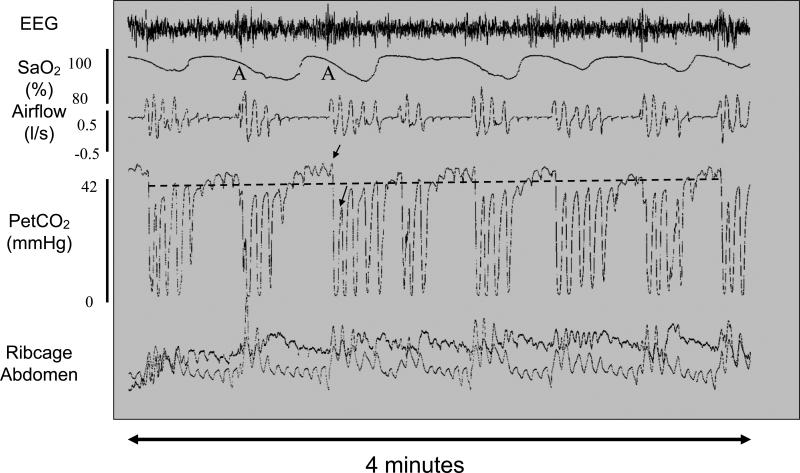
OSA is a disorder of sleep disruption caused by repetitive episodes of upper airway collapse. Sleep onset in OSA patients is associated with a drastic reduction (hypopnea) or even complete elimination (apnea) of airflow, followed by brief awakening with re-establishment of the airway. This cycle may repeat hundreds of times over the course of a single night. OSA severity is quantified by the apnea/hypopnea index (AHI), the number of events per hour that last at least 10 sec and cause blood oxygen desaturation. AHI values greater than 5 are considered to represent OSA, but patients with severe OSA may have an AHI of 30 or greater. Figure 1 shows a typical oscillatory breathing pattern in a person with severe OSA. Note that the breathing cycles between obstructed and unobstructed breaths and that each airway re-opening is associated with EEG arousal. OSA patients are unable to compensate for sleep-related increases in pharyngeal airway resistance without waking up. A portion of OSA morbidity is caused by detrimental effects of chronic intermittent hypoxia, however, sleep fragmentation is responsible for many of the consequences of OSA including excessive daytime sleepiness and cognitive deficits [1].
Figure 1.
Typical OSA breathing pattern with recurrent obstructive events. This polysomnogram from a patient with obstructive sleep apnea shows multiple cycles over a four minute period of airway collapse accompanied by hypercapnia and hypoxia and terminating with arousal (A) and airway restoration. Traces show (from top to bottom) EEG, arterial oxygen saturation (SaO2), airflow (liters/sec), end tidal partial pressure of CO2 (PetCO2), ribcage and abdominal movements. Obstructive apneas are characterized by reduced or absent airflow despite attempts to breathe as shown by rib cage and abdominal movements. Hypoxia is measured by a pulse oximeter. The level of CO2 in exhaled air at the end of an expiratory cycle approximates the partial pressure of CO2 in arterial blood, whereas the signal drops towards zero during inspiration. In this example airflow was reduced but not completely abolished during the obstructions. The dotted line overlying the trace indicates average end tidal CO2. Note the rise in CO2 during the airway obstruction and the large breaths that accompany arousal at apnea termination and that drive the CO2 below baseline. The two arrows on the trace indicate the PetCO2 during the last obstructed breath and the first unobstructed breath. The magnitude of the PetCO2 undershoot is thought to contribute to the likelihood of another obstructive event occurring when the individual falls back to sleep. Adapted from [6].
A low arousal threshold can contribute to OSA pathogenesis
Several interacting traits contribute to OSA susceptibility with the most important of these being airway collapsibility [2,3]. The pharyngeal airway is vulnerable to collapse as this soft tissue structure can be narrowed by fat deposits and is dependent upon dilator muscle activity to retain patency. During wakefulness OSA patients can and do compensate for small airways. Most of this compensation takes the form of increased neuromuscular activity driving enhanced tone in upper airway dilator muscles such as the genioglossus (a tongue protruder) during wake [4]. However, for poorly understood reasons, the ongoing neuromuscular compensation often (but not always) fails during sleep causing OSA [5]. The extent to which the upper airway dilatory muscles can compensate is highly variable among individuals and strongly influences susceptibility to OSA.
Another trait that can influence OSA severity is the inherent stability of one's ventilatory control system. An OSA patient with unstable ventilatory control is prone to larger fluctuations in blood CO2 as the airway obstructs and reopens. Note the difference between the two arrows on the PetCO2 trace during the last obstructed breath and the first unobstructed breath in Figure 1. Hypocapnia is thought to precipitate the next obstruction: most apneas occur during the decline of waxing and waning ventilatory efforts. A new paper by Xie and colleagues nicely demonstrates that administration of CO2 to prevent hypocapnia following an apneic event is able to stabilize breathing in select OSA patients that exhibit not only collapsible airways, but also high CO2 chemosensitivity [6].
Finally, the arousal threshold is a key factor influencing OSA severity. In some cases, the increased respiratory efforts as CO2 rises are sufficient to re-establish breathing without causing arousal. The less one is able to tolerate the increased CO2 and mechanical stimuli that occur in flow-limited breathing without waking up, the more fragmented the individual's sleep will be. Moreover, the arousals themselves tend to perpetuate the cycle by worsening CO2 fluctuations. Specifically, arousals contribute to over-breathing and subsequent CO2 undershoot following an apnea, and these periods of reduced respiratory drive due to hypocapnia may contribute to the next episode of airway collapse [7,8]. Despite the widely held view that arousal is necessary for airway re-opening, evidence suggests that many obstructive events are resolved without arousal [8,9] and exploration of how this may happen is at the cutting edge of OSA research [10]. At least one study suggests that pharmacologically raising the arousal threshold can ameliorate OSA in select groups of patients [11]. Nonetheless for some patients arousal from sleep is the only process that provides sufficient muscle activation to open the airway and reestablish adequate airflow. Clearly arousal is both a blessing and a curse in the context of OSA: a vital survival response in some cases and a contributor to the disorder in others.
What triggers arousal in OSA?
The mechanisms by which airway obstruction causes arousal are uncertain although the available data implicate multiple contributing stimuli including hypercapnia, hypoxia and the mechanical sensations associated with increased ventilatory effort [12]. During an obstructive apnea, airflow is reduced with a commensurate increase in blood CO2 and varying degrees of hypoxia. The accumulating CO2 and hypoxia drive increasing respiratory effort in turn producing progressively greater and greater negative airway pressures as well as proprioceptive feedback from contracting respiratory muscles. When these stimuli reach a critical threshold arousal occurs. Interestingly, arousal is associated with a particular level of respiratory effort (as assessed by mechanical metrics) in a given individual but not a consistent level of either blood CO2 or O2 [13,14]. These studies have been interpreted to emphasize the importance of mechanical stimuli in arousal. However, it is more likely given the complex and interdependent interactions between O2 and CO2 in the chemosensory system [15] and the fact that blood levels do not measure the level of either gas at the tissue levels where chemoreceptors reside, that respiratory effort may simply provide the most accurate and consistent readout of the total contribution of these two gases to simultaneously promote breathing and arousal. In the next sections I will describe the neural systems that control behavioral state and chemoreception as well as how they are linked together to elucidate arousal from OSA.
Arousal system
The arousal system can be defined as the neural substrates for maintenance of the waking state and consciousness. The concept of an ascending arousal system dates back to the experiments of Morruzi and Magoun demonstrating that brainstem lesions can cause coma whereas electrical stimulation can produce a wake-like pattern in the cortical EEG in experimental animals [16]. A wealth of neuroanatomical and physiological studies has subsequently identified a number of brain regions that may have been responsible for Morruzi and Magoun's observations. These areas a) have widespread forebrain projections with axons traversing the lesion and stimulation region and b) promote waking or wake-associated phenomena [17]. However systematic studies placing lesions in these cell groups have identified only two subcortical regions that are critically required for maintaining a behaviorally responsive state: the basal forebrain and the parabrachial/precoeruleus region [18]. It is likely that one or more of the structures in the arousal network mediates OSA arousal.
Sleep system
Although sleep is a state of overall decreased brain activity, several groups of neurons are actually more active during sleep and serve to stabilize this state or promote the transition from wake to sleep. The most well-known of these are located in the preoptic hypothalamus and include the ventrolateral preoptic nucleus (VLPO) and median preoptic nucleus (MnPO) [17,19]. An additional group, the parafacial zone, is located in the medulla [20], and there may be other as yet uncharacterized sleep-active groups. Sleep-active neurons in the VLPO are inhibitory and project to many of the arousal neurons [21]. The VLPO is in turn inhibited by components of the arousal system. This network is hypothesized to act as a flip-flop switch which prevents simultaneous activity in sleep and wake-promoting areas, thereby favoring rapid and complete transitions between behavioral states [17]. VLPO lesions cause significant increases in spontaneous wake time [22].
Since sleep-active GABAergic/galaninergic neurons in the VLPO presumably inhibit several arousal centers, including the PB, acute interruption of this activity would be expected to promote awakening. Therefore one possible mechanism for arousal would be acute withdrawal of the sleep-promoting influence of the VLPO. However, it is not known whether acute withdrawal of VLPO activity might be a wake-promoting influence during arousal from OSA. Evidence investigating this possibility,for example by acutely inhibiting the VLPO neurons optogenetically, would be welcome.
Chemoreceptor system
Blood levels of CO2 and O2 reflect the adequacy of ventilation and dictate ventilatory drive by acting on two sets of chemoreceptors located respectively in the periphery and in the brain. Peripheral chemoreceptors in the carotid body project centrally via the carotid sinus branch of the glossopharyngeal nerve and terminate in the nucleus of the solitary tract (NTS). Chemosensory neurons in the carotid body are more sensitive to a falling O2 than a rising CO2, but their sensitivity to CO2 rises as O2 falls, as occurs during apnea. Given that blood from the heart arrives at the carotid artery before the brain, the peripheral chemoreceptors respond to a change in blood gases with a shorter latency than central chemoreceptors [23]. Several types of central neurons are sensitive to changes in blood CO2, because the bicarbonate buffering in cerebrospinal fluid couples CO2 levels to changes in local pH.
Early experiments in cats demonstrated that the rostral medullary surface just lateral to the pyramidal tract is especially important in CO2 chemoreception. Subsequent work demonstrated two sets of neurons in this area that are responsive to CO2 (and pH) and play an important role in respiratory chemosensitivity. The retrotrapezoid nucleus (RTN) [24,25] which is adjacent to the facial nucleus at the ponto-medullary junction, is both intrinsically CO2 sensitive as well as also receiving signals from peripheral chemoreceptors via the NTS. A potential role of the RTN in arousal is indicated by experiments showing that optogenetic stimulation of the RTN not only increases ventilatory rate and volume, but also enhances the probability of transition from sleep to wake [26]. The RTN projects heavily to medullary respiratory control areas, but has a major ascending projection to the PB, which may contribute to arousal [27]. A second population of serotonin-containing CO2 sensitive neurons is found just caudal to the RTN in the area lateral to the pyramidal tract. Mice lacking serotonin neurons or with acutely inhibited serotonin neurons demonstrate very poor sensitivity to arousal from inspired CO2 [28-31]. These findings initially led to the hypothesis that serotoninergic neurons might carry the hypercapnic signal needed for arousal. However, the CO2 responsiveness of mice lacking serotonin neurons was restored by treating the mice with a serotonin 5HT2a receptor agonist [32]. Hence, serotonin is not required to carry the hypercapnic signal, but probably sensitizes the remaining brain circuitry to hypercapnia. Perhaps serotonin acts to increase the gain of the CO2 arousal pathway by modulating neuronal response properties as it does in the facial motor nucleus [33].
Role of the parabrachial complex in OSA arousal
The parabrachial complex is an attractive candidate for mediating OSA arousal. As a key component of both the central respiratory [34] and arousal [18] networks, the PB is well positioned to access relevant data to determine whether respiratory difficulty warrants arousal. The PB receives intense inputs from both the NTS and the RTN that overlap in the external lateral, lateral crescent and Kolliker-Fuse subnuclei [27,35]. Via the NTS and the trigeminal system, the PB receives mechanosensory information from the airways. Via the NTS and the RTN the PB receives both peripheral and central chemoreceptor inputs. This connectivity suggests that the PB is appropriately positioned to translate visceral sensory stimuli to arousal [36]. To test the hypothesis that the neurons in the PB mediate hypercapnic arousal, Kaur and colleagues [36] developed a mouse model in which mice are periodically exposed to brief increases in CO2 ramping from near zero to 10% over 30 seconds. When applied during NREM sleep, this stimulus is sufficient to cause a brief (often less than 5 sec) awakening on almost all trials. To test the role of glutamatergic PB neurons in hypercapnic arousal, they used mice that were genetically engineered so that the vesicular glutamate transporter (vGluT2) could be eliminated focally in the brain by injections of a viral vector containing the gene for cre-recombinase. When they deleted expression of vGluT2 in the from the external lateral (PBel) and lateral crescent (PBlc) subnuclei, the mice showed substantial deficits in hypercapnic arousal. Upon exposure to hypercapnia mice took on average about 3 times as long as normal to awaken, and in almost 30% of trials they failed to wake up at all during the CO2 presentation. Interestingly, the portion of the PB, which was most important for hypercapnic arousal overlaps with the zone of afferent input from the NTS and the RTN, consistent with the hypothesis that the external lateral PB is a relay for arousal signals to the forebrain.
Parabrachial-to-forebrain pathways that may mediate OSA arousal
The PBel and PBlc have very different output patterns[36]. PBlc primarily projects to the respiratory areas of the medulla, and is likely to be involved in the increased ventilatory efforts during hypercapnia. By contrast the PBel mainly projects to rostral targets, including the basal forebrain [37-39], lateral hypothalamus [40], midline thalamus [41], and the infralimbic cortex [40,42], as well as providing particularly strong projections to the extended amygdala including the bed nucleus of the stria terminalis and the central nucleus of the amygdala [37,40,43]. The PB-amygdala pathway has been associated with autonomic responses, ingestive behavior, conditioned taste aversion [44], and pain [45], but it has not previously been associated with arousal. However, the amygdala may affect the arousal system via projections to the locus coeruleus, infralimbic cortex, tuberomamillary nucleus, or lateral hypothalamus. Furthermore, the amygdala itself has been implicated in promotion of vigilance [46]. Areas of the PB that are adjacent to the PBel also project to the VLPO, but deleting glutamate transmission from these neurons is not likely to have impaired CO2 arousal from sleep, because this would have decreased, not increased sleep propensity [47]. Further work will be necessary to uncover the specific PB pathway that contributes to hypercapnic arousal.
Summary
In summary, despite its ostensible simplicity, OSA is a highly complex disorder with marked inter-individual variability in pathophysiology. The reciprocal interactions between ventilation and behavioral state is thought to contribute to a vicious cycle of arousal and over-breathing, falling back to sleep with under-breathing and airway closure, arousal and over-breathing, etc. An especially low arousal threshold can contribute to OSA in an individual who otherwise would not have it. OSA arousal is mostly likely mediated by PB neurons that receive a convergence of asphyxia-related sensations and project to the arousal system. Finally, a complete understanding of hypercapnic arousal must include elucidation of the role of serotonin, which may modulate responses at any or all of the synapses relaying hypercapnia detection to targets that cause arousal. Through further insights into the pathogenesis of OSA arousal, new therapeutic approaches are likely to emerge.
Highlights.
Arousal threshold is an important trait that determines obstructive sleep apnea severity.
Several respiratory stimuli associated with airway obstruction promote arousal.
Normal hypercapnia-evoked arousal requires intact glutamatergic signaling from the parabrachial nucleus.
Acknowledgements
I thank Drs. Atul Malhotra, Jerry Dempsey, and Veronique Vanderhorst for helpful comments on the manuscript. My work is supported by NHLBI P01 HL095491.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- *1.Djonlagic I, Saboisky J, Carusona A, Stickgold R, Malhotra A. Increased Sleep Fragmentation Leads to Impaired Off-Line Consolidation of Motor Memories in Humans. PLoS One. 2012;7:e34106. doi: 10.1371/journal.pone.0034106. [Important demonstration that not only sleep loss, but also sleep fragmentation per se, can impair memory in humans.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- **2.Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, Gautam S, Owens RL, Malhotra A, White DP. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol. 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [This paper presents a method for noninvasively measuring the four traits that contribute to OSA. This is important because the inter-individual variability in OSA mandates matching treatment with the appropriate physiology. Aside from CPAP, which works to prop the airway open in everyone, interventions such as supplemental oxygen or a sedative affect only subsets of patients that have high loop gain or low arousal threshold, respectively.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. Journal of Applied Physiology. 1988;64:789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 4.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol. 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- *6.Xie A, Teodorescu M, Pegelow DF, Teodorescu MC, Gong Y, Fedie JE, Dempsey JA. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. Journal of Applied Physiology. 2013 doi: 10.1152/japplphysiol.00064.2013. [This paper shows that prevention of hypocapnia due to hyperventilation following obstructive events can stabilize breathing in a subset of OSA patients that exhibit high CO2 sensitivity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow CM, Xi L, Smith CA, Saupe KW, Dempsey JA. A volume-dependent apneic threshold during NREM sleep in the dog. J Appl Physiol. 1994;76:2315–2325. doi: 10.1152/jappl.1994.76.6.2315. [DOI] [PubMed] [Google Scholar]
- 8.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- **9.Younes M, Loewen AHS, Ostrowski M, Laprairie J, Maturino F, Hanly PJ. Genioglossus Activity Available via Non-arousal Mechanisms vs. that Required for Opening the Airway in Obstructive Apnea Patients. Journal of Applied Physiology. 2011 doi: 10.1152/japplphysiol.00312.2011. [This paper clearly shows that the degree of genioglossus muscle activation necessary for airway reopening is typically low with respect to a given patients ability to activate the genioglossus muscle. Thus, delay of arousal should be sufficient to allow for airway reopening in subjects whose genioglossus is responsive to enhanced drive during an obstructive event.] [DOI] [PubMed] [Google Scholar]
- 10.Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–368. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, White DP, Malhotra A. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–514. doi: 10.1042/CS20100588. [Proof of principal that manipulation of arousal threshold can successfully treat OSA in some patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry RB, Gleeson K. Respiratory arousal from sleep: Mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 14.Vincken W, Guilleminault C, Silvestri L, Cosio M, Grassino A. Inspiratory muscle activity as a trigger causing the airways to open in obstructive sleep apnea. Am Rev Respir Dis. 1987;135:372–377. doi: 10.1164/arrd.1987.135.2.372. [DOI] [PubMed] [Google Scholar]
- 15.Dempsey J, Smith C, Blain G, Xie A, Gong Y, Teodorescu M. Role of Central/Peripheral Chemoreceptors and Their Interdependence in the Pathophysiology of Sleep Apnea. In: Nurse CA, Gonzalez C, Peers C, Prabhakar N, editors. Arterial Chemoreception. Springer; Netherlands: 2012. pp. 343–349. Advances in Experimental Medicine and Biology, vol 758.]
- 16.Morruzzi G, Magoun H. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 17.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep State Switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **18.Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. Journal of Comparative Neurology. 2011;519:933–956. doi: 10.1002/cne.22559. [This paper demonstrates that the key substrate for the classic ascending reticular activating system includes neurons in the parabrachial/precoeruleus area that project to the basal forebrain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szymusiak R, McGinty D. Hypothalamic Regulation of Sleep and Arousal. Annals of the New York Academy of Sciences. 2008;1129:275–286. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- 20.Anaclet C, Lin JS, Vetrivelan R, Krenzer M, Vong L, Fuller PM, Lu J. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J Neurosci. 2012;32:17970–17976. doi: 10.1523/JNEUROSCI.0620-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CA, Rodman JR, Chenuel BJA, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- 24.Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JFR, Gourine AV. Essential Role of Phox2b-Expressing Ventrolateral Brainstem Neurons in the Chemosensory Control of Inspiration and Expiration. The Journal of Neuroscience. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbott SBG, Coates MB, Stornetta RL, Guyenet PG. Optogenetic Stimulation of C1 and Retrotrapezoid Nucleus Neurons Causes Sleep State–Dependent Cardiorespiratory Stimulation and Arousal in Rats. Hypertension. 2013;61:835–841. doi: 10.1161/HYPERTENSIONAHA.111.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- 28.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proceedings of the National Academy of Sciences. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respiratory Physiology & Neurobiology. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired Respiratory and Body Temperature Control Upon Acute Serotonergic Neuron Inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith HR, Richerson GB, Buchanan GF. Program #799.07/BBB8 Neuroscience Meeting Planner. Society for Neuroscience; New Orleans, LA: 2012. Activation of 5-HT2A receptors recovers hypercapnia-induced arousal in genetically central 5-HT neuron deficient mice. [Google Scholar]
- 33.Aghajanian GK, Sprouse JS, Sheldon P, Rasmussen K. Electrophysiology of the Central Serotonin System: Receptor Subtypes and Transducer Mechanismsa. Annals of the New York Academy of Sciences. 1990;600:93–103. doi: 10.1111/j.1749-6632.1990.tb16875.x. [DOI] [PubMed] [Google Scholar]
- 34.Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol. 2004;143:115–125. doi: 10.1016/j.resp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Research. 1978;1553:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 36.Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic Signaling from the Parabrachial Nucleus Plays a Critical Role in Hypercapnic Arousal. The Journal of Neuroscience. 2013;33:7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: A phaseolus vulgaris Leucoagglutinin (PHA-L) study in the rat. J. Comp. Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- 38.Shin JW, Geerling JC, Stein MK, Miller RL, Loewy AD. FoxP2 brainstem neurons project to sodium appetite regulatory sites. J Chem Neuroanat. 2011;42:1–23. doi: 10.1016/j.jchemneu.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grove EA. Neural associations of the substantia innominata in the rat: Afferent connections. The Journal of Comparative Neurology. 1988;277:315–346. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- 40.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Research Reviews. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 41.Krout KE, Loewy AD. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. The Journal of Comparative Neurology. 2000;428:475–494. doi: 10.1002/1096-9861(20001218)428:3<475::aid-cne6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Saper CB. Reciprocal parabrachial-cortical connections in the rat. Brain Research. 1982;242:33–40. doi: 10.1016/0006-8993(82)90493-0. [DOI] [PubMed] [Google Scholar]
- 43.Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol. 1988;270:416–426. 398–419. doi: 10.1002/cne.902700310. [DOI] [PubMed] [Google Scholar]
- 44.Sakai N, Yamamoto T. Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs. Neuroreport. 1997;8:2215–2220. doi: 10.1097/00001756-199707070-00025. [DOI] [PubMed] [Google Scholar]
- 45.Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- 46.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 47.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]