Abstract
Low HDL-C levels are associated with atherosclerosis and non-alcoholic steatohepatitis, and increased levels may reduce the risk of these diseases. Inhibition of cholesteryl ester transfer protein (CETP) activity is considered a promising strategy for increasing HDL-C levels. Since CETP is a self-antigen with low immunogenicity, we developed a novel CETP vaccine (Fc-CETP6) to overcome the low immunogenicity of CETP and for long-term inhibition of CETP activity. The vaccine consists of a rabbit IgG Fc domain for antigen delivery to antigen-presenting cells fused to a linear array of 6 repeats of a CETP epitope to efficiently activate B cells. Rabbits were fed a high fat/cholesterol (HFC) diet to induce atherosclerosis and NASH, and immunized with Fc-CETP6 vaccine. The Fc-CETP6 vaccine successfully elicited anti-CETP antibodies and lowered plasma CETP activity. The levels of plasma HDL-C and ApoA-I were higher, and plasma ox-LDL lower, in the Fc-CETP6-immunized rabbits as compared to the unimmunized HFC diet-fed rabbits. Pathological analyses revealed less lipid accumulation and inflammation in the aorta and liver of the Fc-CETP6-immunized rabbits. These results show that the Fc-CETP6 vaccine efficiently elicited antibodies against CETP and reduced susceptibility to both atherosclerosis and steatohepatitis induced by the HFC diet. Our findings suggest that the Fc-CETP6 vaccine may improve atherosclerosis and NASH and has high potential for clinical use.
Introduction
HDL continues to attract interest because its levels are inversely associated with the risk of cardiovascular disease [1]. This may be attributed to its having various potentially anti-atherogenic properties, such as reverse cholesterol transport, anti-inflammatory, anti-oxidative, and anti-thrombotic effects [2]. Clinical studies have shown that low HDL-C is also found in non-alcoholic steatohepatitis (NASH) [3]. In addition, patients with hepatic steatosis also showed higher CETP activity [4]. NASH shares several characteristics with atherosclerosis, including lipid accumulation, inflammation, and macrophage infiltration [5]. Recent studies have suggested that the pathogenesis of NASH involves scavenger receptor-mediated uptake of ox-LDL by macrophages in the liver [6], [7]. This may explain, at least in part, why NASH is an important risk factor for cardiovascular disease [8]. However, it is not known if increasing HDL levels by CETP inhibition can ameliorate NASH.
Cholesteryl ester transfer protein (CETP) is considered a therapeutic target for increasing HDL-C [9]. Interest in CETP as a therapeutic target began as a result of the high HDL-C and low LDL-C observed in Japanese people carrying the homozygous, defective CETP gene who showed no evidence of premature atherosclerosis, even though they had hypercholesterolemia [10]. This CETP is bound mainly to HDL particles and transfers cholesterol ester from HDL to triglyceride-rich lipoproteins. CETP action results in a CE enrichment of non-HDL lipoproteins, which could contribute to atherosclerosis. Small molecule CETP inhibitors, including dalcetrapib, evacetrapib, and anacetrapib, that are at various phases of clinical development inhibit CETP activity and significantly increase HDL-C [11]. However, torcetrapib was failed in phase 3 clinical trial due to compound specific off-target effects [12], and dalcetrapib failed in phase 3 clinical trials due to less meaningful outcomes [13]. Two other CETP inhibitors, evacetrapib and anacetrapib, which showed beneficial effects by increasing HDL-C, are still under clinical trials [14].
Inducing an immune response against specific self-peptides is potentially beneficial for the treatment of certain diseases. The drawbacks of peptide-based immunization include low immunogenicity of self-peptides, a low efficiency of chemical conjugation, and the heterogeneous nature of antigen preparations. To address these problems, we have investigated the use of peptide repeats conjugated to the receptor-binding domain of Fc to induce antibodies that might suppress the function of self-proteins. Since CETP is a self-antigen with low immunogenicity, which may lead to a low immune response [15], we thus designed a novel anti-CETP vaccine (Fc-CETP6) composed of the Fc domain of rabbit IgG fused to a linear repeats epitope within a region responsible for CETP-VLDL/LDL binding. The Fc domain binds to the Fc receptor, facilitating antigen delivery to antigen-presenting cells through receptor-mediated endocytosis [16], [17], which is more efficient than phagocytosis, leading to antigen presentation through the MHC II pathway [18]. It has been demonstrated that carrier proteins bearing linear repeats of epitope are highly effective in inducing strong B cell activation [19]. In this study, linear array epitope (LAE) technology was applied to generate linear repeating epitope [20].
Rabbits have higher CETP levels than humans and are highly susceptible to the induction of developing atherosclerosis [21] and NASH [22]. In this study, the rabbits were fed a high fat/cholesterol (HFC) diet to induce atherosclerosis and NASH. Efficacy of the Fc-CETP6 vaccine in alleviating the progression of atherosclerosis and NASH was evaluated. Our results showed that the Fc-CETP6 vaccine successfully elicited anti-CETP antibodies and lowered plasma CETP activity. Pathological analyses revealed less lipid accumulation and inflammation in the aorta and liver of the Fc-CETP6-immunized rabbits.
Materials and Methods
Construction of DNA fragment, encoding for 6 repeats of human CETP epitope, followed by fusing with rabbit Fc and expression of the fused protein in E. coli BL21 (DE3)
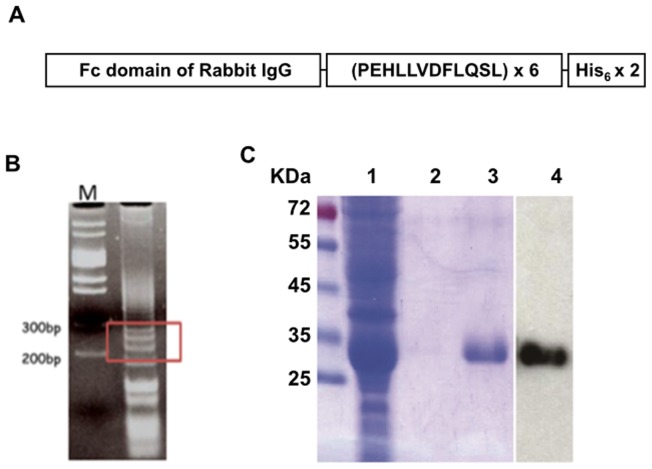
The DNA fragment encoding for 6 repeats of the peptide PEHLLVDFLQSL, a human CETP epitope [23], was generated by the template-repeated polymerase chain reaction (TR-PCR), as described previously [20] (Fig. 1). The TR-PCR products were then subjected to adapter-PCR using adapter primers (Table S2) to create restriction sites at the 5′- and 3′-ends to facilitate further subcloning. The 200–300 bp adapter-PCR products (Fig. 1B) were eluted and cloned into the T-Easy vector (Promega). A clone containing 6 copies of the CETP epitope was identified by sequencing and subcloned into a modified plasmid pET21b vector (Novagen) at the 3′-end of the region coding for the Fc domain of the rabbit IgG. The resulting plasmid, pFc-CETP6, was confirmed by sequencing and introduced into E. coli BL21 (DE3), and the expressed fusion protein (Fc-CETP6) was purified by chromatography on a His-Bind column (Novagen).
Figure 1. The Strategy for produces the Fc-CETP6 vaccine.
(A) Schematic diagram of Fc-CETP6. The CETP vaccine contained the Fc domain of rabbit IgG, followed by 6 repeats of the human CETP epitope, and a double-His6-tag. (B) The products of adapter-PCR. The 200–300 bp products were boxed. M stands for DNA marker. (C) Purity of the His-Bind-purified Fc-CETP6 immunogen. Lanes 1 to 3 show Coomassie blue staining results for the flow-through fraction, wash fraction, and elutes, respectively, while lane 4 shows Western blot analysis of elutes using anti-rabbit IgG (Fc) antibodies.
Assay of anti-CETP antibody titers
The GST and GST-CETP proteins were expressed in E.coli Top10 (Invitrogen), and purified by affinity chromatography. ELISA was applied using plates coated with GST-CETP or GST as tagging control to determine plasma antibody titers to CETP. The titer was defined as the plasma dilution that gave an optical density of 1.0 at 405 nm.
Animal experiments
Two animal studies were carried out; in both, 16-week-old female New Zealand White rabbits (∼2 kg) were allowed an adaptation period of 2 weeks, and then treated as follows.
In the first experiment, the rabbits were randomly allocated to the normal group (n = 4), control group (n = 7), or Fc-CETP6 group (n = 8). Rabbits in the normal group were fed a regular chow diet, while rabbits in the control and Fc-CETP6 groups were fed the HFC diet (chow supplemented with 5% lard and 0.25% cholesterol). Each animal was fed 100 g of the diet per day during the study period and allowed free access to tap water. Rabbits in the Fc-CETP6 group were injected subcutaneously with 0.1 mg of Fc-CETP6 on day 1 and at the end of weeks 2, 4, 6, and 8 (first injection in complete Freund's adjuvant and all others in incomplete Freund's adjuvant), while the control HFC diet-fed group underwent the same schedule of injections with PBS in adjuvant. Plasma samples were collected at the end of weeks 1, 8, 12, 20, and 24 and CETP activity and titers of anti-CETP antibodies were measured. At week 24, the rabbits fasted overnight, and the next morning anesthetized by inhalation of isoflurane and the rabbits' aortas were also collected for atherosclerotic lesion analysis.
In the second experiment, the rabbits were treated as in the first experiment except that the Fc-CETP6 group rabbits (n = 8) received booster injections with 0.1 mg of Fc-CETP6 in incomplete Freund's adjuvant at the end of weeks 24 and 28 and were sacrificed at the end of week 52. After overnight fasting, the rabbits were anesthetized as above and their liver removed after perfusion with physiological saline. Then three samples (each approximately 1 cm3) of the right lobe were taken, fixed in 4% paraformaldehyde, and embedded in paraffin.
All procedures for the care and use of research animals were in accordance with the guidelines of, and approved by, the animal center of the Taiwan Food and Drug Administration, Department of Health, Executive Yuan.
Detection of CETP activity
Plasma CETP activity was measured using CETP Activity Assay Kits (BioVision) according to the manufacturer's guidelines. The data are presented as a percentage of the levels before treatment.
Isolation of lipoprotein fractions and measurement of cholesterol levels
The procedures used to isolate lipoprotein fractions have been described previously [24]. In brief, plasma was prepared by low speed centrifugation at 4°C, then VLDL, LDL, and HDL were isolated by density gradient ultracentrifugation and cholesterol concentrations in the plasma and lipoprotein fractions determined enzymatically (Randox) according to the manufacturer's guidelines.
Quantification of atherosclerotic lesions in the aorta
The aorta was perfused for 20 min with ice-cold PBS, and then pressure-fixed with cold formaldehyde-sucrose solution (10% neutral formalin, 5% sucrose, 20 mM butylated hydroxytoluene, and 2 mM EDTA, pH 7.4). The entire aorta was then dissected out and the aorta opened longitudinally, rinsed briefly in 70% ethanol, and stained with Sudan IV (0.5% Sudan IV in 35% ethanol/50% acetone), and de-stained for 5 min with 80% ethanol. Each aorta was then mounted on a flat surface and images of its surface taken using a digital camera. The area stained with Sudan IV (lipid plaque) was expressed as a percentage of the total surface area of the aorta.
Immunohistochemical analysis
The procedures used have been described previously [25]. In brief, levels of NF-kB and RAM-1 expression in the aorta and liver tissue were determined by immunohistochemical staining using mouse anti-NF-kB and anti-RAM-11 antibodies (Dako). The aortas were analyzed from cross section from each animal and ten random fields (magnification, x200) of each cross-section photographed under a microscope (Olympus, BX60). The liver tissues were analyzed from three tissue sections from right lobe of each animal and ten random fields (magnification, x200) of each section photographed under a microscope (Olympus, BX60).
Liver histology and quantification of steatosis and fibrosis in the liver
Liver sections (4 µm) were stained with hematoxylin and eosin (H&E) and Masson's trichrome stain. An expert pathologist assessed histological steatosis and fibrosis stages with the Histological Scoring System for Nonalcoholic Fatty Liver Disease (NAFLD) [26]. Three tissue sections from each animal were analyzed and ten random fields (magnification, x200) of each section photographed under a microscope (Olympus, BX60).
Measurement of plasma ApoA-I and oxidized–LDL levels
Plasma ApoA-I and plasma ox-LDL were measured using sandwich ELISA kits from CUSABIO (CSB-E09804Rb) and Mercodia (10-1158-01), respectively, according to the manufacturer's guidelines.
Statistical analysis
All data are presented as the mean ± SEM. Normality was examined by the Kolmogorov-Smirnov test, and when the data was normally distributed, the statistical significance of differences was assessed with the independent t test and 1-way ANOVA and by correlation and regression analysis using the SPSS statistical program, version 17. The Mann–Whitney U test was applied when the data was not normally distributed. In all analyses, values of P<0.05 were considered statistically significant.
Results
Generation of the Fc-CETP6 vaccine
DNA encoding 6 repeats of CETP epitope was generated and the fusion protein was induced and expressed in E.coli strain BL21 (DE3) as shown in Fig. 1. The fusion protein Fc-CETP6 was purified by affinity chromatography on a His-Bind column. The purity of the Fc-CETP6 fusion protein examined by Coomassie Blue staining and Western blotting reached 95% (Fig. 1C).
Fc-CETP6 vaccine elicits antibodies against CETP and reduces CETP activity in HFC diet-fed rabbits
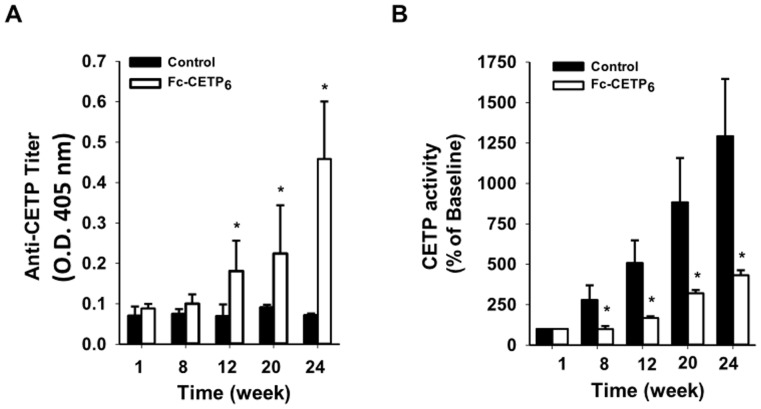
To demonstrate that anti-CETP antibodies were produced and reacted with circulating CETP, the plasma anti-CETP titer and plasma CETP activity were measured. Fig. 2A shows that anti-CETP antibodies were detected in the Fc-CETP6 group from week 12 and that the titer increased up until the end of the study at week 24. Fig. 2B shows that plasma CETP activity in both HFC diet-fed groups increased in a time-dependent manner, but was significantly lower in the Fc-CETP6 group. These results show that injection of Fc-CETP6 induces production of antibodies against CETP, which then reduce CETP activity.
Figure 2. Measurement of plasma anti-CETP titers and CETP activity in rabbits immunized with Fc-CETP6 (experiment 1).
(A) Plasma antibody titers against CETP. Plasma samples were collected as described in material and methods (Control group n = 7; Fc-CETP6 group n = 8) and diluted 2000-fold and the anti-CETP titer measured by ELISA. (B) Plasma CETP activity. Plasma CETP activity was measured and expressed as a percentage of that in the pre-vaccination sample (Control group n = 7; Fc-CETP6 group n = 8). Values are the mean ± SEM for the control rabbits fed the HFC diet and the rabbits fed the HFC diet and immunized with Fc-CETP6. *p<0.05 compared to the control at the same time.
Effects of the Fc-CETP6 vaccine on plasma levels of total-cholesterol and HDL-cholesterol
The effects of vaccination with Fc-CETP6 on levels of total-C and HDL-C and body weight are summarized in Table 1. There was no significant difference between the control and Fc-CETP6 groups in body weight. Levels of total-C and HDL-C increased in a time-dependent manner in both groups. At weeks 12 and 24, total-C levels were significantly lower, and HDL-C levels significantly higher, in the Fc-CETP6 group than in the control group. In addition, at weeks 12 and 24, the non-HDL-C/HDL-C ratio was lower in the Fc-CETP6 group than in the control group. These results show that Fc-CETP6 immunization resulted in a reduced atherogenic lipoprotein profile.
Table 1. Changes in body weight and plasma total cholesterol and HDL-C in rabbits fed the HFC diet with or without vaccination.
| Group | Time (week) | Body wt (kg) | Total C (mg dl−1) | HDL-C (mg dl−1) | Non-HDL-C/HDL-C |
| Control | 0 | 2.6±0.11 | 127.0±2.7 | 24.3±3.7 | 4.5±0.9 |
| 12 | 3.3±0.3 | 342.7±44.5 | 40.4±5.4 | 7.5±0.2 | |
| 24 | 3.9±0.3 | 454.6±10.9 | 46.6±7.2 | 8.8±2.4 | |
| Fc-CETP6 | 0 | 2.5±0.2 | 159.0±19.9 | 29.8±5.0 | 4.9±1.0 |
| 12 | 3.2±0.3 | 262.0±18.8* | 52.8±10.7* | 4.7±0.9* |
Control n = 7; Fc-CETP6 n = 8. 1Values are the mean ± SEM. *p<0.05 compared to the control at the same time.
Fc-CETP6 increases ApoA-I levels and lowers ox-LDL levels
ApoA-I is a major protein in HDL and is known to have anti-inflammatory effects [27]; high levels of ApoA-I have been shown to reduce the progression and even induce regression of atherosclerosis, indicating that ApoA-I is directly protective against atherosclerosis [28]. In addition, ox-LDL is taken up by macrophages in the artery and transforms them into cholesterol-rich foam cells that have been shown to involved in the development of NASH [29]. We therefore measured plasma levels of ApoA-I and ox-LDL in the rabbits (Table 2) and found that ApoA-I levels were significantly higher, and ox-LDL levels significantly lower, in the Fc-CETP6 group than in the control group. In addition, the immunofluorescence stain showed the expression level of ox-LDL around the vein area of liver was significantly reduced in the Fc-CETP6 group compared to control group (Figures S1 and S2). These results may help explain why vaccination with Fc-CETP6 alleviated development of HFC diet-induced atherosclerosis and NASH.
Table 2. Plasma levels of ApoA-I and ox-LDL.
| Plasma levels | Control | Fc-CETP6 |
| ApoA-I (µg mL−1) | 22.2±7.51 | 96.2±30.6* |
| ox-LDL (unit L−1) | 61.6±1.8 | 49.7±5.2* |
Control n = 7; Fc-CETP6 n = 8. 1Values are the mean ± SEM. *p<0.05 compared to control.
Fc-CETP6 ameliorates HFC diet-induced atherosclerotic lesions and inflammation in the aorta
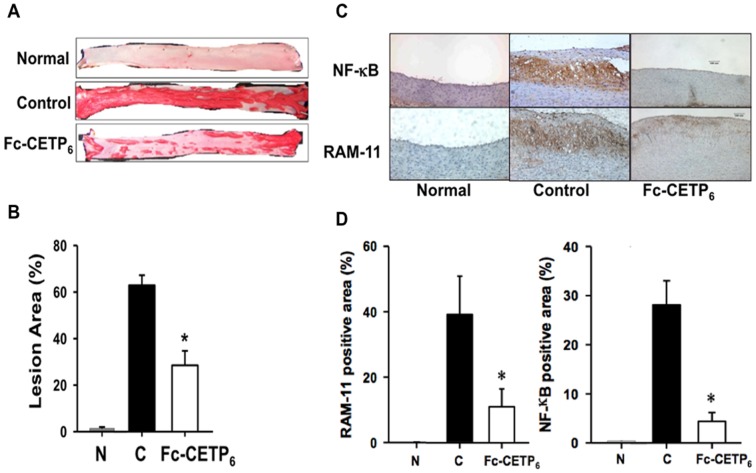
To examine whether vaccination with Fc-CETP6 could ameliorate formation of atherosclerotic plaques, the aorta from each rabbit was isolated and stained with Sudan IV. Representative Sudan IV-stained aorta from each group is shown in Fig. 3A, and the extent of atherosclerosis in the entire aorta, quantified using an image analysis system, is shown in Fig. 3B. In the normal rabbits, only about 1% of the aorta surface area stained by Sudan IV. In contrast, rabbits maintained on the HFC diet for 24 weeks developed atherosclerosis lesions that covered 62.9±4.3% of the aortic surface in the control group, but only 28.5±6.2% in the Fc-CETP6 group (Fig. 3B). Since atherogenesis involves long-term inflammation and the macrophage is considered a key mediator in aortic local inflammation [30], an immunohistochemical analysis was performed to detect the presence of NF-kB, an inflammatory marker, and RAM-11, a macrophage marker, in the aorta. Fig. 3C shows that no NF-kB or RAM-11 staining was detected in the aorta of the normal rabbits, whereas the HFC diet resulted in strong RAM-11 and NF-kB staining in the intima in the control group. Both effects were significantly reduced by vaccination with Fc-CETP6 (Fig. 3D). In summary, our results show that immunization with Fc-CETP6 reduced the extent of aortic inflammation and macrophage infiltration, and thus decreased susceptibility to atherosclerosis progression in the rabbit aorta.
Figure 3. Vaccination with Fc-CETP6 reduces Aortic lesions and inflammation (experiment 1).
(A) Representative Sudan IV-stained aortic specimens at the end of week 24. (B) Quantification of the aortic lesion area. The bar graphs represent the average (N: normal group n = 4; C: control group n = 7; Fc-CETP6 group n = 8) for each group with standard errors. *p<0.05 compared to the control. (C) Expression of NF-κB and RAM-11 (magnification, x200) of the aorta from normal rabbits, control rabbits, and Fc-CETP6 rabbits. (D) Quantification of the RAM-11 and NF-κB positive area of aorta. The bar graphs represent the average (N: normal group n = 4; C: control group n = 7; Fc-CETP6 group n = 8) for each group with standard errors. The positive-stained area was calculated as the (stained area/total area) x 100. Values are the mean ± SEM. *p<0.05 compared to the control.
Fc-CETP6 alleviates HFC diet-induced NASH and fibrosis
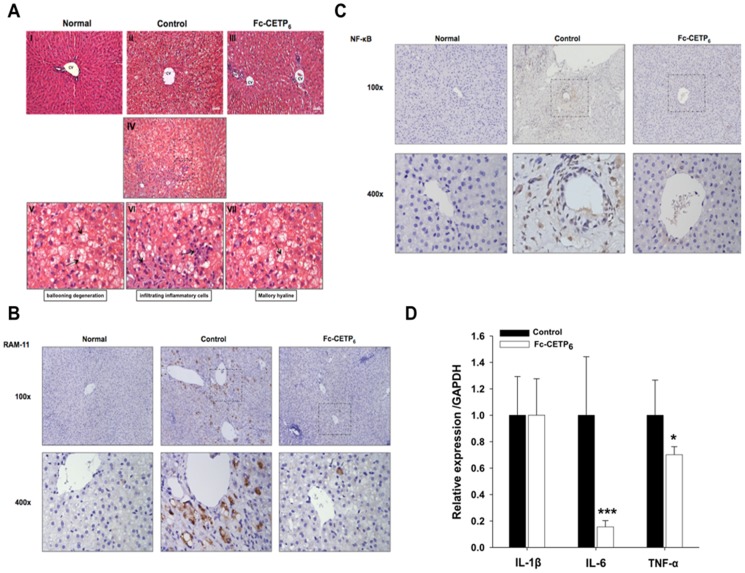
NASH is characterized by hepatic lipid accumulation combined with inflammation, which may ultimately progress into cirrhosis. In the first experiment designed to examine whether the vaccine was able to alleviate the atherosclerosis induced by the HFC diet, we observed that the livers from the control group were pale in color, while those from the normal group and Fc-CETP6 group were red, suggesting that Fc-CETP6 injection alleviated HFC-induced nonalcoholic fatty liver disease. Ogawa et al. found that a human-type NASH with advanced fibrosis is induced in rabbits by feeding a diet supplemented with 0.75% cholesterol and 12% corn oil [22]. In the second experiment, to test whether the Fc-CETP6 vaccine could reduce susceptibility to NASH and fibrosis in rabbits fed a HFC diet, we examined the effects of the Fc-CETP6 vaccine on hepatic lipid accumulation and NASH in rabbits after 12 months of HFC diet feeding by staining of liver sections with H&E and Masson's trichrome. As shown in Fig. 4, liver sections from the control group showed evidence of parenchymatous lipid accumulation, microvesicular steatosis with ballooning degeneration in the perivenular area, infiltrating inflammatory cells, and Mallory hyaline (Fig. 4A V-VII), whereas those from the Fc-CETP6 group showed hepatocytes with only mild fat accumulation, and those from normal group showed no evidence of steatosis. To further evaluate the effects of Fc-CETP6 on liver inflammation, immunohistochemistry staining were performed using antibodies against RAM-11, recognizes activated kupffer cells [31], and NF-κB, an inflammation marker. Compared to the liver of control rabbit, the liver of Fc-CETP6 showed decreased number of RAM-11 positive cell around the central vein area at sites where fatty degeneration of hepatocytes was evident. Normal group was shown as RAM-11 negative control (Fig. 4B). At higher magnification, large RAM-11 positive cells contained vacuoles (lipid droplet). Moreover, NF-κB stained cells around central vein area in the liver of control rabbits, and NF-κB stained cells were significantly reduced in the Fc-CETP6 group. Normal group was shown as NF-κB negative control (Fig. 4C). The changes of inflammation and fibrosis were further confirmed by detection of hepatic mRNA expression levels of pro-inflammatory cytokines and genes associated with fibrosis. Fig. 4D shows that mRNA levels of tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6) were significantly lower in the Fc-CETP6 group than in the control group; whereas (interleukin 1β) IL-1β was not different between the two groups. These results support that Fc-CETP6 reduces hepatic inflammation in the HFC diet fed rabbits.
Figure 4. Vaccination with Fc-CETP6 attenuates high HFC diet-induced steatosis and NASH.
(A) Representative liver sections stained with H&E (magnification, x200). Liver section stained with H&E from control rabbits showing NASH characteristics, such as ballooning degeneration, infiltrating inflammatory cells and Mallory hyaline were observed in the control (magnification, x200). (B) Expression of RAM-11 (magnification, upper panel x100 and lower panel x400) of the liver from control and Fc-CETP6 rabbits. (C) Expression of NF-kB (magnification, upper panel x100 and lower panel x400) of the liver from control and Fc-CETP6 rabbits. (D) Expression of inflammation related genes in control rabbits (black bars) and Fc-CETP6 rabbits (white bars). The bar graphs represent the average (C: control group n = 7; Fc-CETP6 group n = 8) for each group with standard errors. Values are the mean ± SEM. *p<0.05; ***p<0.001 compared to the control.
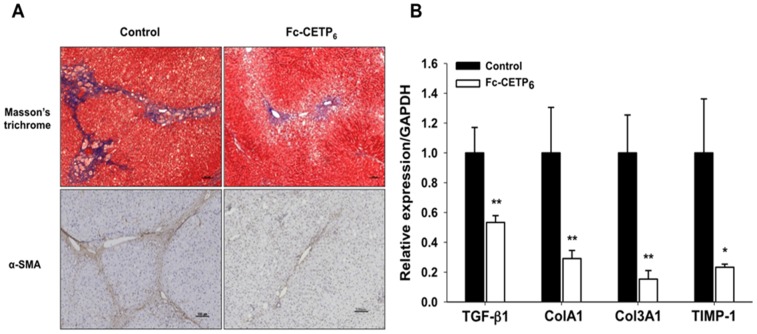
Liver sections from the control group showed positive trichrome staining, prominent bridging fibrosis with fibrous bands extending from the perivenular area to the pericellular area, whereas the Fc-CETP6 group showed diminished, or no, bridging fibrosis. The fibrotic septa were composed of cells positive for α-SMA, a marker of activated stellate cells and myofibroblasts (Fig. 5A); whereas the Fc-CETP6 group showed diminished bridging fibrosis that limited in central vein area. And hepatic mRNA levels of fibrosis associate genes, transforming growth factor β1 (TGF-β1), collagens 1A1 (Col1A1), 3A1 (Col3A1) and tissue inhibitors of metalloproteinases-1 (TIMP-1) were all significantly lower in the liver of Fc-CETP6 rabbits than in the liver of control rabbits (Fig. 5B). These results support that Fc-CETP6 reduces hepatic fibrosis in the HFC diet fed rabbits.
Figure 5. Vaccination with Fc-CETP6 reduces inflammation related gene and fibrosis related gene expression profile (experiment 2).
(A) Representative liver sections stained with Masson's trichrome stain and immunohistochemistry stained for α–SMA, a marker of activated stellate cells and myofibroblasts. α–SMA positive cells were expressed near the fibrotic septa (magnification, upper panel x100 and lower panel x100). Three sections from right lobe liver of each rabbit (control group n = 7; Fc-CETP6 group n = 8) were analyzed and ten random fields (magnification, x200) of each section photographed under a microscope (Olympus, BX60). (B) Expression of fibrosis related genes in control rabbits (black bars) and Fc-CETP6 rabbits (white bars) *p<0.05; **p<0.01compared to the control.
The grades of steatosis and fibrosis were summarized in the Table S1. In terms of steatosis, the results showed that, of the liver specimens from the control group, none were grade 0 or 1, 7% were grade 2, and 93% grade 3, whereas the values for the Fc-CETP6 group were 12% grade 0, 56% grade 1, 32% grade 2, and none grade 3. In terms of fibrosis, in the control group, none were grade 0 or 1, while 25% were grade 2, 62.5% grade 3, and 12.5% grade 4, whereas in the Fc-CETP6 group, 14% of the liver specimens presenting no fibrosis, while 29% were grade 1a, 43% grade 1b, 14% grade 2, and none grade 3 or 4 (Table S1).
Discussion
In this study, we designed and produced a novel Fc-CETP6 vaccine. This vaccine induced the generation of anti-CETP antibodies that reduced plasma CETP activity, alleviating the development of both atherosclerosis and NASH in HFC diet-fed rabbits. Rittershaus et al. showed that a decrease in CETP activity in vivo by vaccination with TT/CETP vaccine could enhance HDL-C and reduced atherosclerosis [32]. To our knowledge, our study provides the first evidence showing that a vaccine targeted at CETP is able to delay the development of both atherosclerosis and NASH in HFC diet-fed rabbits. Atherosclerosis and NASH are both metabolic syndrome and there is evidence that both are associated with lower HDL-C levels and higher ox-LDL levels [29] and involve macrophage activation and infiltration, accompanied by chronic inflammation [33].
In this study, we observed that the Fc-CETP6 vaccine could increase circulating levels of HDL-C and ApoA-I, and decrease levels of non-HDL-C and ox-LDL. Furthermore, Fc-CETP6 vaccine can reduce macrophage infiltration (RAM-11) and activation (NF-kB) in the aorta and liver tissue. Patients with hepatic steatosis also showed higher CETP activity [3], indicating that CETP inhibition may be regarded as a target to improve NASH [34]. In addition, ApoA-I, the most abundant protein in HDL, has been demonstrated to be a potent anti-oxidative and anti-inflammatory agent in in vivo studies [35], [36]. In this study, plasma ApoA-I levels were 4.5 times higher in the Fc-CETP6 group than in the control group and these high levels of ApoA-I may result in increased anti-oxidative and anti-inflammatory effects. It is not fully revealed whether CETP inhibition improves the progression of NASH and atherosclerosis, but it is worth further investigation.
Since an increased CETP level can reduce HDL-C and CETP deficiency is associated with elevated HDL-C, CETP inhibitors have been investigated. However, clinical trial results showed that the CETP inhibitors torcetrapib and anacetrapib significantly increase HDL-C levels, but do not reduce the risk of recurrent cardiovascular events [37], [38]. Several lines of evidence indicate that the increase in HDL levels caused by torcetrapib is due to a reduction in the HDL-ApoA-I catabolic rate, rather than increased HDL production [39], [40]. Unlike small molecule inhibitors [41], antibodies are non-permeable large molecules and only work at the plasma level. When the anti-CETP antibody binds to C-terminus of CETP, this process blocks the transfer of cholesterol ester from HDL to VLDL and LDL [42].
Higher CETP activity has been shown to be closely associated with hepatic steatosis in patients with metabolic syndrome [4]. Moreover, over-expression of simian CETP in 57BL/6 mice accelerates the development of fatty liver [43]. In this study, we showed that inhibition of CETP by the Fc-CETP6 vaccine markedly reduced the occurrence of HFC diet-induced hepatic steatosis and steatohepatitis in rabbits. Recent reports have shown that the pathogenesis of NASH involves scavenger receptor-mediated uptake of ox-LDL by macrophages in the liver [6], [7], and that NASH is strongly associated with risk of cardiovascular disease [8], [44]. It is highly likely that atherosclerosis and NASH result from the same etiology and that increasing HDL levels and reducing ox-LDL accumulation in macrophage may be beneficial in both diseases. However, the underlying mechanisms by which inhibition of CETP prevents hepatic steatosis and steatohepatitis deserve further investigation.
Based on these findings, linear array epitope with Fc as a vaccine was proven to amplify immunogenicity, even in our adjuvant-free trail (data not shown). Our Fc-CETP6 vaccine may have potential application in the development of a therapeutic agent for both atherosclerosis and NASH in humans. However, the underlying cause of NASH is still not clear. Obesity, diabetes, insulin resistance, or intestinal bacteria have been proposed as risk factors for its development [45]. In this study, the HFC diet-fed rabbits did not develop obesity and diabetes. It is, therefore, not clear whether an anti-CETP vaccine can prevent NASH induced by other different factors in addition to the HFC diet, but this approach definitely merits further investigation.
Supporting Information
Immunofluorescence staining of ox-LDL in liver specimens. (A) Representative immunofluorescence staining of ox-LDL in liver specimens at the end of week 52nd. Control n = 7, Fc-CETP6 n = 8. Specimens stained with DAPI to visualize the nuclei (magnification, x100).
(TIF)
Quantification of liver ox-LDL. Quantification of ox-LDL positive stain area. The relative intensity in Y-axis of figure was calculated as “Mean Intensity/image captured area(µm xµm)”x100. Values are the mean ± SEM. **p<0.01.
(TIF)
Effects of the Fc-CETP6 vaccine on Hepatic Histology at 52 Weeks. Vaccination with Fc-CETP6 attenuates HFC diet-induced NASH and Fibrosis on Hepatic Histology at the end of week 52nd. Three sections (each approximately 1 cm3) were obtained from the right lobe liver of each rabbit (control group n = 7, Fc-CETP6 group n = 8). Ten images were taken randomly from each section. Randomly select 200 samples out of each group for data quantification.
(TIF)
Primer list of TR-PCR, AD-PCR and Quantitative-PCR.
(TIF)
Method S1. Immunofluorescence staining of the liver tissue. The presence of ox-LDL in the liver tissue sections was evaluated by immunofluorescence staining. The right lobe of each rabbit liver was obtained and embedded in OCT compound then stored at −20°C. The slides were cut to a thickness of 6 µm and fixed with ice-cold acetone. Primary antibody in 30 µl (rabbit polyclonal anti ox-LDL immunoglobulin (IgG, Calbiochem, Germany) was added. Slides incubated in the absence of primary antibody were used as negative control. After incubating for 30 min in a humid chamber at room temperature, slides were washed with PBS, and a fluorescein isothiocyanate labeled antihuman IgG (30 µl) was administered as a conjugate substance. After another 30 min at room temperature, the slides were washed with the standard PBS solution. After drying, the slides were covered with a mounting medium and examined under a fluorescence microscope (Leica DMRX, Wetzlar, Germany). Method S2. Quantification of ox-LDL staining procedure. The images were obtained from three sections of the right lobe liver from each rabbit (control group n = 7, Fc-CETP6 group n = 8). Ten images were taken randomly from each section. The relative intensity of ox-LDL positive staining was calculated from the program, Zen2010 (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA). The relative intensity in Y-axis of figure was calculated as “Mean Intensity/image captured area (µm xµm)” x100.
(DOC)
Acknowledgments
We thank Dr. Chang-Wen Chi for his great help of animal study.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by research grants NHRI-EX101-10153SI (http://goo.gl/bx7Wul) and NHRI-EX102-10153SI (http://goo.gl/w3824A) from the National Health Research Institutes of Taiwan. Dr. Shao-Chun Lu and Dr. Jaulang Hwang received the funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Glass CK, Witztum JL (2001) Atherosclerosis. the road ahead. Cell 104:503–516. [DOI] [PubMed] [Google Scholar]
- 2. Rader DJ (2006) Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest 116:3090–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, et al. (2010) Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 52:913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lucero D, Zago V, Lopez GI, Graffigna M, Lopez GH, et al. (2011) Does non-alcoholic fatty liver impair alterations of plasma lipoproteins and associated factors in metabolic syndrome? Clin Chim Acta 412:587–592. [DOI] [PubMed] [Google Scholar]
- 5. Musunuru K (2010) Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids 45:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bieghs V, van Gorp PJ, Walenbergh SM, Gijbels MJ, Verheyen F, et al. (2012) Specific immunization strategies against oxidized low-density lipoprotein: a novel way to reduce nonalcoholic steatohepatitis in mice. Hepatology 56:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieghs V, Wouters K, van Gorp PJ, Gijbels MJ, de Winther MP, et al. (2010) Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology 138:: 2477–2486, 2486 e2471–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Targher G, Day CP, Bonora E (2010) Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 363:1341–1350. [DOI] [PubMed] [Google Scholar]
- 9. Assmann G, Schulte H, von Eckardstein A, Huang Y (1996) High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 124 Suppl: S11–20 [DOI] [PubMed] [Google Scholar]
- 10. Inazu A, Brown ML, Hesler CB, Agellon LB, Koizumi J, et al. (1990) Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med 323:1234–1238. [DOI] [PubMed] [Google Scholar]
- 11. Barter PJ, Rye KA (2012) Cholesteryl ester transfer protein inhibition as a strategy to reduce cardiovascular risk. J Lipid Res 53:1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honey K (2007) Drug designed to raise HDL levels falls down. J Clin Invest 117:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhainds D, Arsenault BJ, Brodeur MR, Tardif JC (2012) An update on the clinical development of dalcetrapib (RO4607381), a cholesteryl ester transfer protein modulator that increases HDL cholesterol levels. Future Cardiol 8:513–531. [DOI] [PubMed] [Google Scholar]
- 14. Rader DJ, deGoma EM (2014) Future of cholesteryl ester transfer protein inhibitors. Annu Rev Med 65:385–403. [DOI] [PubMed] [Google Scholar]
- 15. Davidson MH, Maki K, Umporowicz D, Wheeler A, Rittershaus C, et al. (2003) The safety and immunogenicity of a CETP vaccine in healthy adults. Atherosclerosis 169:113–120. [DOI] [PubMed] [Google Scholar]
- 16. Swanson JA, Hoppe AD (2004) The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol 76:1093–1103. [DOI] [PubMed] [Google Scholar]
- 17. Gosselin EJ, Wardwell K, Gosselin DR, Alter N, Fisher JL, et al. (1992) Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J Immunol 149:3477–3481. [PubMed] [Google Scholar]
- 18. Ota H, Takashima Y, Hayashi Y, Matsumoto Y (2006) A fusion protein of IgG fc and mouse-derived antigen on the surface of pseudorabies virus particles does not accelerate production of harmful auto-reactive antibodies. J Vet Med Sci 68:1179–1183. [DOI] [PubMed] [Google Scholar]
- 19. Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL (2007) Activating B cell signaling with defined multivalent ligands. ACS Chem Biol 2:252–262. [DOI] [PubMed] [Google Scholar]
- 20. Hsu CT, Ting CY, Ting CJ, Chen TY, Lin CP, et al. (2000) Vaccination against gonadotropin-releasing hormone (GnRH) using toxin receptor-binding domain-conjugated GnRH repeats. Cancer Res 60:3701–3705. [PubMed] [Google Scholar]
- 21. Tall AR (1986) Plasma lipid transfer proteins. J Lipid Res 27:361–367. [PubMed] [Google Scholar]
- 22. Ogawa T, Fujii H, Yoshizato K, Kawada N (2010) A human-type nonalcoholic steatohepatitis model with advanced fibrosis in rabbits. Am J Pathol 177:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaofu Q, Dan M, Jie W, Liao Z, Li Z, et al. (2004) Long-lasting specific antibodies against CETP induced by subcutaneous and mucosal administration of a 26-amino acid CETP epitope carried by heat shock protein 65 kDa in the absence of adjuvants. Vaccine 22:3187–3194. [DOI] [PubMed] [Google Scholar]
- 24. Chang PY, Lu SC, Su TC, Chou SF, Huang WH, et al. (2004) Lipoprotein-X reduces LDL atherogenicity in primary biliary cirrhosis by preventing LDL oxidation. J Lipid Res 45:2116–2122. [DOI] [PubMed] [Google Scholar]
- 25. Chiang HL, Lin CY, Jan FD, Lin YS, Hsu CT, et al. (2012) A novel synthetic bipartite carrier protein for developing glycotope-based vaccines. Vaccine 30:7573–7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, et al. (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 28. Smith JD (2010) Apolipoprotein A-I and its mimetics for the treatment of atherosclerosis. Curr Opin Investig Drugs 11:989–996. [PMC free article] [PubMed] [Google Scholar]
- 29. Chalasani N, Deeg MA, Crabb DW (2004) Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 99:1497–1502. [DOI] [PubMed] [Google Scholar]
- 30. Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874. [DOI] [PubMed] [Google Scholar]
- 31. Buyssens N, Kockx MM, Herman AG, Lazou JM, Van den Berg K, et al. (1996) Centrolobular liver fibrosis in the hypercholesterolemic rabbit. Hepatology 24:939–946. [DOI] [PubMed] [Google Scholar]
- 32. Rittershaus CW, Miller DP, Thomas LJ, Picard MD, Honan CM, et al. (2000) Vaccine-induced antibodies inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol 20:2106–2112. [DOI] [PubMed] [Google Scholar]
- 33. Bieghs V, Rensen PC, Hofker MH, Shiri-Sverdlov R (2012) NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis 220:287–293. [DOI] [PubMed] [Google Scholar]
- 34. Brea A, Mosquera D, Martin E, Arizti A, Cordero JL, et al. (2005) Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol 25:1045–1050. [DOI] [PubMed] [Google Scholar]
- 35. Cheng AM, Handa P, Tateya S, Schwartz J, Tang C, et al. (2012) Apolipoprotein A-I attenuates palmitate-mediated NF-kappaB activation by reducing Toll-like receptor-4 recruitment into lipid rafts. PLoS One 7:e33917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patel S, Di Bartolo BA, Nakhla S, Heather AK, Mitchell TW, et al. (2010) Anti-inflammatory effects of apolipoprotein A-I in the rabbit. Atherosclerosis 212:392–397. [DOI] [PubMed] [Google Scholar]
- 37. Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE (2008) Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation 118:2506–2514. [DOI] [PubMed] [Google Scholar]
- 38. Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, et al. (2010) Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 363:2406–2415. [DOI] [PubMed] [Google Scholar]
- 39. Brousseau ME, Millar JS, Diffenderfer MR, Nartsupha C, Asztalos BF, et al. (2009) Effects of cholesteryl ester transfer protein inhibition on apolipoprotein A-II-containing HDL subspecies and apolipoprotein A-II metabolism. J Lipid Res 50:1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brousseau ME, Diffenderfer MR, Millar JS, Nartsupha C, Asztalos BF, et al. (2005) Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler Thromb Vasc Biol 25:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clark RW, Ruggeri RB, Cunningham D, Bamberger MJ (2006) Description of the torcetrapib series of cholesteryl ester transfer protein inhibitors, including mechanism of action. J Lipid Res 47:537–552. [DOI] [PubMed] [Google Scholar]
- 42. Zhang L, Yan F, Zhang S, Lei D, Charles MA, et al. (2012) Structural basis of transfer between lipoproteins by cholesteryl ester transfer protein. Nat Chem Biol 8:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blake WL, Ulrich RG, Marotti KR, Melchior GW (1994) The development of fatty liver is accelerated in transgenic mice expressing cynomolgus monkey cholesteryl ester transfer protein. Biochem Biophys Res Commun 205:1257–1263. [DOI] [PubMed] [Google Scholar]
- 44. Bhatia LS, Curzen NP, Calder PC, Byrne CD (2012) Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J 33:1190–1200. [DOI] [PubMed] [Google Scholar]
- 45. Farrell GC, Larter CZ (2006) Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43:S99–S112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunofluorescence staining of ox-LDL in liver specimens. (A) Representative immunofluorescence staining of ox-LDL in liver specimens at the end of week 52nd. Control n = 7, Fc-CETP6 n = 8. Specimens stained with DAPI to visualize the nuclei (magnification, x100).
(TIF)
Quantification of liver ox-LDL. Quantification of ox-LDL positive stain area. The relative intensity in Y-axis of figure was calculated as “Mean Intensity/image captured area(µm xµm)”x100. Values are the mean ± SEM. **p<0.01.
(TIF)
Effects of the Fc-CETP6 vaccine on Hepatic Histology at 52 Weeks. Vaccination with Fc-CETP6 attenuates HFC diet-induced NASH and Fibrosis on Hepatic Histology at the end of week 52nd. Three sections (each approximately 1 cm3) were obtained from the right lobe liver of each rabbit (control group n = 7, Fc-CETP6 group n = 8). Ten images were taken randomly from each section. Randomly select 200 samples out of each group for data quantification.
(TIF)
Primer list of TR-PCR, AD-PCR and Quantitative-PCR.
(TIF)
Method S1. Immunofluorescence staining of the liver tissue. The presence of ox-LDL in the liver tissue sections was evaluated by immunofluorescence staining. The right lobe of each rabbit liver was obtained and embedded in OCT compound then stored at −20°C. The slides were cut to a thickness of 6 µm and fixed with ice-cold acetone. Primary antibody in 30 µl (rabbit polyclonal anti ox-LDL immunoglobulin (IgG, Calbiochem, Germany) was added. Slides incubated in the absence of primary antibody were used as negative control. After incubating for 30 min in a humid chamber at room temperature, slides were washed with PBS, and a fluorescein isothiocyanate labeled antihuman IgG (30 µl) was administered as a conjugate substance. After another 30 min at room temperature, the slides were washed with the standard PBS solution. After drying, the slides were covered with a mounting medium and examined under a fluorescence microscope (Leica DMRX, Wetzlar, Germany). Method S2. Quantification of ox-LDL staining procedure. The images were obtained from three sections of the right lobe liver from each rabbit (control group n = 7, Fc-CETP6 group n = 8). Ten images were taken randomly from each section. The relative intensity of ox-LDL positive staining was calculated from the program, Zen2010 (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA). The relative intensity in Y-axis of figure was calculated as “Mean Intensity/image captured area (µm xµm)” x100.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.