Abstract
Background
Predictors of death in hospitalized HIV-infected patients have not been previously reported in Bangladesh.
Objective
The primary aim of this study was to determine predictors of death among hospitalized HIV-infected patients at a large urban hospital in Bangladesh.
Methods
A study was conducted in the HIV in-patient unit (Jagori Ward) of icddr,b's Dhaka Hospital. Characteristics of patients who died during hospitalization were compared to those of patients discharged from the ward. Bivariate analysis was performed to determine associations between potential risk factors and death. Multivariable logistic regression was used to identify factors independently associated with death.
Results
Of 293 patients admitted to the Jagori Ward, 57 died during hospitalization. Most hospitalized patients (67%) were male and the median age was 35 (interquartile range: 2–65) years. Overall, 153 (52%) patients were diagnosed with HIV within 6 months of hospitalization. The most common presumptive opportunistic infections (OIs) identified were tuberculosis (32%), oesophageal candidiasis (9%), Pneumocystis jirovecii pneumonia (PJP) (8%), and histoplasmosis (7%). On multivariable analysis, independent predictors of mortality were CD4 count ≤200 cells/mm3 (adjusted odds ratio [aOR]: 16.6, 95% confidence interval [CI]: 3.7–74.4), PJP (aOR: 18.5, 95% CI: 4.68–73.3), oesophageal candidiasis (aOR: 27.5, 95% CI: 5.5–136.9), malignancy (aOR:15.2, 95% CI: 2.3–99.4), and bacteriuria (aOR:7.9, 95% CI: 1.2–50.5). Being on antiretroviral therapy prior to hospitalization (aOR: 0.2, 95% CI: 0.06–0.5) was associated with decreased mortality.
Conclusion
This study showed that most patients who died during hospitalization on the Jagori Ward had HIV-related illnesses which could have been averted with earlier diagnosis of HIV and proper management of OIs. It is prudent to develop a national HIV screening programme to facilitate early identification of HIV.
Background
As of 2012, an estimated 35.3 million people were living with human immunodeficiency virus (HIV) globally and among them 2.3 million were newly infected [1]. In the Asia Pacific region, the estimated number of new HIV infections was 350,000 (220,000–550,000) and the number of AIDS-related deaths across the region was 270,000 (190,000–360,000) [2]. By the end of 2013, the Ministry of Health and Family Welfare (MOHFW) of Bangladesh had documented 3,241 persons with confirmed HIV, including 1,299 persons who developed AIDS and 472 persons who had died [3]. With an estimated HIV prevalence of <0.1% in the general population [3], Bangladesh has been considered a low HIV prevalence country [4] since the first case was detected in 1989 [5]. Despite these low numbers, Bangladesh is one of the nine countries in the world in which the estimated HIV incidence increased by >25% between 2001 and 2011 [6].
Diagnosis of HIV in Bangladesh is often delayed for a variety of reasons, including lack of awareness of HIV infection due to its low prevalence, the limited number of HIV testing and counseling facilities, which is often a critical entry point for engagement into care and prevention, and the unwillingness of many people to be tested [7], [8]. As a result, HIV infected persons are diagnosed in late stages of the disease and most hospitalized patients with HIV have a CD4 count of <200 cells/mm3 [9]. The Dhaka Hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) opened the Jagori Ward, an inpatient unit for the management and treatment of people living with HIV (PLHIV), in May 2008. The experience from the Jagori Ward's first 21 months (from May 2008 to February 2010) has been previously reported; we found that 38% of those who died had active tuberculosis (TB) and that the majority of patients admitted to the ward had a CD4 cell count <50 cells/mm3 [9]. However, we were unable to determine independent risk factors associated with death due to low sample size and the limited number of variables collected. Little information is available regarding the clinical presentation of AIDS in Bangladesh and there is no literature on predictors of mortality among HIV-infected persons hospitalized in Bangladesh. The primary objective of this study was to determine independent predictors of death during hospitalization. A secondary objective was to estimate the proportion of deaths among those hospitalized on the Jagori Ward. Such data could provide critical information about severity of HIV in PLHIV and thus lead to improvement of the clinical management of PLHIV in this low-prevalence country.
Materials and Methods
Study design
This was a retrospective observational study conducted from February 2009 –December 2012. Data were collected after extraction of electronic medical records of patients hospitalized on the Jagori ward. Patient's information was anonymized and de-identified prior to analysis. This study (protocol number: PR-12078) was approved by icddr,b's Research Review and Ethical Review Committees. It also received approval from the Centers for Disease Control and Prevention, USA, as not involving human subject research.
Study setting
Dhaka Hospital's Jagori Ward has provided in-patient services for HIV-infected patients and served as a major referral center for PLHIV since May 2008. Currently, it admits over 100 PLHIV annually. In February 2009, Dhaka Hospital implemented an electronic medical-record system whereby patients are provided a unique patient identification number on each admission. All personal and clinical data collected are recorded in this access-protected medical record system. The majority of patients admitted to the Jagori Ward are referred by peer-led non-governmental HIV care and support groups (hereafter referred to as nongovernmental organizations or NGOs), and the most of the remaining patients were referred from government and private hospitals. If not already engaged with an NGO, patients are introduced to NGOs prior to discharge and the NGOs are then responsible for providing antiretroviral (ARV) medication and primary care to the patients. Following medical record extraction of variables described below, all identifiable patient's information was deleted from the research database.
Study population
All confirmed HIV-infected patients who were admitted to the Jagori Ward during the study period were enrolled in this study. Data were collected only for first admissions during the study period and included data obtained until discharge or death during that admission, though information about deaths during subsequent hospitalizations in the course of the study period were collected. Unique study identification numbers were generated to distinguish re-admission and follow-up visits. For this analysis, we only used data collected from patients that were entered into the electronic medical record system.
Study definitions
During admission, a trained counselor obtained social histories from each patient and entered these into their medical records. The self-reported route of HIV transmission, self-reported high-risk sexual behaviors (including having multiple sex partners, sex with commercial sex workers, or male-male sex), receipt of blood and blood products, injection drug use, and for children, having a parent with HIV infection, were recorded. Information on migrant work history was obtained. Migrant work history was defined as history of work outside of Bangladesh by patients or their spouses or by parents of pediatric patients. By the term “HIV-related conditions” we understood characteristics consistent with World Health Organization (WHO) stage IV AIDS defining illnesses/infection [10]. Clinical staging of HIV and management of opportunistic infections (OIs) were performed according to WHO guidelines [10], [11].
Diagnostic criteria for presumptive OIs were as follows: TB – clinical features suggestive of TB with characteristic radiological features and/or smear-positive for acid-fast bacilli; Pneumocystis jirovecii pneumonia (PJP) – suggestive clinical features including hypoxemia (SaO2 <90%) [12], CD4 cell count <200 cells/mm3, bilateral, diffuse interstitial infiltrates on chest radiograph, and/or high lactate dehydrogenase enzyme (LDH) in the absence of TB or fungal infection [10]; histoplasmosis – characteristic umbilicated skin eruptions with prolonged fever and/or features of bone marrow suppression with CD4 cell count <100/mm3 and high LDH and/or detection of fungal elements consistent with Histoplasma capsulatum by histology from skin, lymph nodes, or bone marrow [10]; oesophageal candidiasis – creamy white, plaque-like lesions extending beyond the oropharynx with odynophagia, anorexia, or burning sensation in the throat with CD4 cell count <200/mm3 [11]; cytomegalovirus infection (retinitis, colitis, oesophagitis) – GI symptoms (bloody diarrhea, abdominal pain, colitis) or visual impairment and characteristic fundoscopy features and CD4 cell count <50/mm3 [10]; cerebral toxoplasmosis – features of encephalitis with focal neurological signs and multiple lesions in neuroimaging of brain and/or positive anti-toxoplasma immunoglobulin G (IgG) and CD4 cell count <100/µl [11]; cryptococcal meningitis – features of meningitis with CD4 cell count <100/µl and cerebrospinal fluid findings suggestive of meningitis with suggestive neuroimaging [10], [11].
Data management and analysis
The following variables were extracted from the medical records of Jagori Ward patients: age, gender, exposure history, duration of HIV diagnosis, presenting symptoms, physical examination findings, WHO staging at discharge/death, AIDS-defining illnesses, nadir and most recent CD4 cell counts, taking ARV medications at the time of admission, and taking co-trimoxazole at the time of admission. For deceased patients, duration of hospitalization was recorded and causes of death were extracted from death certificates and for the discharged patients duration of hospitalization and diagnosis on discharge were extracted from the discharge certificate of the same medical records. All data were entered into a database (SPSS version 17.0; Chicago, IL, USA). Differences in proportions were compared by the Chi-square test or Fisher-Exact test as appropriate. Differences in means were compared by Student's t test (for normally distributed data) or Mann-Whitney test (for data that were not normally distributed). P-values less than 0.05 were considered statistically significant. Strength of association was determined by calculating adjusted odds ratios (aOR) and their 95% confidence intervals (CIs).
In identifying independent factors associated with deaths in patients, variables were initially analyzed using a bivariate model, then independent predictors were identified using a logistic regression model using a backward, stepwise approach beginning with inclusion of all variables significantly associated with death on bivariate analysis (p-value ≤0.05) and including only those variables with p-values ≤0.05 in the final model.
Results
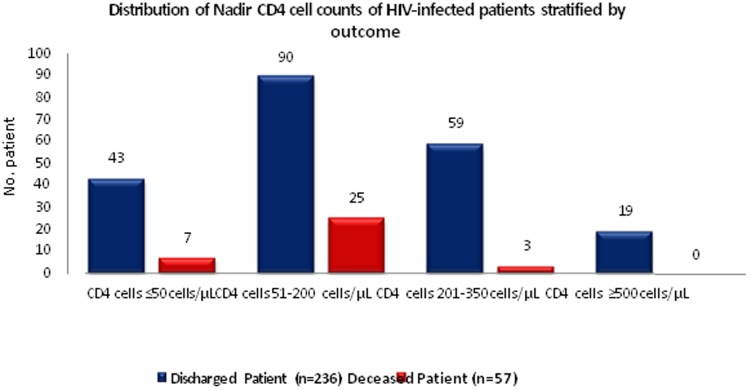
We reviewed 651 hospital admission records of the Jagori Ward by HIV-infected individuals during February 2009 to December 2012; this included admissions of 293 unique and 358 repeat admissions by these individuals. Of the 293 unique patients admitted to the Jagori Ward, there were 57 (19%) deaths during the first visit. Sixty-seven percent of the hospitalized patients were male and the median age was 35 (range: 2–65) years. The median duration of hospitalization was 6 (interquartile range: 1–87) days. Seventy-seven percent of patients had a history of migrant work. A total of 24 (8%) of patients reported a history of injection drug use. Comparison of baseline characteristics and laboratory findings for deceased and discharged patients are shown in Table 1. The nadir CD4 cell counts and WHO stages of deceased and discharged patients are shown in Fig. 1.
Table 1. Baseline characteristics of the HIV-infected patients stratified by outcome.
| Characteristic | Deceased (n = 57) | Discharged (n = 236) | OR (95% CI) | P-value | |
| Gender | |||||
| Male (%) | 40 (70) | 155 (66) | 1.2 (0.6–2.4) | 0.62 | |
| Female (%) | 17 (30) | 81 (34) | |||
| Age in years, median (range) | 36 (9–60) | 35 (2–65) | ---- | 0.19 | |
| Exposure history | |||||
| High-risk sexual exposure | 47 (83) | 193 (82) | 1.0 (0.4–2.4) | 0.89 | |
| Injection drug user | 7 (12) | 17 (7) | 0.5 (0.2–1.57) | 0.21 | |
| Mother-to child transmission | 1 (2) | 21 (9) | 5.4 (0.7–111.5) | 0.06 | |
| Blood product | 1 (2) | 4 (2) | 0.9 (0.1–23.1) | 0.97 | |
| Unknown | 0 | 2(1) | undefined | ||
| History of migrant work | |||||
| Migrant worker | 33 (58) | 120 (51) | 0.75 (0.4–1.4) | 0.33 | |
| Spouse of migrant worker | 7 (12) | 46 (20) | 1.73 (0.7–4.5) | 0.2 | |
| Child of migrant worker | 1 (2) | 16 (7) | 4.07 (0.5–84.1) | 0.14 | |
| None (no relation with migrant work) | 16 (28) | 54 (23) | 0.76 (0.4–1.5) | 0.4 | |
| New HIV diagnosis (<6 months prior to admission) | 38(67) | 115(49) | 2.1(1.1–4.0) | 0.02 | |
| WHO clinical staging on admission | |||||
| Stage I | 0 | 52 (22) | undefined | 0.001 | |
| Stage II | 0 | 79 (34) | undefined) | 0.001 | |
| Stage III | 9 (16) | 79 (34) | 2.7 (1.2–6.2) | 0.001 | |
| Stage IV | 48 (84) | 26 (11) | 0.02 (0.01–0.06) | <0.001 | |
| Nadir CD4 cell count (≤200/mm 3) | 54 (95) | 130 (55) | 14.5 (4.4–60.6) | <0.001 | |
| Current ART use | 23 (40) | 145 (67) | 0.3 (0.2–0.6) | <0.001 | |
| Most recent CD4 cell count/mm3 (median, IQR) | 47 (3–236) | 161 (5–1549) | ---- | <0.001 | |
| Haemoglobin (gm/dl) (median, IQR) | 7.3 (2.8–13.8) | 10.30 (2.7–15.90) | ----- | <0.001 | |
| Total white cell count(1000/mm3) (median, IQR) | 6.5 (0.50–42.11) | 6.0 (0.88–37.84) | ----- | <0.54 | |
Figures represent n (%), unless specified. OR: odds ratio. CI: confidence interval. IQR: interquartile range.
Figure 1. Distribution of Nadir CD4 cell counts of HIV-infected patients stratified by outcome.
Diagnoses stratified by outcome are shown in Table 2. Among the 93 patients with presumptive TB diagnoses, 48 (53%) had pulmonary TB, 5 (6%) had TB meningitis and the rest had various other forms of extrapulmonary TB. A total of 11 patients were diagnosed with malignancies: four had non-Hodgkin's lymphoma, two had central nervous system tumours, and one each had Kaposi's sarcoma, mediastinal sarcoma, cervical cancer, carcinoma of the tongue and adenocarcinoma of the colon. The mean nadir CD4 cell count was lower for patients who died (69±60 cells/mm3) than for those who were discharged (223±216 cells/mm3). Cytomegalovirus infection, cerebral Toxoplasmosis, Cryptococcal meningitis and Herpes viral infection were not associated with death on bivariate analysis.
Table 2. Diagnoses of HIV-infected patients stratified by outcome.
| Clinical condition | Deceased | Discharged | OR (95% CI) | P-value |
| (n = 57) | (n = 236) | |||
| Presumptive tuberculosis | 26 (46) | 67 (28) | 2.1 (1.1–3.9) | 0.02 |
| Presumptive Pneumocystis jiroveci pneumonia | 15 (26) | 8 (3) | 10.2 (3.8–28.2) | <0.001 |
| Presumptive histoplasmosis | 12 (21) | 9 (4) | 6.7 (2.5–18.6) | <0.001 |
| Presumptive oesophasial candidiasis | 15 (26) | 12 (5) | 6.7 (2.7–16.5) | <0.001 |
| Malignancy * | 5 (9) | 6 (3) | 3.7 (0.9–14.3) | 0.04 |
| Presumptive Cytomegalovirus infection | 4 (7) | 11 (5) | 1.5 (0.4–5.5) | 0.5 |
| Presumptive Cerebral Toxoplasmosis | 2 (4) | 1 (1) | 8.6 (0.5–242.6) | 0.09 |
| Presumptive Cryptococcal meningitis | 1 (2) | 5 (2) | — | 0.48 |
| Herpes viral infection (H. simplex & H. zoster) | 0 | 8 (3) | — | 0.36 |
| Bacteremia | 21 (37) | 22 (9) | 5.7 (2.7–12.0) | <0.001 |
| Isolation of multiple organisms in blood | 5 (9) | 1 (1) | 22.6 (2.5–522.1) | <0.001 |
| Bacteriuria | 21 (38) | 43 (18) | 2.7 (1.4–5.3) | 0.003 |
*Non-Hodgkin's lymphoma (4 cases), central nervous system tumors (2 cases), and Kaposi's sarcoma (1 case), mediastinal sarcoma (1 case), cervical cancer (1 case), carcinoma of the tongue (1 case) and adenocarcinoma of the colon (1 case).
Figures represent n (%), unless specified. OR: odds ratio. CI: confidence interval. IQR: interquartile range.
Factors independently associated with death included CD4 count ≤200 cells/µl (OR = 16.6, 95% CI = 3.72–74.38), Pneumocystis jiroveci pneumonia (OR = 18.5, 95% CI = 4.68–73.26), esophageal candidiasis (OR = 27.5, 95% CI = 5.54–136.90), malignancy (OR = 15.21, 95% CI = 2.33–99.42), and bacteriuria (OR = 7.9, 95% CI = 1.25–50.51). Patients getting ARV prior to admission was found to be a negative predictor (OR = 0.17, 95% CI = 0.06–0.46). [Table 3]
Table 3. Independent predictors of death in hospitalized HIV-infected patients.
| Unadjusted | Adjusted | |||
| Predictors | OR (95%CI) | p value | OR (95% CI) | p value |
| Nadir CD4 cell count ≤200/mm3 | 14.5 (4.4–60.6) | <0.001 | 16.6 (3.7–74.4) | <0.0001 |
| Presumptive Pneumocystis jiroveci pneumonia | 10.2 (3.8–28.2) | <0.001 | 18.5 (4.7–73.3) | <0.0001 |
| Presumptive osophageal candidiasis | 6.7 (2.7–16.5) | <0.001 | 27.5 (5.5–136.9) | <0.0001 |
| Malignancy | 3.7 (0.9–14.3) | 0.04 | 15.2 (2.3–99.4) | <0.004 |
| Bacteriuria | 2.7 (1.4–5.3) | 0.003 | 7.9 (1.2–50.5) | 0.02 |
| Current antiretroviral use | 0.3 (0.2–0.6) | <0.001 | 0.2 (0.06–0.46) | <0.001 |
Adjustment variables: New HIV diagnosis, presumptive TB, presumptive histoplasma, bacteremia and isolation of multiple organisms in blood.
OR indicates odds ratio. CI indicates confidence interval.
Discussion
This report identifies important clinical characteristics and predictors of mortality in hospitalized HIV-infected persons in Bangladesh. Our study demonstrated rates of OIs and inpatient deaths similar to those reported in resource-limited countries with high HIV burden [13], [14]. We also found that most deaths in hospitalized HIV-infected patients are associated with OIs, AIDS-related malignancies, and bacterial infections, similar to reports from other low-income countries [13], [14]. Although available data did not allow us to determine actual causes of death (due to the absence of autopsy facilities and lack of societal acceptance of routine use of autopsies), the high proportions of patients with AIDS-related illnesses who died during hospitalization suggest that most deaths were AIDs-related. This is in contrast to reports from high–income countries, where deaths in HIV-infected patients are now mostly due to non-AIDS related causes [15], [16] and where national screening and early intervention programs are much more widespread.
As expected, nadir CD4 cell count ≤200/mm3 was found to be an independent predictor of death, which is similar to findings described in other reports [17], [18]. Individuals hospitalized within six months of HIV diagnosis with were more likely to die during hospitalization. This may be due to diagnosis at an advanced stage of HIV infection. Delays in HIV diagnosis in Bangladesh are likely primarily due to limited access to testing, unwillingness of many to be tested and difficulty identifying individuals who do not have commonly recognized risk factors for infection and thus escaped the routine screening among risk groups [19], [20].
Since the first case of HIV was identified in Bangladesh, the responses and initiatives taken to control and prevent the spread of the disease have included safe needle exchange programs, free condom distribution to sex workers in brothels, safe sex education among peer groups and, recently, the implementation of a methadone program for injection drug users [21]. More than three-quarters of patients in this study had a history of migrant work (Table 1), which has recently been identified as a risk factor for HIV infection in Bangladesh [22], [23]. A recent report from Dhaka Hospital found that among persons diagnosed with HIV infection by provider-initiated HIV Testing and Counselling, 67% of HIV-infected patients had a history of migrant work [24]. Until recently, persons with a history of migrant work were not targeted for HIV prevention interventions as they had not been identified as a group at high-risk for HIV infection [22], [24]. However, it is not known what proportion of HIV-infected persons with history of migrant work were infected abroad or what the risk behaviours of many of those who acquired HIV abroad were. It has been reported that extramarital sex is 2–3 times higher in spouses who live apart, and there is higher proportion of reported sex with female sex workers among male migrant workers [25]. A total of 8% of patients in this study reported injection drug use, compared to 10% of Jagori Ward patients who reported such behaviour in a 2011 report [9].
Consistent with reports from other resource-limited settings, we found that OIs were associated with death among hospitalized HIV-infected persons [14], [20]. Despite a demonstrated reduction of PJP-related mortality after the introduction of ARV medications in other high-income countries [26], [27], our study demonstrated that PJP is an independent predictor of death in HIV-infected patients. In studies from Africa, it has been reported that PJP is the primary cause of death in patients with low CD4 cell counts, despite the availability of prophylaxis against and effective treatment for PJP [17], [28], underscoring the need for early suspicion and treatment of PJP even in the absence of adequate laboratory facilities for confirming the diagnosis.
Our study also showed that having a malignancy was an independent predictor for death. Most malignancies identified were AIDS-related. Our findings are consistent with findings that malignancies are common in HIV-infected individuals, even in those with CD4 cell count >200/mm3 [28], and underscore the need for surveillance for malignancies among all HIV-infected individuals.
We found bacteriuria to be independently associated with death. Urinary tract infections occur at increased rates among HIV-infected persons [29], and bacteriuria is more common in those with very low CD4 cell counts [30], which could explain our findings. However, it is also possible bacteriuria is a surrogate marker of disease severity, as urine analyses and urine culture are more commonly performed among those presenting with more serious illnesses.
TB was the most common OI identified among patients in this study, though it was not found to be an independent predictor for death. TB is endemic in the general population in Bangladesh, which may explain why it was not more common among those who died than among those who were discharged in this study. Although we did not find that TB was an independent predictor of death, coinfection with HIV and TB is an important issue in Bangladesh and all persons who are infected with HIV should be screened for active TB.
There are several limitations of this study. First, this was a retrospective, observational study and may have been subject to incorrect interpretation of information. Second, data were missing for some variables and little information available about causes of death. Third, due to laboratory constraints, we typically had to rely on clinical manifestations for OI diagnoses, because confirmatory diagnostic testing results were frequently not available; this could have led to misclassification of some presumptive OI diagnoses and failure to detect OIs in some patients. Finally, this report reflects the experience of just one urban hospital that primarily provides care for patients with late stage HIV disease and so may not be generalizable to HIV-infected patients admitted to other hospitals in Dhaka or elsewhere in Bangladesh.
In conclusion, this study describes factors associated with mortality among hospitalized HIV-infected patients in a country with low HIV prevalence. Most of the factors identified have already been reported in medical literature but original as local data, and could be mitigated or prevented by earlier diagnosis of HIV infection and improved access to care. Expansion of voluntary counselling and testing, and widespread education about HIV and AIDS might lead to increased access to and acceptability of HIV screening. Dissemination of information to providers regarding HIV risk behaviours and clinical features of HIV should also be promoted to increase provider-initiated testing and counselling in healthcare facilities. In addition, it will be critical to increase and improve the training of providers in Bangladesh on HIV care practices, including treatment with ARV medications, prophylaxis against OIs, and the treatment and prevention of other AIDS-related illnesses.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. We express our sincere thanks and gratitude to all physicians including clinical fellows, nurses, counsellors, members of feeding team and cleaners of Jagori Ward and the nongovernmental organizations that provide care and support for HIV-infected persons for their valuable support and contributions during data collection.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data are available from the icddrb Institutional Research Unit for researchers who meet the criteria for access to confidential data. The following is the institutional website link- www.icddrb.org
Funding Statement
This research protocol was funded by the icddr,b and its donors who provide unrestricted support to this organization for its infrastructure (such as Dhaka Hospital) and research. Current donors providing unrestricted support include the following: Australian Agency for International Development (AusAID), Government of the People's Republic of Bangladesh, Canadian International Development Agency, Embassy of the Kingdom of Netherlands, Swedish International Development Cooperation Agency, Swiss Agency for Development and Cooperation and Department for International Development, UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: United Nations Programme on HIV/AIDS, 2013. Available: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf; Accessed: 2013 Dec 24.
- 2.UNAIDS (2013) HIV in Asia and the Pacific: UNAIDS report 2013. Available: http://www.aidsdatahub.org/HIV-in-Asia-and-the-Pacific-UNAIDS-Report-2013 Accessed: 2014 Jan 10.
- 3.Dhaka Tribune. World AIDS day observed:370 new HIV cases detected in the country. Dhaka tribune 2 Dec 2 2013. Available: http://www.dhakatribune.com/health/2013/dec/02/world-aids-day-observed; Accessed: 2013 Dec 31.
- 4. Azim T, Rahman M, Alam M, Chowdhury I, Khan R, et al. (2008) Bangladesh moves from being a low-prevalence nation for HIV to one with a concentrated epidemic in injecting drug users. International journal of STD & AIDS 19:327–331. [DOI] [PubMed] [Google Scholar]
- 5. Mamtaz A (1999) HIV/AIDS: response to the pandemic in Bangladesh. Journal of preventive and social medicine 18:74–83. [PubMed] [Google Scholar]
- 6.UNAIDS (2012) A progress report on the Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Available: www.unaids.org/en/media/…/JC2385_ProgressReportGlobalPlan_en.pdf. 1–24 p. Accessed: 2013 Jan 02.
- 7.Azim T, Khan SI, Nahar Q, Reza M, Alam N, et al. (2009) 20 years of HIV in Bangladesh: experiences and way forward. Dhaka: World Bank, 2009:, 1–201 p. Available: www.hnpinfobangladesh.com/…/di_220_Consolidated%20Report%20N. Accessed: 2013 Mar 23. [Google Scholar]
- 8. Bunnell R, Opio A, Musinguzi J, Kirungi W, Ekwaru P, et al. (2008) HIV transmission risk behavior among HIV-infected adults in Uganda: results of a nationally representative survey. Aids 22:617–624. [DOI] [PubMed] [Google Scholar]
- 9. Matin N, Shahrin L, Pervez MM, Banu S, Ahmed D (2011) Clinical profile of HIV/AIDS-infected patients admitted to a new specialist unit in Dhaka, Bangladesh–a low-prevalence country for HIV. J Health Popul Nutr 29:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (2006) WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. HIV/AIDS Programme. Strengthening health services to fight HIV/AIDS. Available: www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. Geneva. pp. 1–52. Accessed: 2013 Feb 13.
- 11.Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, et al. (2009) Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Pp. 1–207. [PubMed]
- 12.World Health Organization (2013) Pocket book for hospital care of children: guidelines for the managemnet of common illness with limited resources. Geneva. Available: http://www.who.int/maternal_child_adolescent/documents/child_hospital_care/en/. Accessed: 2014 Apr 09.
- 13. Jerene D, Endale A, Hailu Y, Lindtjørn B (2006) Predictors of early death in a cohort of Ethiopian patients treated with HAART. BMC infectious diseases 6:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agaba PA, Digin E, Makai R, Apena L, Agbaji OO, et al. (2011) Clinical characteristics and predictors of mortality in hospitalized HIV-infected Nigerians. The Journal of Infection in Developing Countries 5:377–382. [DOI] [PubMed] [Google Scholar]
- 15. Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, et al. (2006) Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. JAIDS Journal of Acquired Immune Deficiency Syndromes 41:194–200. [DOI] [PubMed] [Google Scholar]
- 16. Krentz H, Dean S, Gill M (2006) Longitudinal assessment (1995–2003) of hospitalizations of HIV-infected patients within a geographical population in Canada. HIV Med 7:457–466. [DOI] [PubMed] [Google Scholar]
- 17.Schneider M, Zwahlen M, Egger M (2005) Natural history and mortality in HIV-positive individuals living in resource-poor settings: A literature review. UNAIDS Obligation HQ/03/463871 UNAIDS Obligation HQ/03/463871.
- 18. Kumarasamy N, Solomon S, Flanigan TP, Hemalatha R, Thyagarajan S, et al. (2003) Natural history of human immunodeficiency virus disease in southern India. Clinical Infectious Diseases 36:79–85. [DOI] [PubMed] [Google Scholar]
- 19. Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV (2006) Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Annals of Internal Medicine 145:397–406. [DOI] [PubMed] [Google Scholar]
- 20. Sani MU, Mohammed AZ, Adamu B, Yusuf SM, Samaila AA, et al. (2006) AIDS mortality in a tertiary health institution: A four-year review. Journal of the National Medical Association 98:862. [PMC free article] [PubMed] [Google Scholar]
- 21. Azim T, Khan SI, Haseen F, Huq NL, Henning L, et al. (2008) HIV and AIDS in Bangladesh. Journal of health, population, and nutrition 26:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weine SM, Kashuba AB (2012) Labor migration and HIV risk: a systematic review of the literature. AIDS Behav 16:1605–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Organization for Migration (IOM) (2013) Situation Analysis on Migration and HIV in Bangladesh. pp. 1–43. Available: http://hpnconsortium.org/materials/material-detail/69/1/2. Accessed: 2012 Jun 23.
- 24. Pervez MM (2013) HIV counselling and testing at icddr,b's Dhaka Hospital. Health and Science Bulletin 11:15–21. [Google Scholar]
- 25. Mercer A, Khanam R, Gurley E, Azim T (2007) Sexual risk behaviour of married men and women in Bangladesh associated with husbands' work migration and living apart. Sex Transm Dis 34:265–73. [DOI] [PubMed] [Google Scholar]
- 26. Morris A, Lundgren JD, Masur H, Walzer PD, Hanson DL, et al. (2004) Current epidemiology of Pneumocystis pneumonia. Emerging infectious diseases 10:1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fei MW, Kim EJ, Sant CA, Jarlsberg LG, Davis JL, et al. (2009) Predicting mortality from HIV-associated Pneumocystis pneumonia at illness presentation: an observational cohort study. Thorax 64:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Charles BHEL, Rochelle PW, Yazdan Y, Kenneth AF (2003) Review of Human ImmunodeficiencyVirus Type 1–Related Opportunistic Infections in Sub-Saharan Africa. HIV/AIDS 2003:652–663. [Google Scholar]
- 29. Pinho AMF, Lopes GS, Ramos CF, Santos OD, Oliveira MPB (1994) Urinary tract infection in men with AIDS. Genitourin Med 70:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoepelman AIM, BarenMV, Brock JVD (1992) Bacteriuria in men with HIV-1 is related to their immune status(CD4+ cell count). AIDS 6:179–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data are available from the icddrb Institutional Research Unit for researchers who meet the criteria for access to confidential data. The following is the institutional website link- www.icddrb.org